CaO/MgO modified perovskite type oxides for chemical-looping steam reforming of methane
-
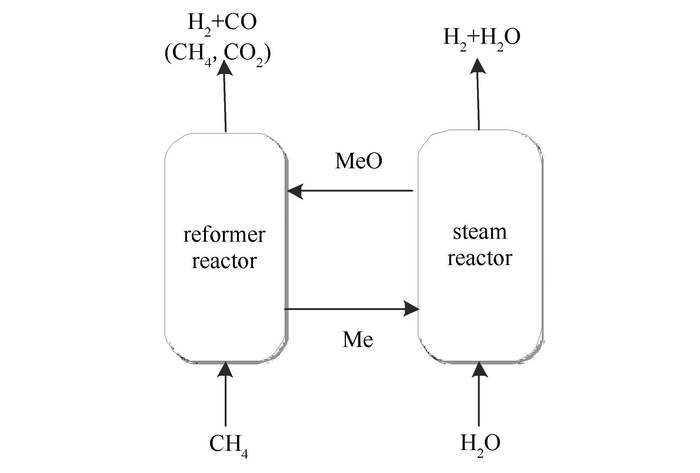
摘要: 甲烷化学链蒸汽重整(Chemical-looping steam methane reforming,CL-SMR)是基于化学链燃烧的概念而提出的一种新颖的技术。在重整反应器中,甲烷与载氧体中的晶格氧发生部分氧化反应生成合成气(H2/CO物质的量比为2.0),还原后的载氧体进入到水蒸气反应器中,与水蒸气反应恢复晶格氧的同时生成H2。以钙钛矿型氧化物LaFeO3为载氧体用于甲烷化学链蒸气重整过程,同时通过碱金属CaO和MgO对LaFeO3进行负载,以增大载氧体的比表面积、热稳定性和抗积炭能力。通过X射线衍射(XRD)、H2程序升温还原(H2-TPR)、BET比表面积分析(BET)和X光电子能谱(XPS)对载氧体进行表征。结果表明,三种载氧体均表现出较高的反应活性和合成气选择性,循环后仍能保持钙钛矿的结构。从反应性能、选择性和抗积炭能力等方面综合考虑,LaFeO3-CaO的效果最好,五次循环后具有很好的再生性。Abstract: Chemical-looping steam methane reforming (CL-SMR) is a novel method proposed on the base of chemical looping combustion (CLC) technology. In the CL-SMR scheme, methane is partially oxidized to syngas (H2/CO(molar ratio)=2.0) by the lattice oxygen in reformer reactor in the absence of gaseous oxidant, and then the reduced oxygen carrier is oxidized by steam to produce hydrogen in steam reactor. The use of perovskite type oxide LaFeO3 as an oxygen carrier in CL-SMR was studied. While the basicity of CaO/MgO modified oxygen carriers, LaFeO3-CaO and LaFeO3-MgO, were also synthesized aiming to increase specific surface area, thermostability, and resistance to coke formation. The synthesized oxides were characterized by X-ray diffraction (XRD), H2-temperature-programmed reduction (H2-TPR), Brunauer-Emmett-Teller (BET) surface area and X-ray photoelectron spectroscopy (XPS). Three oxygen carriers exhibited high active and selective for syngas production from methane, and maintained perovskite type over cyclic redox operations. The LF-CaO sample is the best candidate for the CL-SMR of the three samples judging from the reactivity, selectivity, and resistance to carbon formation. It showed good regenerability during 5 redox reactions.
-
Key words:
- chemical-looping /
- methane reforming /
- perovskite /
- CaO/MgO modified /
- redox
-
Table 1 Position and area percentage of the major reduction peaks for the three samples
Sample Peak position t/℃ Fractional peak area /% LF 515 3.3 702 96.7 LF-CaO 403 41.8 545 8.3 640 49.9 LF-MgO 435 2.9 560 45.4 780 51.7 Table 2 Specific surface area of perovskites
Oxygen carriers LF LF-CaO LF-MgO Specific surface area A/(m2·g-1) 3.5 20.3 21.7 Average pore diameter d/nm 28.4 3.7 11.2 Table 3 Surface elemental composition and relative proportion for the samples measured by XPS
Oxygen carrier Surface compositions /% La Fe Ca Mg OI OII OIII LF 20.2 7.7 - - 27.3 17.1 27.7 LF-CaO 13.7 5.5 7.8 - 24.8 26 22.2 LF-MgO 15.4 5.4 - 9.6 15.6 27.2 26.7 -
[1] GO K S, SON S R, KIM S D, KANG K S, PARK C S. Hydrogen production from two-step steam methane reforming in a fluidized bed reactor[J]. Int J Hydrogen Energy, 2009,34:1301-1309. doi: 10.1016/j.ijhydene.2008.11.062 [2] ZHU X, WANG H, WEI Y G, LI K Z, CHENG X M. Hydrogen and syngas production from two-step steam reforming of methane using CeO2 as oxygen carrier[J]. J Nat Gas Chem, 2011,20(3):281-286. doi: 10.1016/S1003-9953(10)60185-5 [3] 李然家, 余长春, 代小平, 沈师孔. 用晶格氧为氧源的甲烷部分氧化制合成气[J]. 催化学报, 2002,23(4):381-387. https://www.researchgate.net/publication/287915640_Partial_oxidation_of_methane_to_synthesis_gas_using_lattice_oxygen_instead_of_molecular_oxygenLI Ran-jia, YU Chang-chun, DAI Xiao-ping, SHEN Shi-kong. Partial oxidation of methane to synthesis gas using lattice oxygen instead of molecular oxygen[J]. Chin J Catal, 2002,23(4):381-387. https://www.researchgate.net/publication/287915640_Partial_oxidation_of_methane_to_synthesis_gas_using_lattice_oxygen_instead_of_molecular_oxygen [4] LI R J, YU C C, ZHU G R, SHEN S K, ZHANG Z X. Partial oxidation of natural gas to synthesis gas using lattice oxygen from A FeO3 (A=La, Nd, Sm, Eu) perovskite[J]. Chem Eng Oil Gas, 2004,33:5-8. https://www.researchgate.net/publication/286896492_Partial_oxidation_of_methane_to_synthesis_gas_using_lattice_oxygen [5] DAI X P, YU C C, LI R J, WU Q, SHI K J, HAO Z P. Effect of calcination temperature and reaction conditions on methane partial oxidation using lanthanum-based perovskite as oxygen donor[J]. J Rare Earths, 2008,26(3):341-346. doi: 10.1016/S1002-0721(08)60092-7 [6] MAGNUS R, ANDERS L, TOBIAS M, CHEN D, ANDERS H and ERLEND B. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1-xFeyCo1-yO3-δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4[J]. Int J Greenh Gas Con, 2008,2(1):21-36. doi: 10.1016/S1750-5836(07)00107-7 [7] DAI X P, YU C C, WU Q. Comparison of LaFeO3, La0.8Sr0.2FeO3, and La0.8Sr0.2Fe0.9Co0.1O3 perovskite oxides as oxygen carrier for partial oxidation of methane[J]. J Nat Gas Chem, 2008,17(4):415-418. doi: 10.1016/S1003-9953(09)60019-0 [8] 赵坤, 何方, 黄振, 郑安庆, 赵增立. La1-xSrxFeO3钙钛矿型氧化物中的晶格氧用于甲烷部分氧化制合成气[J].催化学报, 2014,35(7):1196-1205. doi: 10.1016/S1872-2067(14)60084-XZHAO K, HE F, HUANG Z, ZHENG A Q, LI H B, ZHAO Z L. La1-xSrxFeO3 perovskites as oxygen carriers for the partial oxidation of methane to syngas[J]. Chin J Catal, 2014,35(7):1196-1205. doi: 10.1016/S1872-2067(14)60084-X [9] ZHAO K, HE F, HUANG Z, ZHENG A Q, LI H B, ZHAO Z L. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane[J]. Int J Hydrogen Energy, 2014,39(7):3243-3252. doi: 10.1016/j.ijhydene.2013.12.046 [10] LI K Z, WANG H, WEI Y G, YAN D X. Partial oxidation of methane to syngas with air by lattice oxygen transfer over ZrO2-modified Ce-Fe mixed oxides[J]. Chem Eng J, 2011,173(2):574-582. doi: 10.1016/j.cej.2011.08.006 [11] ARYA S, NATHAN G, HUANG Y, CHEN Y, LI F. Fe2O3@LaxSr1-xFeO3 core-shell redox catalyst for methane partial oxidation[J]. Chem Cat Chem, 2014,6:790-799. [12] ROSSETTI I, FORNI L. Catalytic flameless combustion of methane over perovskites prepared by flame-hydrolysis[J]. Appl Catal B:Environ, 2001,33(4):345-352. doi: 10.1016/S0926-3373(01)00194-1 [13] HE F, LI X A, ZHAO K, HUANG Z, WEI G Q, LI H B. Catalytic flameless combustion of methane over perovskites prepared by flame-hydrolysis[J]. Fuel, 2013,108:465-473. doi: 10.1016/j.fuel.2012.11.035 [14] LI K Z, WANG H, WEI Y G, YAN D X. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2-Fe2O3 complex oxides[J]. Chem Eng J, 2010,156(3):512-518. doi: 10.1016/j.cej.2009.04.038 [15] PRITI V G, RAJESH B B. Pure phase LaFeO3 perovskite with improved surface area synthesized using different routes and its characterization[J]. Mater Chem Phys, 2010,119(1/2):324-329. https://www.researchgate.net/publication/248264027_Pure_phase_LaFeO_3_perovskite_with_improved_surface_area_synthesized_using_different_routes_and_its_characterization [16] MA H Q, TAN X, ZHU H M, ZHANG J Y, ZHANG L. XPS Characterization of La1-xCexFeO3 perovskite as high-temperture water-gas shift catalysts[J]. J Chin Rare Earth Soc, 2003,21(4):445-448. [17] LI X, ZHANG H B, LIU X X, LI S J, ZHAO M Y. XPS study on O(1s) and Fe(2p) for nanocrystalline composite oxide LaFeO3 with the perovskite structure[J]. Mater Chem Phys, 1994,38:355-362. doi: 10.1016/0254-0584(94)90213-5 [18] KEE Y K, HYUN S R, YU T S, DONG J S, WANG L Y, SEUNG B P. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process[J]. Appl Catal A:Gen, 2008,340(2):183-190. doi: 10.1016/j.apcata.2008.02.009 [19] QUINCOCES C E, DICUNDO S, ALVAREZ A M, GONZALEZ M G. Effect of addition of CaO on Ni/Al2O3 catalysts over CO2 reforming of methane[J]. Mater Lett, 2001,50(1):21-27. doi: 10.1016/S0167-577X(00)00406-7 [20] CHOUDHARY V R, UPHADE B S, MAMMAN A S. Large enhancement in methane-to-syngas conversion activity of supported Ni catalysts due to precoating of catalyst supports with MgO, CaO or rare-earth oxide[J]. Catal Lett, 1995,32(3-4):387-390. doi: 10.1007/BF00813233 [21] DUANE D M, RANJANI S. Fluidized-bed and fixed-bed reactor testing of methane chemical looping combustion with MgO-promoted hematite James Poston[J]. Appl Energy, 2015,146:111-121. doi: 10.1016/j.apenergy.2015.02.047 [22] HU J B, YU C L, ZHOU X C. Research progress of carbon deposition on catalysts during the partial oxidation of methane[J]. Nonferrous Met Sci Eng, 2012,3(2):5-11. [23] YU C L, XU H Y, GE Q J. Characterization of the metallicphase of Zn-doped Pt and Pt-Sn catalysts for propane dehydrogenation[J]. J Mol Catal A, 2007,266:80-89. doi: 10.1016/j.molcata.2006.10.025 -





 下载:
下载: