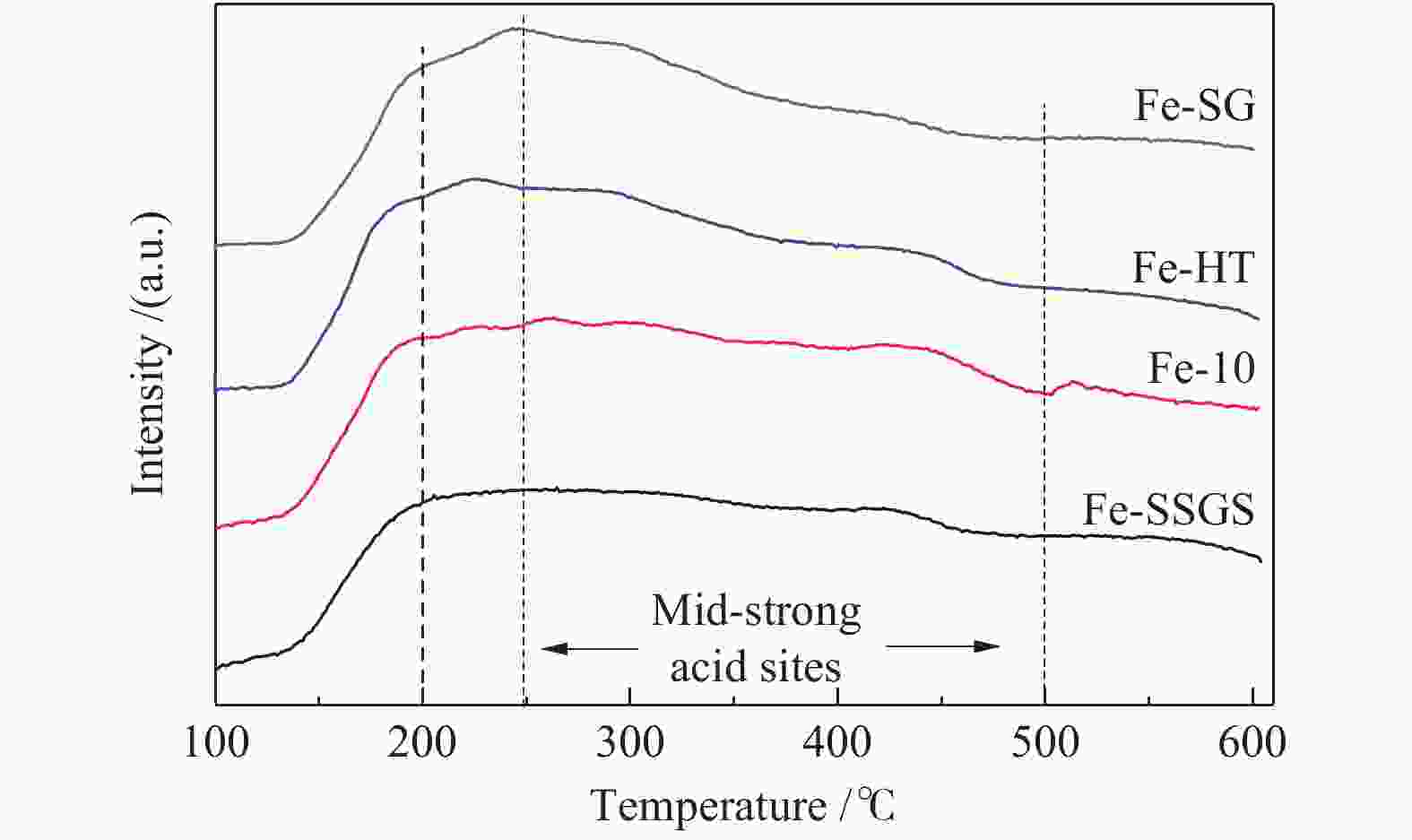
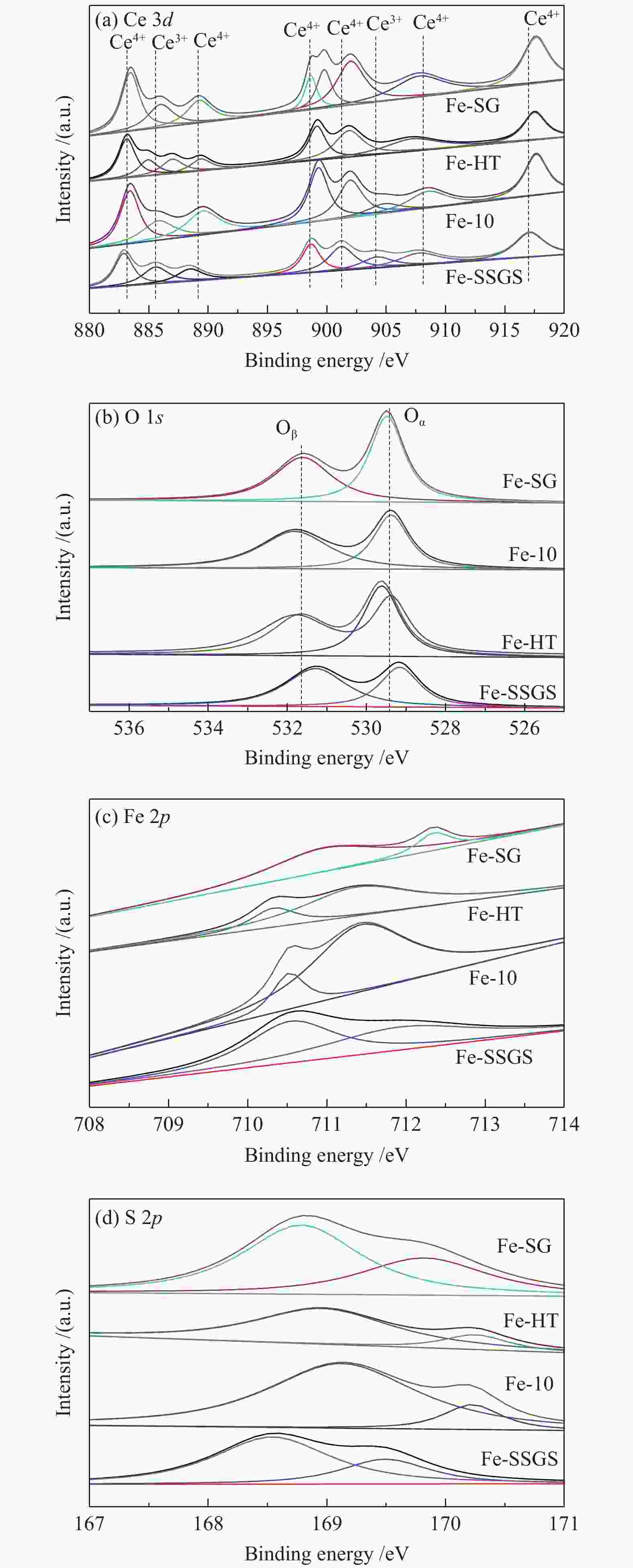
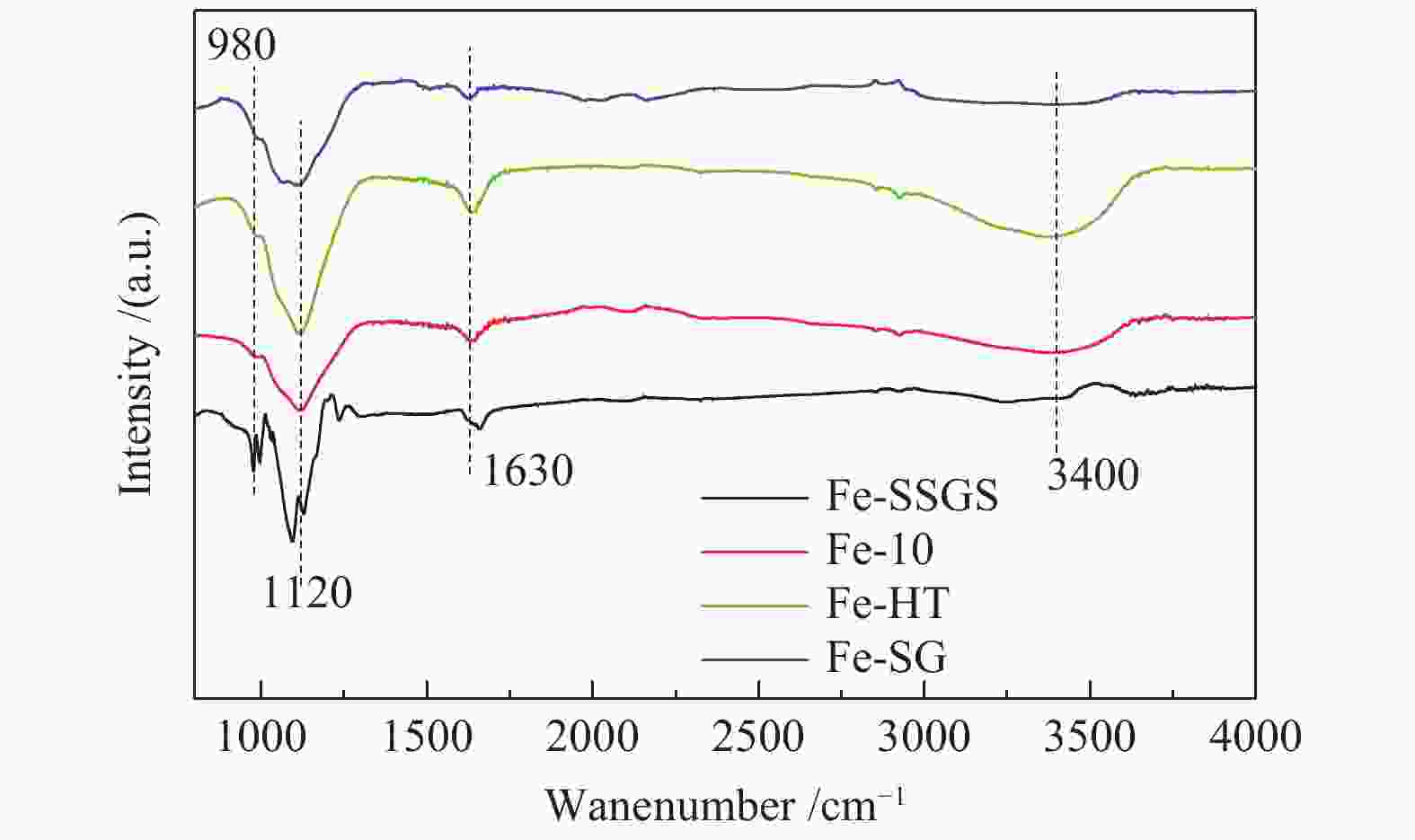
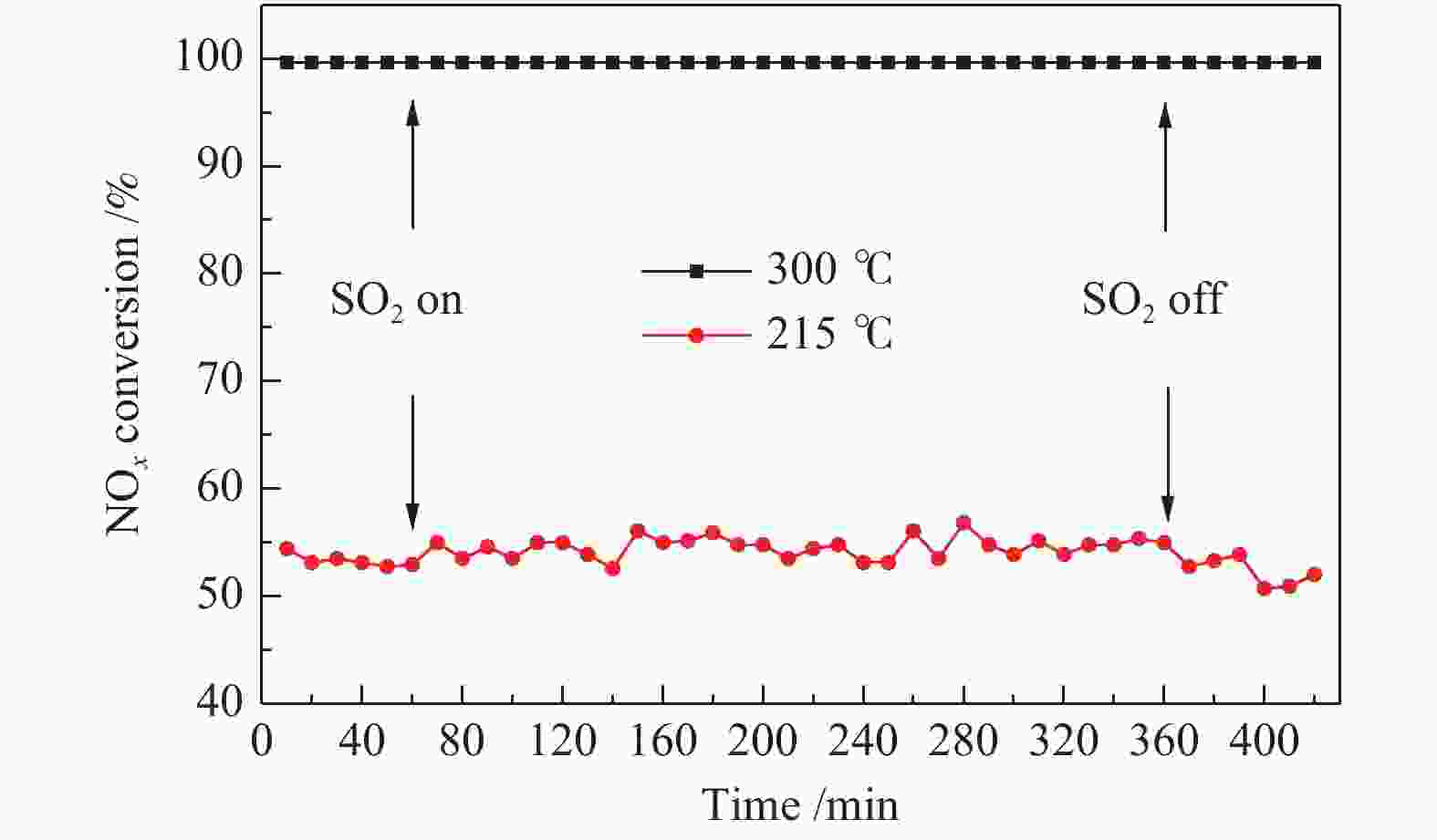
Effect of sulfate species on the performance of Ce-Fe-Ox catalysts in the selective catalytic reduction of NO by NH3
-
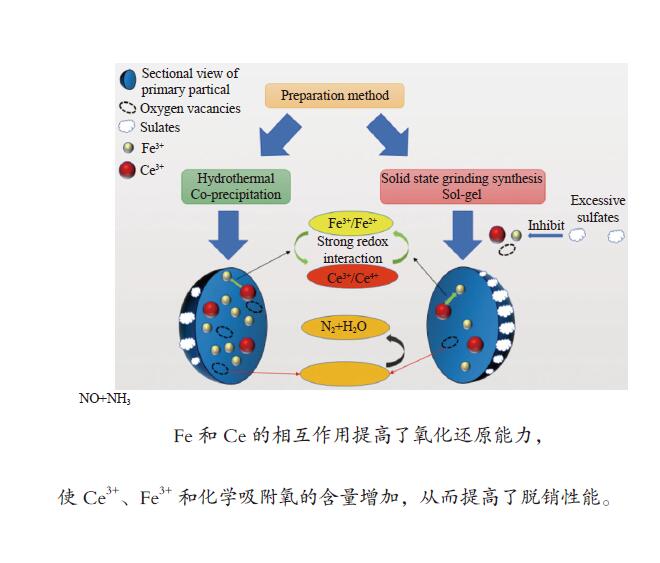
摘要: 采用四种不同的方法制备了一系列含
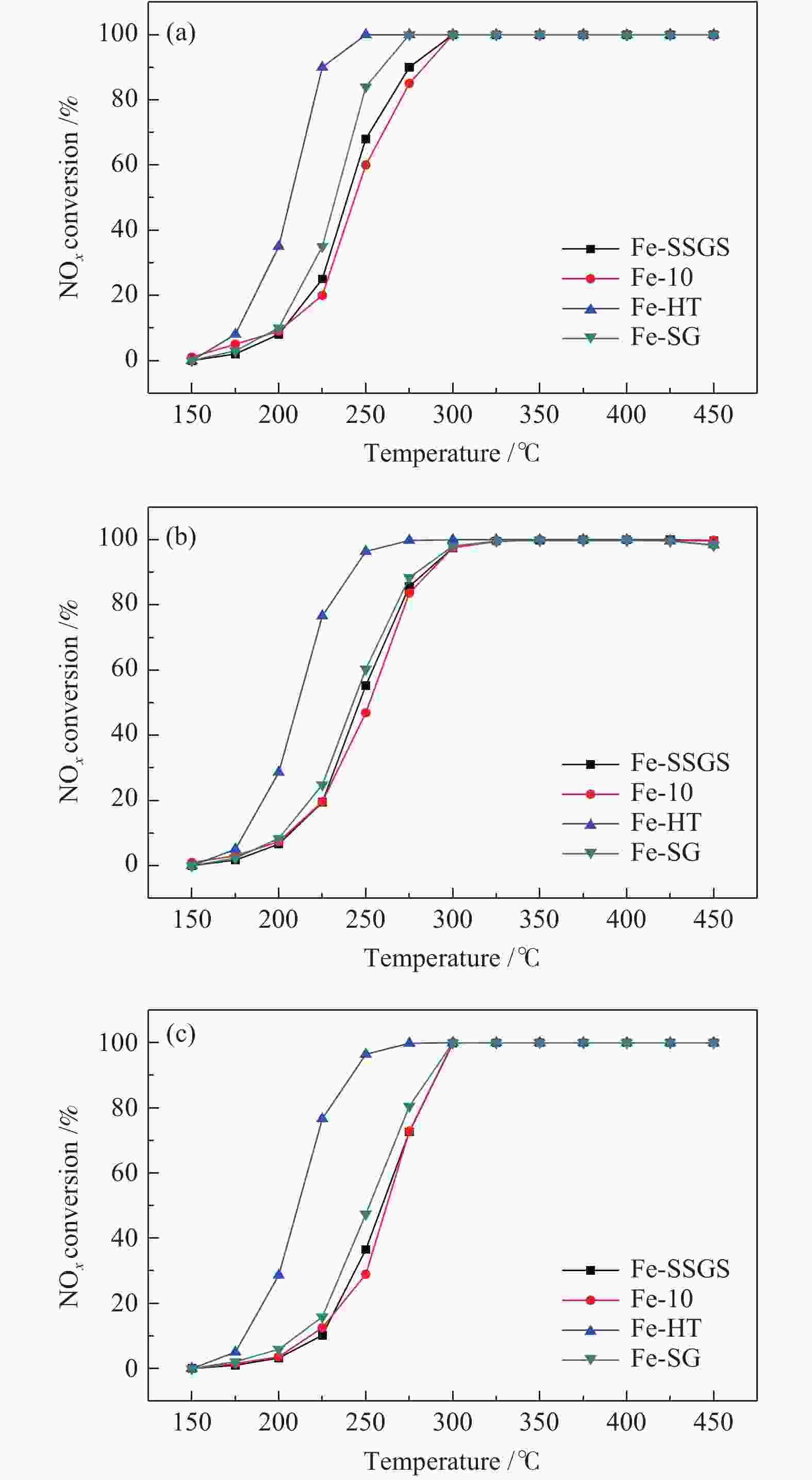
$ {\rm{SO}}^{2-}_{4} $ 改性Ce-Fe-Ox催化剂,并研究其NH3选择性催化还原NOx的催化活性。结果表明,水热法制备的Ce-Fe-Ox(Fe-HT)可提高其催化性能。其优异的SCR性能与硫酸的加入有关,$ {\rm{SO}}^{2-}_{4} $ 的加入会导致CeO2晶体的弱化,提高其催化活性。Fe和Ce协同作用可提高催化剂的氧化还原能力,进而提高化学吸附氧的含量、Ce3+/(Ce4+ + Ce3+)和Fe3+/(Fe3+ + Fe2+)的比例,从而提高催化性能。过量的硫酸盐会导致Fe3+和Ce3+的下降,降低催化性能。Abstract: A series of Ce-Fe-Ox catalysts containing sulfate species was prepared by four different methods including sol-gel (Fe-SG), hydrothermal (Fe-HT), co-precipitation (Fe-10) and solid state grinding synthesis (Fe-SSGS) methods. The Ce-Fe-Ox catalysts were used in the selective catalytic reduction (SCR) of NOx by NH3 and the effect of sulfate species on the catalytic performance was investigated. The results indicate that the Fe-HT catalyst prepared by the hydrothermal method exhibits excellent performance in the NH3-SCR of NO, with a NO conversion of nearly 100% even at 250 °C. The Fe-HT catalyst contains proper amount of sulfate, which can decrease the CeO2 crystallinity and enhance the catalytic performance in the NH3-SCR of NO. The Fe-HT catalyst has a high content of surface Ce3+ and Fe3+ and there is a synergy between the Fe and Ce species, which can improve the redox capacity and greatly increase the quantity of chemisorbed oxygen. In contrast, excessive amount of sulfate species in Fe-SSGS and Fe-SG may reduce the synergy between Fe and Ce and then impair the catalytic performance of Ce-Fe-Ox in NH3-SCR.-
Key words:
- selective catalytic reduction /
- sulfates /
- surface acidity /
- chemisorption oxygen
-
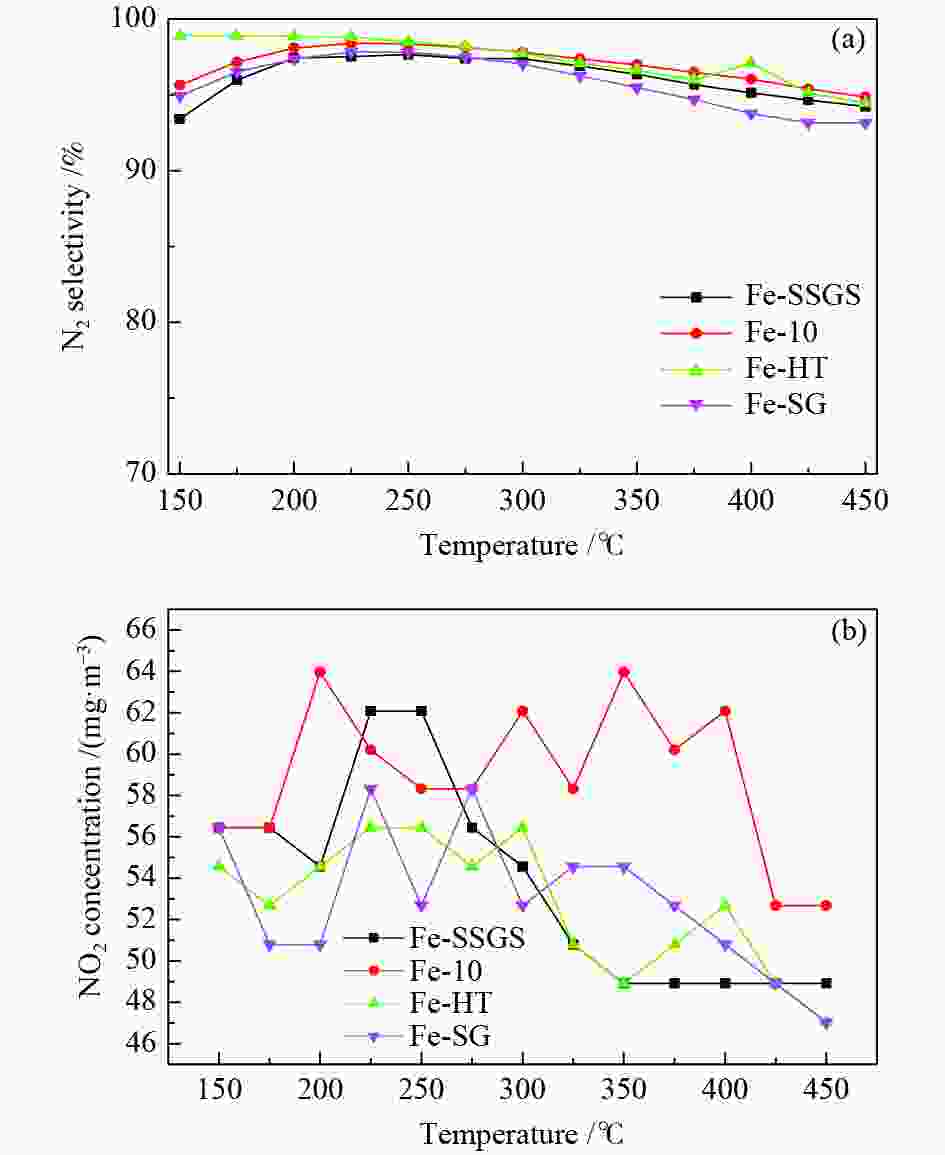
图 2 Fe-SSGS、Fe-10、Fe-HT和Fe-SG催化剂(a)N2选择性,(b) 催化NO氧化成NO2的性能
Figure 2 (a) N2 selectivity and (b) performance test diagram of NO oxidation to NO2 over the Fe-SSGS, Fe-10, Fe-HT and Fe-SG catalysts. Reaction conditions: 0.2 mL catalyst; inlet gas = 0.05% NO, 0.05% NH3, 5% O2, N2 as balance; total flow rate = 200 mL/min
表 1 Fe-SSGS、Fe-10、Fe-HT和Fe-SG催化剂的比表面积和总孔体积
Table 1 Specific surface area and total pore volume of Fe-SSGS, Fe-10, Fe-HT and Fe-SG samples
Sample SBET/
(m2·g−1)Total pore volume/
(cm3·g−1)Average pore
diameter/nmFe-SSGS 33 0.20 12.3 Fe-10 50 0.40 15.9 Fe-HT 47 0.37 15.6 Fe-SG 13 0.08 12.0 表 2 XPS结合能和表面原子比
Table 2 Binding energies and surface atomic ratios derived from XPS
Sample Surface atomic concentration/atomic% Ce3+/
(Ce4+ + Ce3+)Oα/
(Oα + Oβ)Fe3+/
(Fe3+ + Fe2+)Fe-SSGS 19.3 41.8 48.7 Fe-10 29.2 45.2 84.7 Fe-HT 34.1 51.6 79.2 Fe-SG 24.6 45.3 18.2 -
[1] SCHNEIDER H, SCHARF U, WOKAUN A, BAIKER A. Chromia on titania: IV. Nature of active sites for selective catalytic reduction of NO by NH3[J]. J Catal,1994,146(2):545−556. doi: 10.1006/jcat.1994.1093 [2] BRANDENBERGER S, KRÖCHER O, TISSLER A, ALTHOFF R. The state of the art in selective catalytic reduction of NOx by Ammonia using metal-exchanged zeolite catalysts[J]. Catal Rev Sci Eng,2008,50(4):492−531. doi: 10.1080/01614940802480122 [3] GU T, JIN R, LIU Y, WENG X, WU Z. Promoting effect of calcium doping on the performances of MnOx/TiO2 catalysts for NO reduction with NH3 at low temperature[J]. Appl Catal B: Environ,2013,129:30−38. doi: 10.1016/j.apcatb.2012.09.003 [4] LI J, CHEN J, KE R, LUO C, HAO J. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts[J]. Catal Commun,2007,8(12):1896−1900. doi: 10.1016/j.catcom.2007.03.007 [5] GAO X, JIANG Y, FU Y, ZHONG Y, LUO Z, CEN K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3[J]. Catal Commun,2010,11(5):465−469. doi: 10.1016/j.catcom.2009.11.024 [6] SONG Z, ZHANG Q, NING P, FAN J, DUAN Y, LIU X, HUANG Z. Effect of CeO2 support on the selective catalytic reduction of NO with NH3 over PW/CeO2[J]. J Taiwan Inst Chem Eng,2016,65:149−161. doi: 10.1016/j.jtice.2016.04.034 [7] SONG Z, NING P, ZHANG Q, LIU X, ZHANG J, WANG Y, DUAN Y, HUANG Z. The role of surface properties of silicotungstic acid doped CeO2 for selective catalytic reduction of NOx by NH3: Effect of precipitant[J]. J Mol Catal A: Chem,2016,413:15−23. doi: 10.1016/j.molcata.2015.12.009 [8] SONG Z, YIN L, ZHANG Q, NING P, DUAN Y, WANG J, LIU X, LONG K, HUANG Z. Relationship between the WO3 states and reaction pathway over CeO2-ZrO2-WO3 catalysts for selective catalytic reduction of NO with NH3[J]. Mol Catal,2017,437:95−104. doi: 10.1016/j.mcat.2017.04.033 [9] TOPSØE N Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy[J]. Science,1994,265(5176):1217−1219. doi: 10.1126/science.265.5176.1217 [10] SUN Y, GUO Y, SU W, WEI Y. Low-temperature selective catalytic reduction of NO with NH3 over Fe-Ce-Ox catalysts[J]. Trans Tianjin Univ,2017,23(1):35−42. doi: 10.1007/s12209-016-0017-y [11] HU W, GAO X, DENG Y, QU R, ZHENG C, ZHU X, CEN K. Deactivation mechanism of arsenic and resistance effect of SO42− on commercial catalysts for selective catalytic reduction of NOx with NH3[J]. Chem Eng J,2016,293:118−128. doi: 10.1016/j.cej.2016.02.095 [12] YAO X, WANG Z, YU S, YANG F, DONG L. Acid pretreatment effect on the physicochemical property and catalytic performance of CeO2 for NH3-SCR[J]. Appl Catal A: Gen,2017,542:282−288. doi: 10.1016/j.apcata.2017.06.003 [13] AZAMBRE B, ZENBOURY L, WEBER J V, BURG P. Surface characterization of acidic ceria–zirconia prepared by direct sulfation[J]. Appl Surf Sci,2010,256(14):4570−4581. doi: 10.1016/j.apsusc.2010.02.049 [14] LIU Z, XING L, MA H, CHENG L, LIU J, YANG J, ZHANG Q. Sulfated Ce-doped TiO2 as visible light driven photocatalyst: Preparation, characterization and promotion effects of Ce doping and sulfation on catalyst performance[J]. Environ Prog Sustain Energy,2017,36(2):494−504. doi: 10.1002/ep.12511 [15] 张秋林, 张金辉, 宁平, 宋忠贤, 王燕彩, 徐利斯, 唐小苏. SO42-改性对Ce、Ti基催化剂NH3-SCR脱硝性能的影响[J]. 昆明理工大学学报(自然科学版),2014,(6):110−115.ZHANG Qiu-lin, ZHANG Jin-hui, NING Ping, SONG Zhong-xian, WANG Yan-cai, XU Li-si, TANG Xiao-su. Enhanced effect of SO42− on Ce and Ti-based catalysts for selective catalytic reduction of NOx with NH3[J]. J Kunming Univ Sci Technol (Nat Sci),2014,(6):110−115. [16] ZHANG X, WANG J, SONG Z, ZHAO H, XING Y, ZHAO M, ZHAO J, MA Z, ZHANG P, TSUBAKI, N. Promotion of surface acidity and surface species of doped Fe and SO42− over CeO2 catalytic for NH3-SCR reaction[J]. Mol Catal,2019,463:1−7. doi: 10.1016/j.mcat.2018.11.002 [17] 边雪, 肖坤宇, 王书豪, 邱保龙. Fe、Ce改性锰钛催化剂的制备及其低温脱硝性能研究[J]. 功能材料,2019,50(7):07090−07095. doi: 10.3969/j.issn.1001-9731.2019.07.016BIAN Xue, XIAO Kun-yu, WANG Shu-hao, QIU Bao-long. Preparation and the study on low-temperature denitrification of Fe, Ce modified manganese titanium catalyst[J]. J Funct Mater,2019,50(7):07090−07095. doi: 10.3969/j.issn.1001-9731.2019.07.016 [18] LIU J, LI X, ZHAO Q, KE J, XIAO H, LV X, LIU S, TADÉ M, WANG, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FT-IR analysis for structure-activity correlation[J]. Appl Catal B: Environ,2017,200:297−308. doi: 10.1016/j.apcatb.2016.07.020 [19] QU R, GAO X, CEN K, LI J. Relationship between structure and performance of a novel cerium-niobium binary oxide catalyst for selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ,2013,142:290−297. [20] TAKITA Y, MORIYAMA J, NISHIGUCHI H, ISHIHARA T, HAYANO F, NAKAJO T. Decomposition of CCl2F2 over metal sulfate catalysts[J]. Catal Today,2004,88(3/4):103−109. doi: 10.1016/j.cattod.2003.11.003 [21] YAO X, CHEN L, CAO J, YANG F, TAN W, DONG L. Morphology and crystal-plane effects of CeO2 on TiO2/CeO2 catalysts during NH3-SCR reaction[J]. Ind Eng Chem Res,2018,57(37):12407−12419. doi: 10.1021/acs.iecr.8b02830 [22] PETROV K I, IVANOV V I, PERVYKH V G. Vibrational spectra of lanthanum, cerium, praseodymium, and neodymium sulfate pentahydrates[J]. J Struct Chem,1967,8(2):310−312. doi: 10.1007/BF00745659 [23] GAO S, WANG P, CHEN X, WANG H, WU Z, LIU Y, WENG X. Enhanced alkali resistance of CeO2/SO42−–ZrO2 catalyst in selective catalytic reduction of NOx by ammonia[J]. Catal Commun,2014,43:223−226. doi: 10.1016/j.catcom.2013.10.017 [24] YU Y, CHEN C, MA M, DOUTHWAITE M, HE C, MIAO J, CHEN J, LI C. SO2 promoted in situ recovery of thermally deactivated Fe2(SO4)3/TiO2 NH3-SCR catalysts: from experimental work to theoretical study[J]. Chem Eng J,2019,361:820−829. doi: 10.1016/j.cej.2018.12.149 [25] LI Y, SONG W, LIU J, ZHAO Z, GAO M, WEI Y, WANG Q, DENG J. The protection of CeO2 thin film on Cu-SAPO-18 catalyst for highly stable catalytic NH3-SCR performance[J]. Chem Eng J,2017,330:926−935. doi: 10.1016/j.cej.2017.08.025 [26] MEUNIER F C, ROSS J R H. Effect of ex situ treatments with SO2 on the activity of a low loading silver–alumina catalyst for the selective reduction of NO and NO2 by propene[J]. Appl Catal B: Environ,2000,24(1):23−32. doi: 10.1016/S0926-3373(99)00088-0 [27] SCHILL L, PUTLURU S S R, FEHRMANN R, JENSEN A D. Low-temperature NH3-SCR of NO on mesoporous Mn0.6Fe0.4/TiO2 prepared by a hydrothermal method[J]. Catal Lett,2014,144(3):395−402. doi: 10.1007/s10562-013-1176-2 [28] WANG D, PENG Y, XIONG S C, LI B, GAN L N, LU C M, CHEN J J, MA Y L, LI J H. De-reducibility mechanism of titanium on maghemite catalysts for the SCR reaction: an in situ DRIFTS and quantitative kinetics study[J]. Appl Catal B: Environ,2018,221:556−564. doi: 10.1016/j.apcatb.2017.09.045 [29] FEYZI M, IRANDOUST M, MIRZAEI A A. Effects of promoters and calcination conditions on the catalytic performance of iron-manganese catalysts for fischer-tropsch synthesis[J]. Fuel Process Technol,2011,92(5):1136−1143. doi: 10.1016/j.fuproc.2011.01.010 [30] ABDELSAYED V, SHEKHAWAT D, SMITH M W. Effect of Fe and Zn promoters on Mo/HZSM-5 catalyst for methane dehydroaromatization[J]. Fuel,2015,139:401−410. doi: 10.1016/j.fuel.2014.08.064 [31] LIU X L, GUO J X, CHU Y H, LUO D M, YIN H Q, SUN M C, YAVUZ R. Desulfurization performance of iron supported on activated carbon[J]. Fuel,2014,123:93−100. doi: 10.1016/j.fuel.2014.01.068 [32] SOH B W, NAM I S. Effect of support morphology on the sulfur tolerance of V2O5/Al2O3 catalyst for the reduction of NO by NH3[J]. Ind Eng Chem Res,2003,42(13):2975−2986. doi: 10.1021/ie020861b [33] KIJLSTRA W S, BIERVLIET M, POELS E K, BLIEK A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B: Environ,1998,16(4):327−337. doi: 10.1016/S0926-3373(97)00089-1 [34] LEE K J, KUMAR P A, MAQBOOL M S, RAO K N, SONG K H, HA H P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3-SCR: Physico-chemical properties and catalytic activity[J]. Appl Catal B: Environ,2013,142/143(10):705−717. [35] CHANG H, LI J, YUAN J, CHEN L, DAI Y, ARANDIYAN H, XU J, HAO J. Ge, Mn-doped CeO2-WO3 catalysts for NH3-SCR of NOx: effects of SO2 and H2 regeneration[J]. Catal Today,2013,201(1):139−144. [36] SHEN Y, MA Y, ZHU S. Promotional effect of zirconium additives on Ti0.8Ce0.2O2 for selective catalytic reduction of NO[J]. Catal Sci Technol,2012,2(3):589−599. doi: 10.1039/C2CY00363E [37] SULTANA A, SASAKI M, HAMADA H. Influence of support on the activity of Mn supported catalysts for SCR of NO with ammonia[J]. Catal Today,2012,185(1):284−289. doi: 10.1016/j.cattod.2011.09.018 [38] DAMYANOVA S, PEREZ C A, SCHMAL M, BUENO J M C. Characterization of ceria-coated alumina carrier[J]. Appl Catal A: Gen,2002,234(1/2):271−282. doi: 10.1016/S0926-860X(02)00233-8 [39] MURUGAN B, RAMASWAMY A V. Chemical states and redox properties of Mn/CeO2−TiO2 nanocomposites prepared by solution combustion route[J]. J Phys Chem C,2008,112(51):20429−20442. doi: 10.1021/jp806316x [40] OTSUKA K, WANG Y, NAKAMURA M. Direct conversion of methane to synthesis gas through gas-solid reaction using CeO2-ZrO2 solid solution at moderate temperature[J]. Appl Catal A: Gen,1999,183(2):317−324. doi: 10.1016/S0926-860X(99)00070-8 [41] LI Y, SUN Q, KONG M, SHI W, HUANG J, TANG J, ZHAO X. Coupling oxygen ion conduction to photocatalysis in mesoporous nanorod-like ceria significantly improves photocatalytic efficiency[J]. J Phys Chem C,2011,115(29):14050−14057. doi: 10.1021/jp202720g [42] ZHANG Z, CHEN L, LI Z, LI P, YUAN F, NIU X, ZHU Y. Activity and SO2 resistance of amorphous CeaTiOx catalysts for the selective catalytic reduction of NO with NH3: in situ DRIFT studies[J]. Catal Sci Technol,2016,6(19):7151−7162. doi: 10.1039/C6CY00475J [43] LIU X, ZHOU K, WANG L, WANG B, LI Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. J Am Chem Soc,2009,131(9):3140−3141. doi: 10.1021/ja808433d [44] CHANG H, CHEN X, LI J, MA L, WANG C, LIU C, SCHWANK J W, HAO J. Improvement of activity and SO2 tolerance of Sn-modified MnOx-CeO2 catalysts for NH3-SCR at low temperatures[J]. Environ Sci Technol,2013,47(10):5294−5301. doi: 10.1021/es304732h [45] GUO M, LIU Q, ZHAO P, HAN J, LI X, HA Y, FU Z, SONG C, JI N, LIU C, MA D, LI Z. Promotional effect of SO2 on Cr2O3 catalysts for the marine NH3-SCR reaction[J]. Chem Eng J,2019,361:830−838. doi: 10.1016/j.cej.2018.12.100 [46] GUO R T, LU C Z, PAN W G, ZHEN W L, WANG Q S, CHEN Q L, DING H L, YANG N Z. A comparative study of the poisoning effect of Zn and Pb on Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3[J]. Catal Commun,2015,59:136−139. doi: 10.1016/j.catcom.2014.10.006 [47] DU X, ZHANG D, SHI L, GAO R, ZHANG J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane[J]. J Phys Chem C,2012,116(18):10009−10016. doi: 10.1021/jp300543r [48] KRISHNA K, BUENO-LÓPEZ A, MAKKEE M, MOULIJN J A. Potential rare-earth modified CeO2 catalysts for soot oxidation part Ⅱ: Characterisation and catalytic activity with NO + O2[J]. Appl Catal B: Environ,2007,75(3/4):201−209. doi: 10.1016/j.apcatb.2007.04.007 [49] HU W, ZHANG Y, LIU S, ZHENG C, GAO X, NOVA I, TRONCONI E. Improvement in activity and alkali resistance of a novel V-Ce(SO4)2/Ti catalyst for selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ,2017,206:449−460. doi: 10.1016/j.apcatb.2017.01.036 [50] DELAHAY G, VALADE D, GUZMAN-VARGAS A, COQ B. Selective catalytic reduction of nitric oxide with ammonia on Fe-ZSM-5 catalysts prepared by different methods[J]. Appl Catal B: Environ,2005,55(2):149−155. doi: 10.1016/j.apcatb.2004.07.009 [51] GENG Y, CHEN X, YANG S, LIU F, SHAN W. Promotional Effects of Ti on a CeO2-MoO3 Catalyst for the Selective Catalytic Reduction of NOx with NH3[J]. ACS Appl Mater Interfaces,2017,9(20):16951−16958. doi: 10.1021/acsami.6b05380 [52] ZHANG Q, ZHANG J, SONG Z, NING P, LI H, LIU X. A novel and environmentally friendly SO42−/CeO2 catalyst for the selective catalytic reduction of NO with NH3[J]. J Ind Eng Chem,2016,34:165−171. doi: 10.1016/j.jiec.2015.11.006 [53] 中本一雄, 黄德如. 无机和配位化合物的红外和拉曼光谱[M]. 北京: 化学工业出版社, 1986.NAKAMOTO K, HUANG De-ru. Infrared and Raman Spectra of Inorganic Coordination Compound[M]. Beijing: Chemical Indusry Press, 1986. -






 下载:
下载: