In-situ study of effect of migrating alkali metals on gasification reactivity of coal char
-
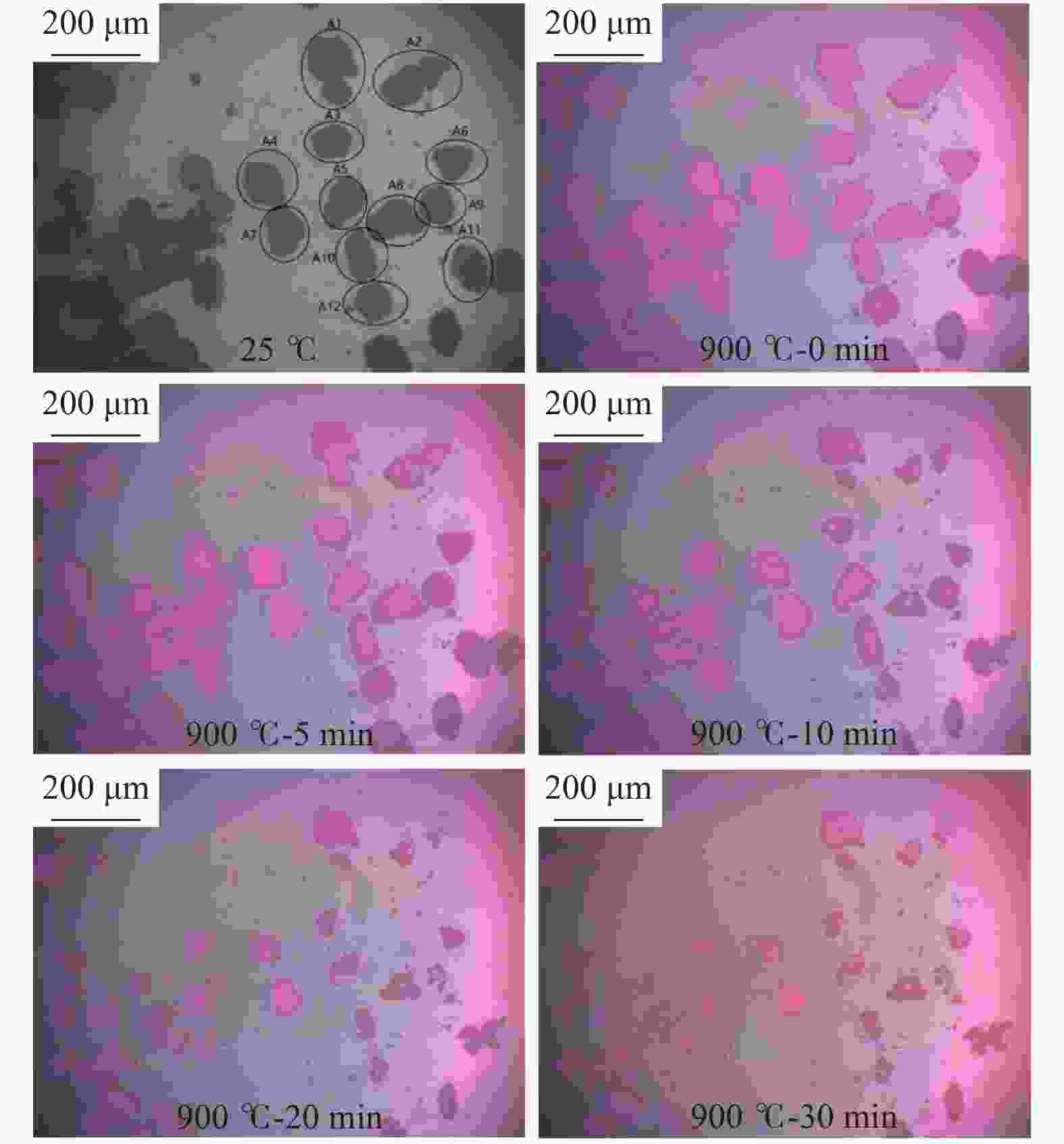
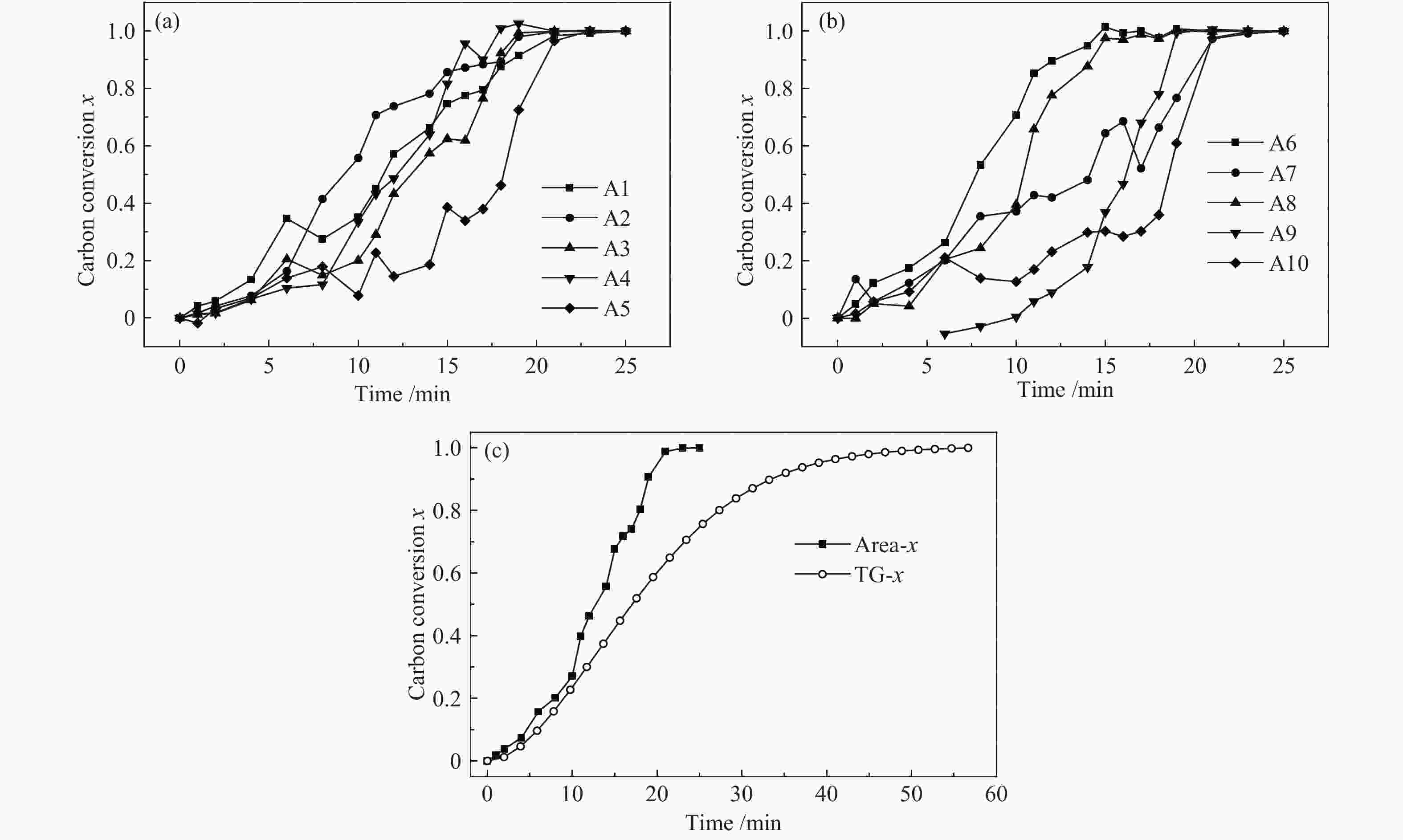
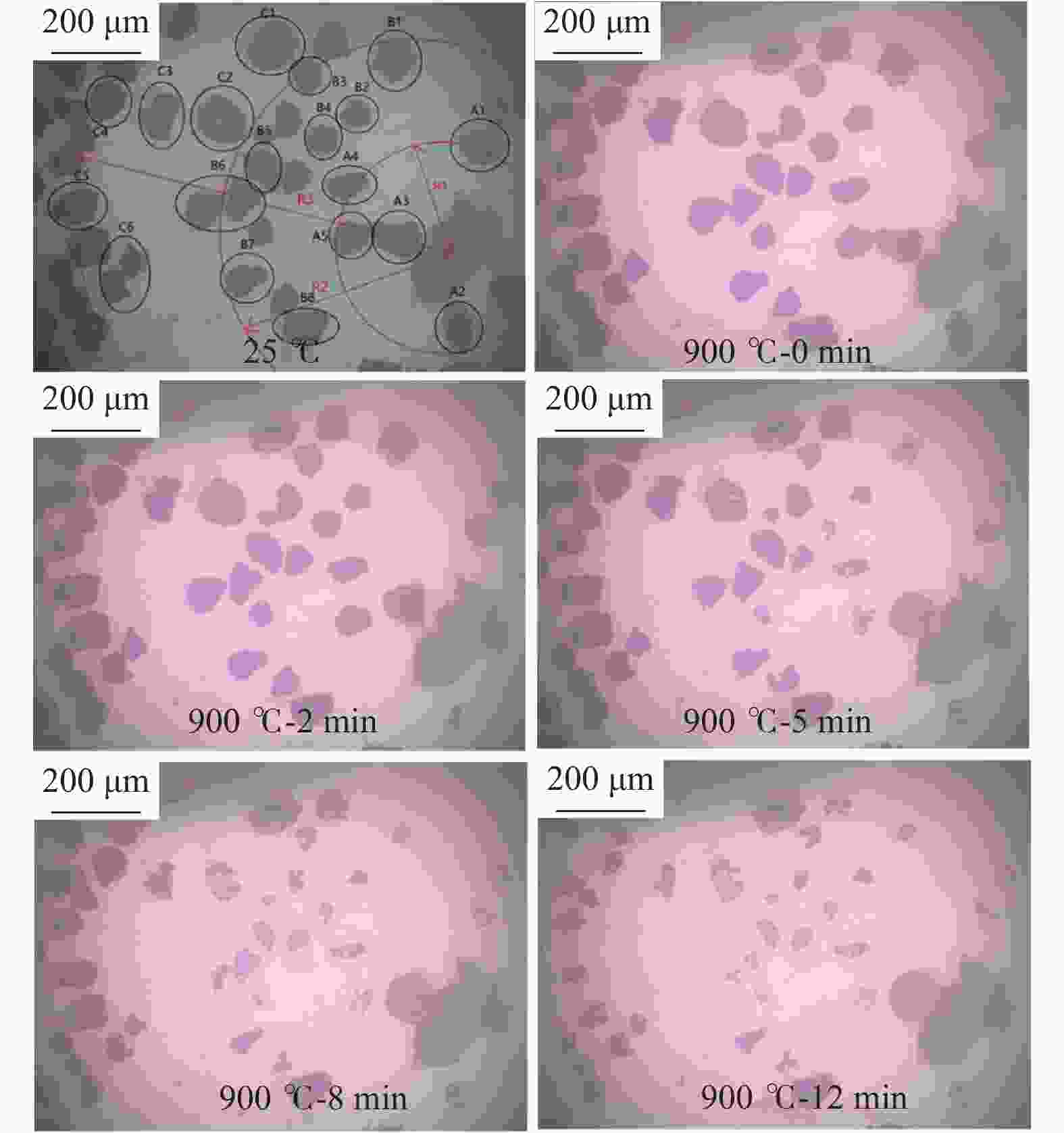
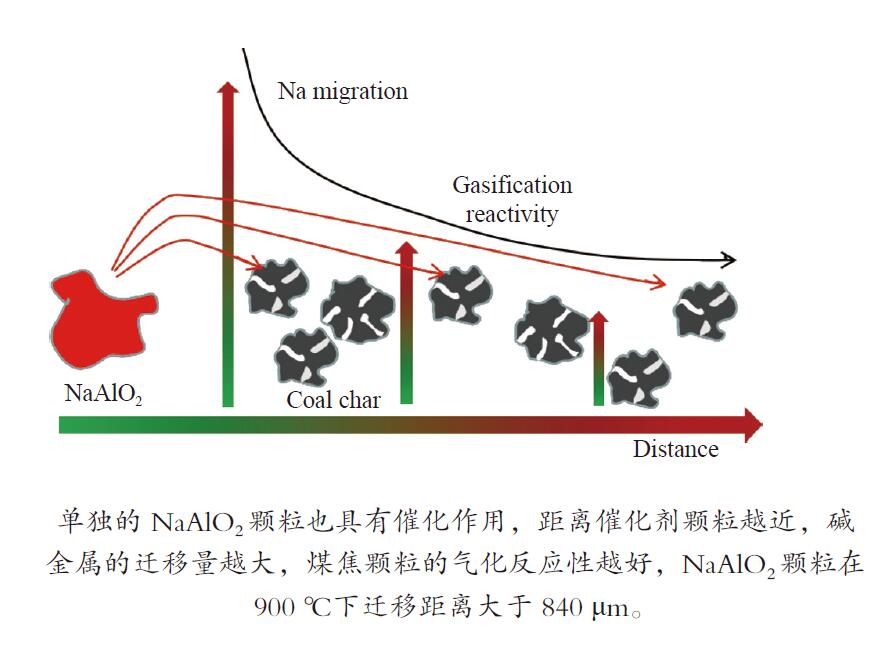
摘要: 本研究通过热重与高温热台显微镜分析了神木煤焦颗粒的原位气化行为,探究了单颗粒NaAlO2催化剂的原位催化作用,并结合SEM-EDX探究了碱金属的分布。结果表明,在气化初期,面积法与热重法得到的碳转化率曲线较为一致;在气化后期,煤焦中灰分会形成颗粒的骨架,在气化过程中使煤焦颗粒面积不发生变化,灰分阻碍气化剂向煤焦扩散,使气化速率降低,通过面积法计算的碳转化率小于热重法。单独的NaAlO2颗粒也具有催化作用,距离催化剂颗粒越近,碱金属的迁移量越大,煤焦颗粒的气化反应性越好,NaAlO2颗粒在900 ℃下迁移距离大于840 μm。Abstract: The migrating and diffusion of alkali metals affect catalytic gasification of coal char. The paper investigated in-situ gasification behaviors of coal char by TG and hot stage microscope, and studied catalytic performance of single NaAlO2 particle and Na distribution by SEM-EDX. The results show that in the beginning stage, the curve of carbon conversion obtained by area method is consistent with that by TG method. In the ending stage, the ash in coal char acts as framework of the particles, as a result, area of the particles keeps unchanged. Meanwhile, the ash hinders diffusion of gasifying agent into coal char, and decreases the gasification rate, so carbon conversion rate calculated by area change is lower than that from TG. The single NaAlO2 particle has catalytic performance. The coal char particles, which are closer to NaAlO2, have higher gasification reactivity, because the amount of migrating alkali is higher. The migrating distance of NaAlO2 is higher than 840 μm at 900 ℃ .
-
Key words:
- catalytic gasification /
- alkali metals /
- gasification /
- migrating of alkali metals
-
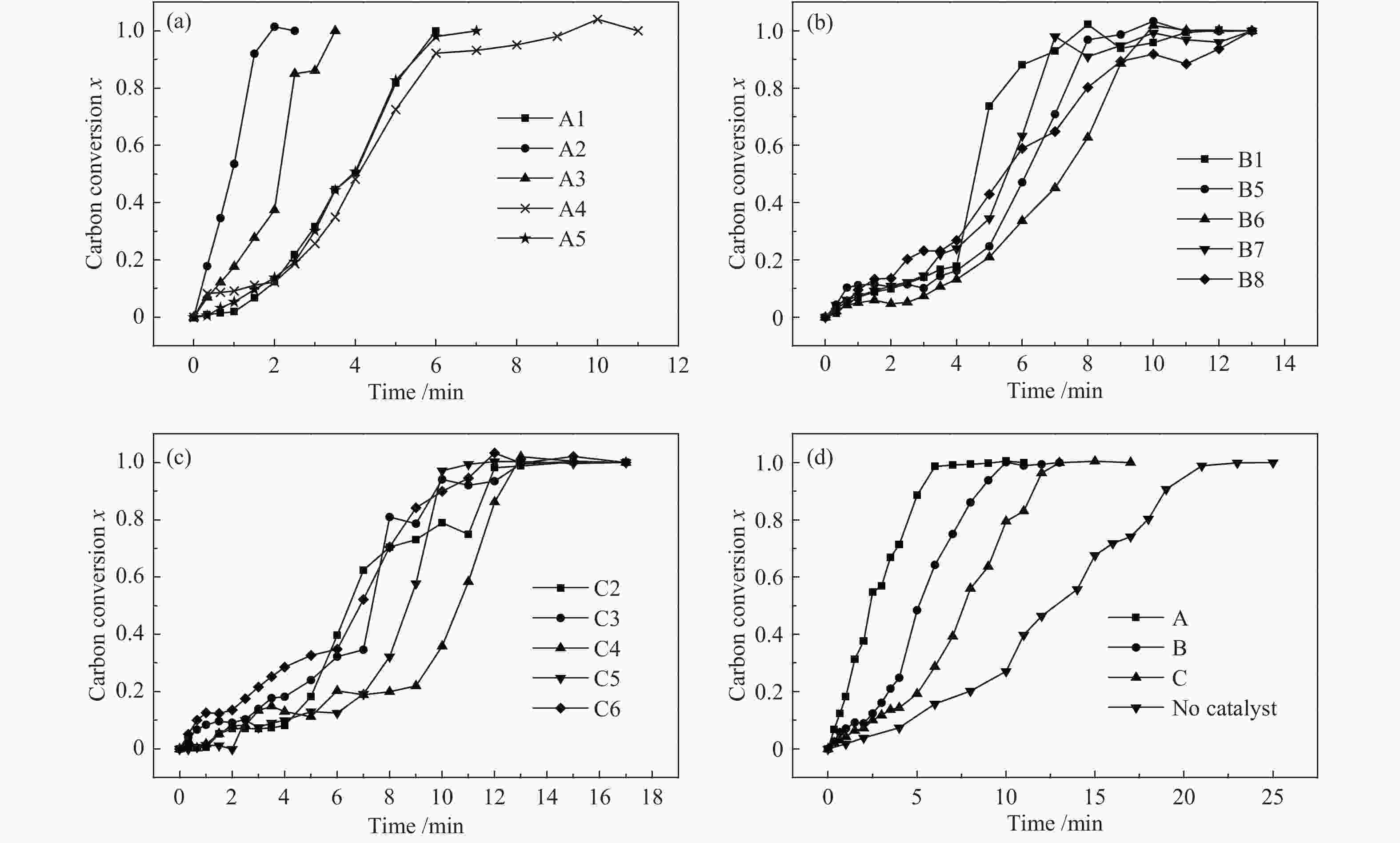
图 4 距离NaAlO2颗粒不同距离煤焦颗粒的气化行为
Figure 4 Gasification behaviors of char particles with different distance to catalyst particles
(a): the gasification behaviors of char particles in A area; (b): the gasification behaviors of char particles in B area; (c): the gasification behaviors of char particles in C area; (d): the comparison of the mean gasification behaviors between different area and uncatalytic gasification
表 1 神木烟煤与煤焦的工业分析和元素分析
Table 1 Proximate and ultimate analyses of SM coal and SMC
Sample Proximate analysis
wad/%Ultimate analysis
wdaf/%V FC A M C H N S O* SM coal 32.46 59.25 6.73 1.56 82.63 5.07 1.10 0.16 11.04 SMC 1.40 87.72 10.31 0.57 96.36 0.79 1.39 0.15 1.31 ad: air-dried basis; daf: dry ash-free basis; *: by difference 表 2 神木烟煤煤灰的矿物质组成
Table 2 Ash compositions of SM coal
Sample Content w/% Al2O3 SiO2 Fe2O3 CaO TiO2 MgO Na2O SO3 K2O P2O5 SM coal 16.85 48.07 5.79 19.42 0.68 1.04 0.6 3.65 0.81 0.22 -
[1] 李风海, 房倚天. 煤焦气化影响因素及其反应特性研究进展[J]. 菏泽学院学报,2010,32(5):69−73. doi: 10.3969/j.issn.1673-2103.2010.05.018LI Feng-hai, FANG Yi-tian. Research progress on influencing factors and reaction characteristics of coal gasification[J]. J Heze Univ,2010,32(5):69−73. doi: 10.3969/j.issn.1673-2103.2010.05.018 [2] KIM YK, PARK J, JUNG D, MOCHIDA I. Low-temperature catalytic conversion of lignite: 2. Recovery and reuse of potassium carbonate supported on perovskite oxide in steam gasification[J]. J Ind Eng Chem,2014,20(1):194−201. doi: 10.1016/j.jiec.2013.04.007 [3] 梅艳钢, 王志青, 高松平, 郑洪岩, 张郃, 房倚天. 碱金属与碱土金属在煤炭热转化过程中的影响研究进展[J]. 燃料化学学报,2020,48(4):385−394. doi: 10.3969/j.issn.0253-2409.2020.04.001MEI Yan-gang, WANG Zhi-qing, GAO Song-ping, ZHENG Hong-yan, ZHANG He, FANG Yi-tian. Research progress of the influence of alkali metals and alkaline earth metals on coal thermal chemical conversion[J]. J Fuel Chem Technol,2020,48(4):385−394. doi: 10.3969/j.issn.0253-2409.2020.04.001 [4] MEI Y, WANG Z, BAI J, HE C, LI W, LIU T, HUANG J, FANG Y. Mechanism of Ca additive acting as a deterrent to Na2CO3 deactivation during catalytic coal gasification[J]. Energy Fuels,2019,33(2):938−945. doi: 10.1021/acs.energyfuels.8b03861 [5] 高松平, 赵建涛, 王志青, 王建飞, 房倚天, 黄戒介. CO对褐煤快速热解行为的影响[J]. 燃料化学学报,2013,41(5):550−557. doi: 10.3969/j.issn.0253-2409.2013.05.005GAO Song-ping, ZHAO Jiao-tao, WANG Zhi-qing, WANG Jian-fei, FANG Yi-tian, HUANG Jie-jie. Effect of CO on fast pyrolysis behaviors of lignite[J]. J Fuel Chem Technol,2013,41(5):550−557. doi: 10.3969/j.issn.0253-2409.2013.05.005 [6] 向银花, 王洋, 张建民, 黄戒介, 赵建涛. 煤气化动力学模型研究[J]. 燃料化学学报,2002,30(1):21−26. doi: 10.3969/j.issn.0253-2409.2002.01.005XIANG Yin-hua, WANG Yang, ZHANG Jian-min, HUANG Jie-jie, ZHAO Jian-tao. Study on dynamic model of coal gasification[J]. J Fuel Chem Technol,2002,30(1):21−26. doi: 10.3969/j.issn.0253-2409.2002.01.005 [7] ZONG N, LIU Y. Learning about the mechanism of carbon gasification by CO2 from DSC and TG data[J]. Thermochim Acta,2012,527:22−26. doi: 10.1016/j.tca.2011.09.025 [8] YUAN N, FAN S, ZHAO Q, ZHAO L, KIM H T. Investigations of both catalytic steam gasification of Indonesian Lanna coal and potassium catalyst recovery using K2CO3 as a catalyst[J]. Energy Fuels,2016,30(3):2492−2502. doi: 10.1021/acs.energyfuels.5b02536 [9] 孟繁锐, 李伯阳, 李先春, 邱爽. K2CO3对兰炭催化气化特性的影响[J]. 化工学报,2019,70(S1):99−109.MENG Fan-rui, LI Bo-yang, LI Xian-chun, QIU Shuang. Catalysis effects of K2CO3 for gasification of semi-coke[J]. CIESC J,2019,70(S1):99−109. [10] DING L. ZHOU Z, GUO Q, HUO W, YU G. Catalytic effects of Na2CO3 additive on coal pyrolysis and gasification[J]. Fuel,2015,142:134−144. doi: 10.1016/j.fuel.2014.11.010 [11] 陈杰, 陈凡敏, 王兴军, 于广锁, 王辅臣. 煤催化气化过程中钾催化剂回收的实验研究[J]. 化学工程,2012,40(6):68−71. doi: 10.3969/j.issn.1005-9954.2012.06.017CHEN jie, CHEN Fan-min, WANG Xing-jun, YU Guang-suo, WANG Fu-chen. Experimental study on potassium catalyst recovery in coal catalytic gasification[J]. Chem Eng,2012,40(6):68−71. doi: 10.3969/j.issn.1005-9954.2012.06.017 [12] 廖洪强, 邓德敏, 李保庆, 姚强. 煤催化气化研究进展与煤-纸浆黑液共气化[J]. 煤炭转化,2000,23(3):1−5. doi: 10.3969/j.issn.1004-4248.2000.03.001LIAO Hong-qiang, DENG De-min, LI Bao-qing, YAO Qiang. Research progress of coal catalytic gasification and coal-pulp black liquor gasification[J]. Coal Convers,2000,23(3):1−5. doi: 10.3969/j.issn.1004-4248.2000.03.001 [13] 王丽杰, 孙飞, 皮信信, 李隆昕, 李思佳, 高继慧. 微量Ca对准东煤基多孔碳孔隙结构调控[J]. 工程热物理学报,2018,39(11):2559−2567.WANG Li-jie, SUN Fei, PI Xin-xin, LI Long-xin, LI Si-jia, GAO Ji-hui. Adjusting porosity of Zhundong coal-based activated carbons based on catalytically physical activation process with trace calcium source[J]. J Eng Thermophys,2018,39(11):2559−2567. [14] 陈凡敏, 王兴军, 王西明, 周志杰. 煤催化气化过程中钾的迁移及其对气化反应特性的影响[J]. 燃料化学学报,2013,41(3):265−270. doi: 10.3969/j.issn.0253-2409.2013.03.002CHEN Fan-min, WANG Xing-jun, WANG Xi-ming, ZHOU Zhi-jie. Transformation of potassium during catalytic gasification of coal and the effect on gasification[J]. J Fuel Chem Technol,2013,41(3):265−270. doi: 10.3969/j.issn.0253-2409.2013.03.002 [15] MIN Z, YIMSIRI P, ASADULLASH M, ZHANG S, LI C Z. Catalytic reforming of tar during gasification. Part II. Char as a catalyst or as a catalyst support for tar reforming[J]. Fuel,2011,90(7):2545−2552. doi: 10.1016/j.fuel.2011.03.027 [16] LI C Z. Some recent advances in the understanding of the pyrolysis and gasification behavior of Victorian brown coal[J]. Fuel,2007,86(12):1664−1683. -




 下载:
下载: