Performance of Hg0 removal from coal-fired flue gas over coal gasification slag
-
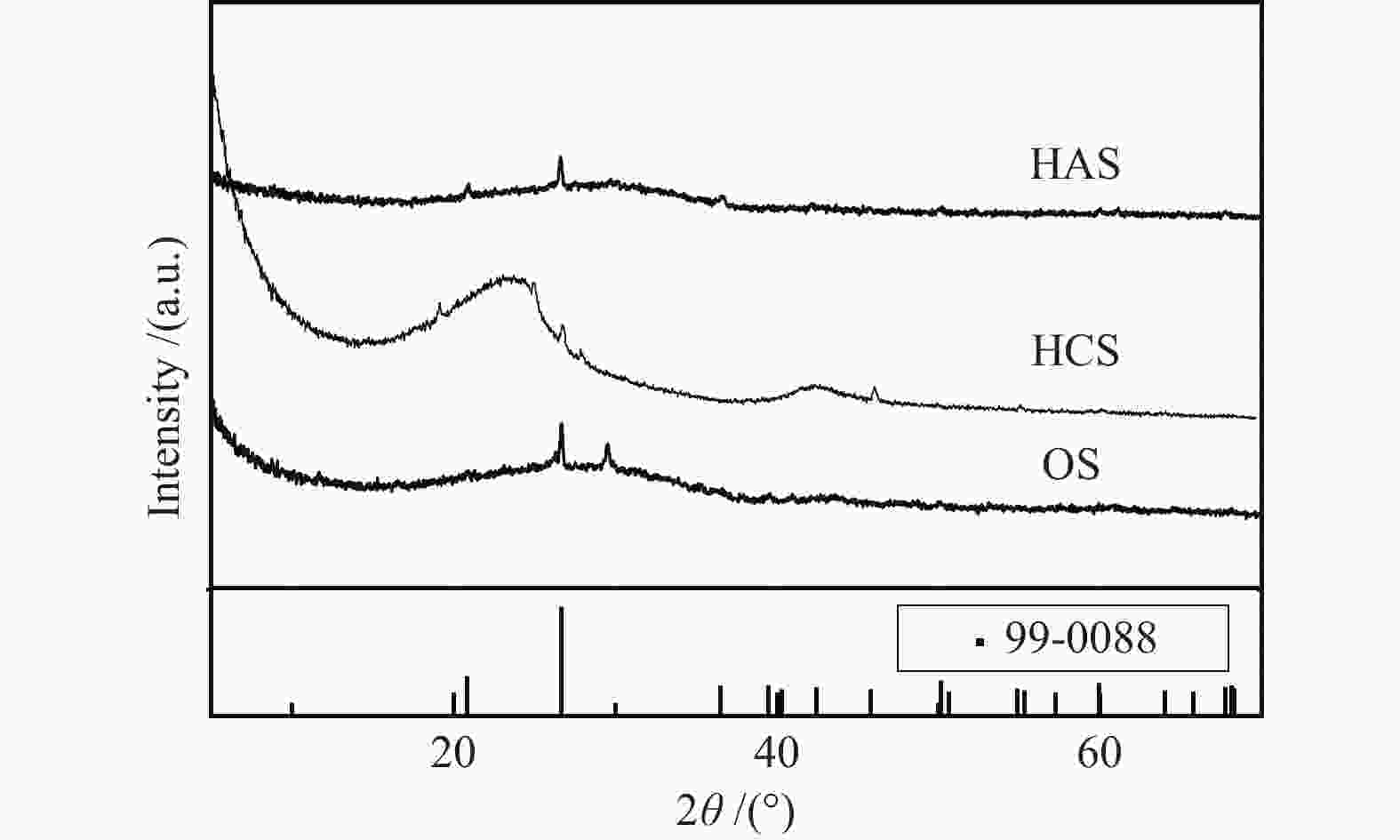
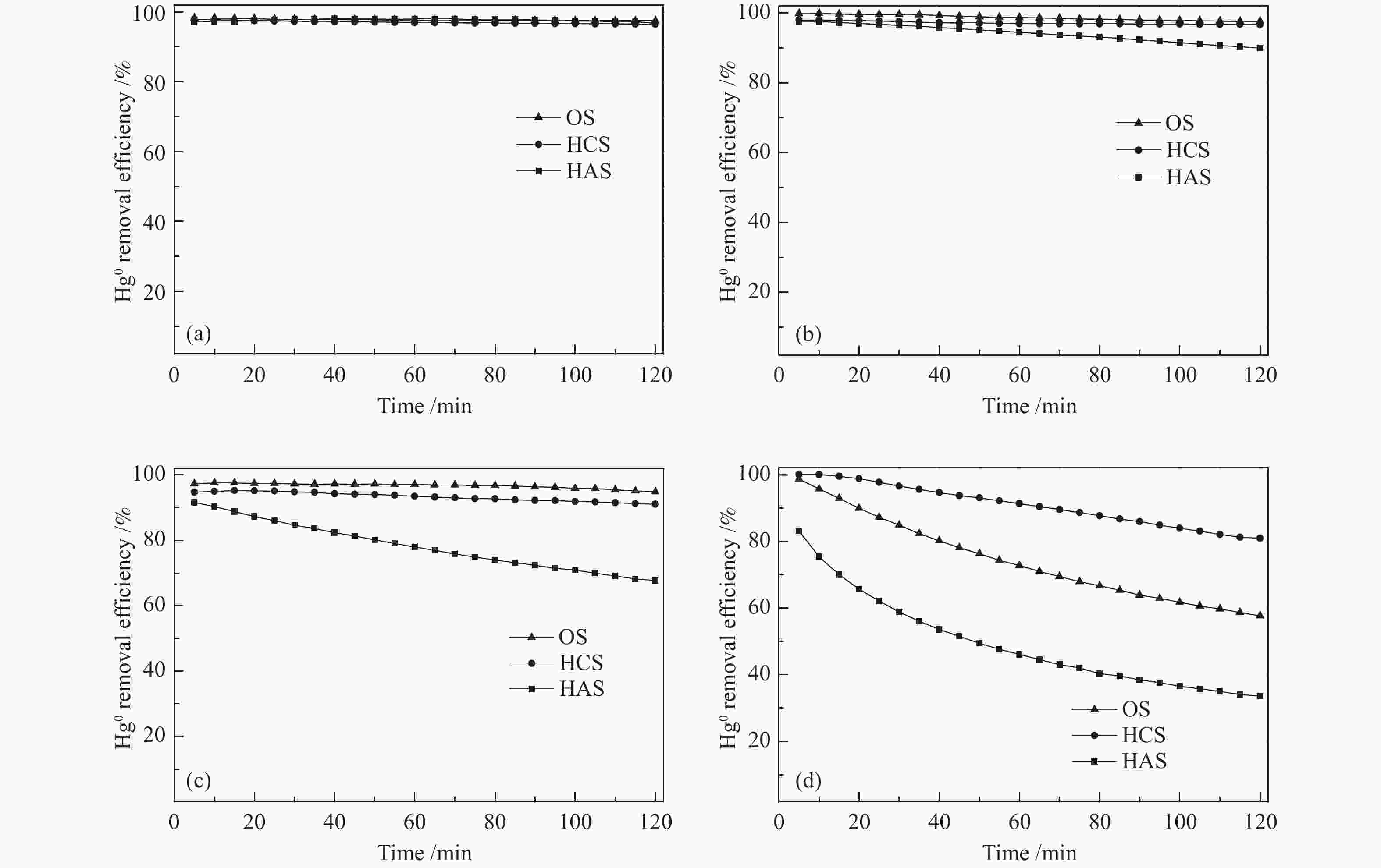
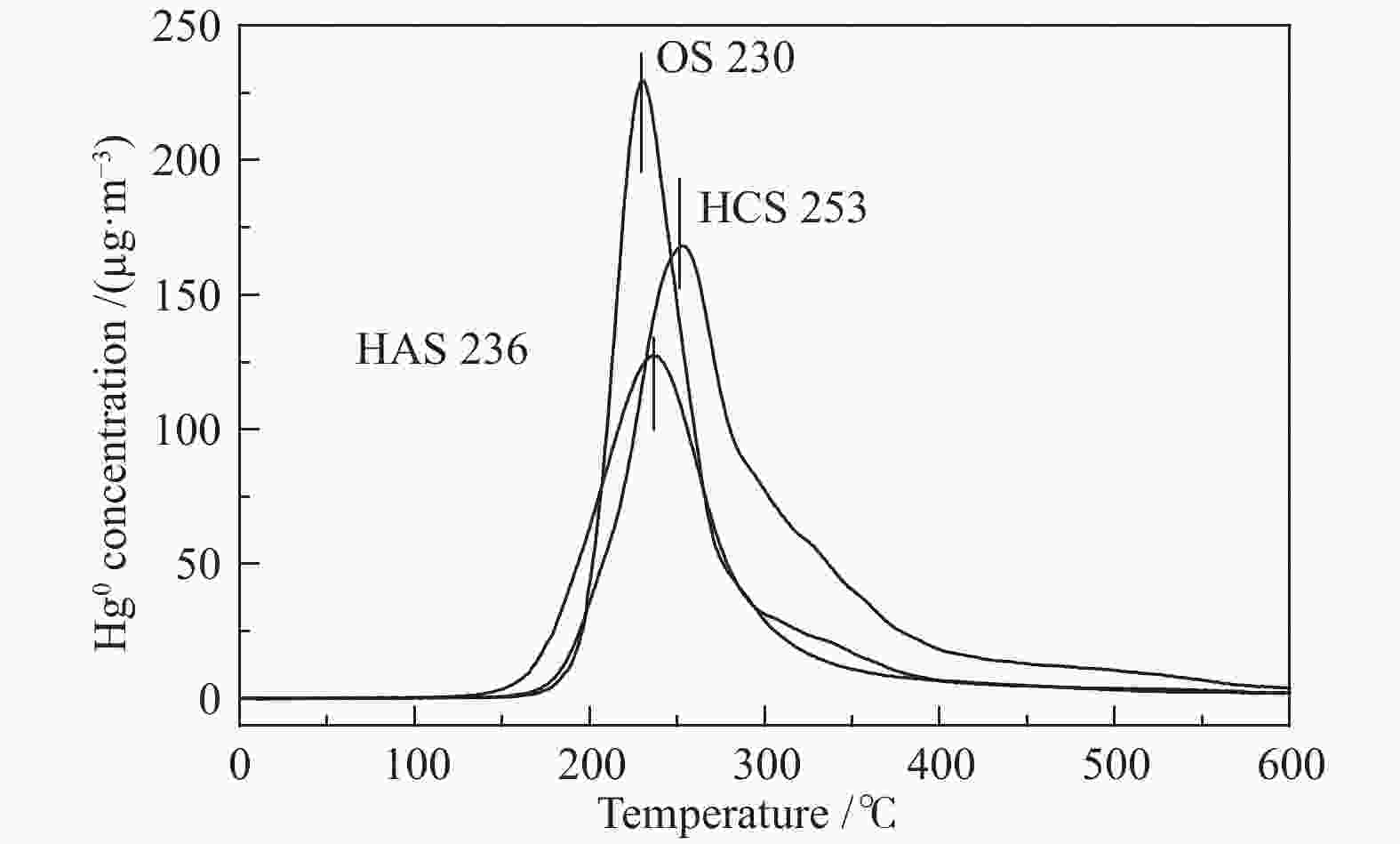
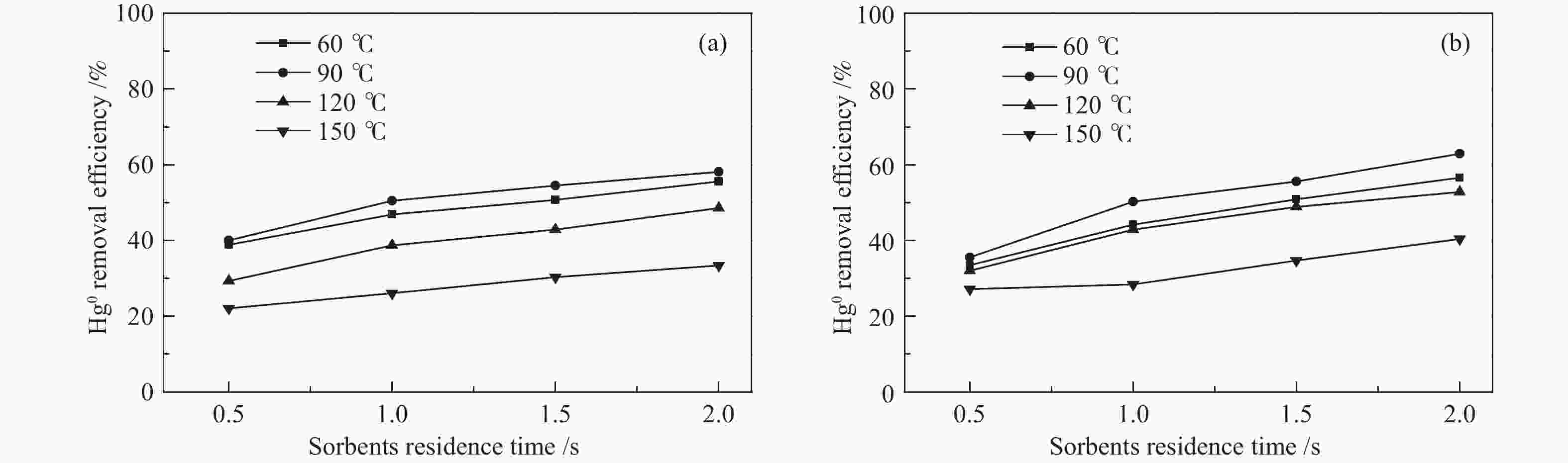
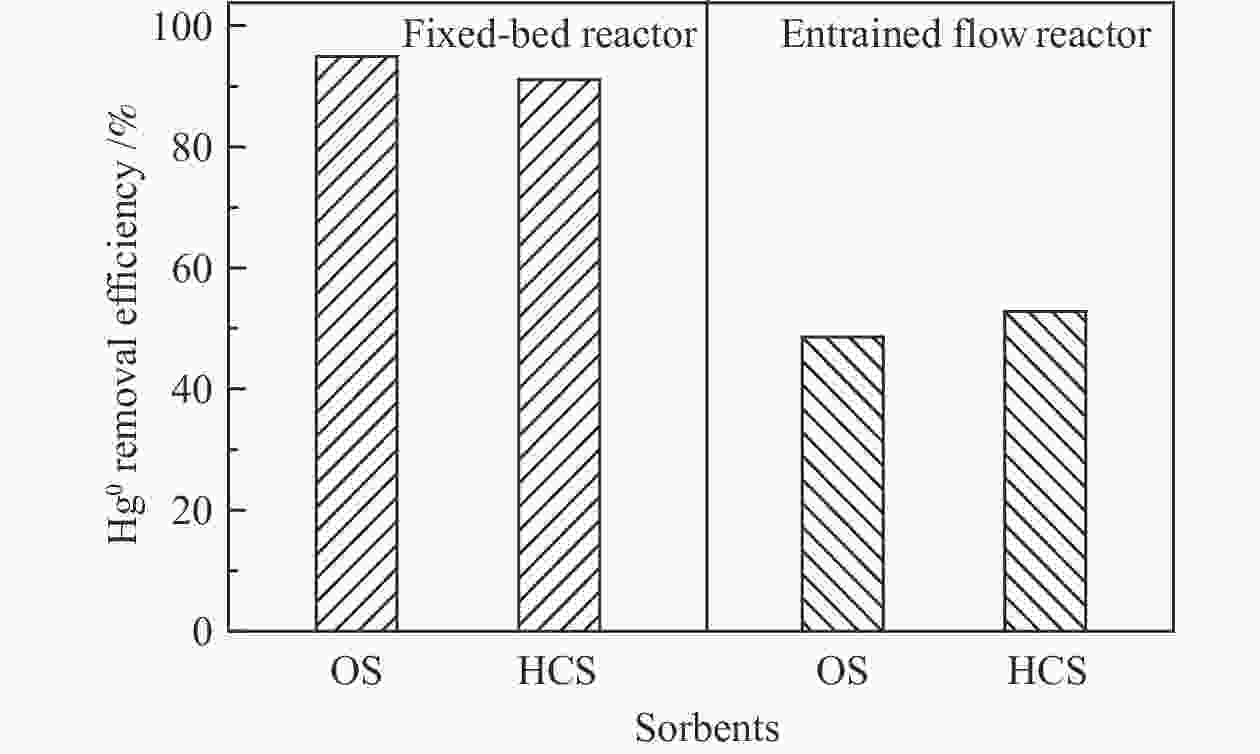
摘要: 汞作为一种重金属污染物,对环境和人体健康影响很大,如何对其高效脱除已引起了研究者的广泛关注。本研究使用煤气化渣及其分选后样品作为脱汞吸附剂,通过固定床和气流床脱汞实验考察了吸附剂的脱汞性能,利用N2吸附-脱附、X射线衍射(XRD)、X射线光电子能谱(XPS)、扫描电镜(SEM)等表征手段分析了吸附剂的物化特性。固定床脱汞评价结果显示,OS和HCS在60−120 ℃保持91%以上的脱汞效率;HAS在60 ℃有最高97%的脱汞效率,HAS的脱汞活性受脱汞温度影响较大。Hg-TPD和XPS表征结果表明,吸附剂中的化学吸附氧参与了汞的氧化,在吸附剂表面生成HgO。气流床脱汞评价结果表明,OS和HCS在碳汞比为40000,脱汞温度为60 ℃时,脱汞效率分别为56%、57%;当碳汞比为80000,脱汞温度为60 ℃时,脱汞效率分别为100%、82%。Abstract: Mercury (Hg), a kind of heavy metal pollutant, has a great impact on the environment and human health. The highly efficient technology for Hg removal has attracted widespread attention from researchers. In this study, the coal gasification slag (CGS) and the slag after sorting were used as sorbents, and the Hg0 removal performance of the sorbents were investigated through the fixed-bed reactor and entrained flow reactor. The characterization methods such as N2 adsorption-desorption, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) were performed to analyze the physical and chemical characteristics of the sorbents. According to the results of fixed-bed Hg0 removal experiment, the Hg0 removal efficiency of the origin slag (OS) and high carbon slag (HCS) could maintain more than 91% at 60−120 ℃. The high ash slag (HAS) showed the highest Hg0 removal efficiency of 97% at 60 ℃, which was greatly affected by the Hg0 removal temperature. The Hg-TPD (Hg-temperature program desorption) and XPS results indicated that the chemisorbed oxygen on the sorbent participated in the Hg0 oxidation process with HgO formed on the surface of the sorbent. According to the results of entrained flow Hg0 removal experiments, the Hg0 removal efficiencies of OS and HCS were 56% and 57% at C/Hg ratio of 40000 and the Hg0 removal temperature of 60 ℃, respectively. When the C/Hg ratio was 80000 and the Hg0 removal temperature was 60 ℃, the Hg0 removal efficiencies of OS and HCS were 100% and 82%, respectively.
-
Key words:
- mercury /
- coal gasification slag /
- coal-fired flue gas /
- injecting mercury removal
1) #: 高春新和井云环贡献相同 -
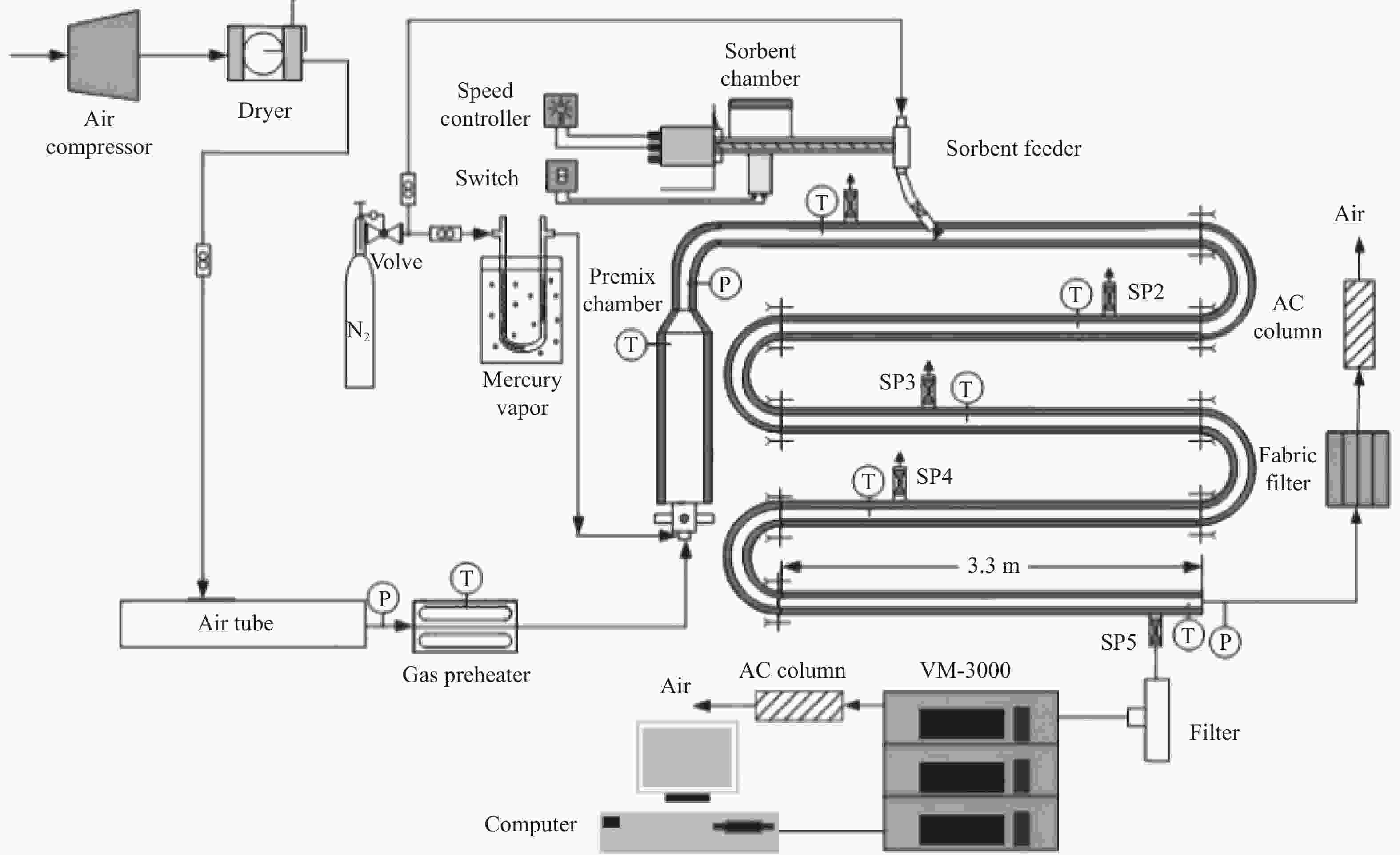
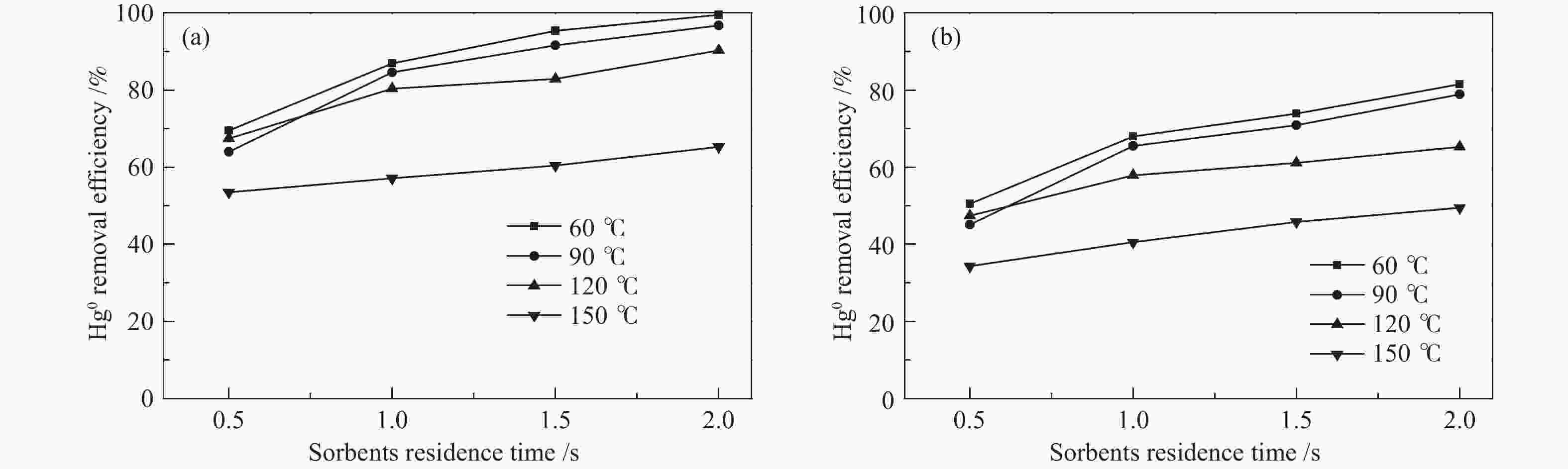
图 10 不同反应器下的脱汞效率
Figure 10 Hg0 removal efficiency at different reactors
(reaction conditions of the fix-bed reactor: t = 120 ℃, N2 + 4% O2, GHSV = 1.0 × 105 h−1; the reaction conditions of entrained flow reactor: t = 120 ℃, initial Hg0 concentration: 9.5 μg/m3, air + 25 L/min N2, θ = 40000, the residence time is 2.0 s)
表 1 样品的工业分析和元素分析
Table 1 Proximate and ultimate analyses of samples
Sample Proximate analysis w/% Ultimate analysis wad/% Mad Aad Vad Vdaf C H O* N S OS 0.47 72.36 2.65 9.75 24.22 0.32 1.98 0.13 0.52 HCS 0.71 12.66 2.99 3.45 82.44 0.29 3.06 0.54 0.30 HAS 0.15 93.44 1.46 22.77 4.17 0.05 − 0.03 0.40 *:by difference 表 2 样品的孔结构参数
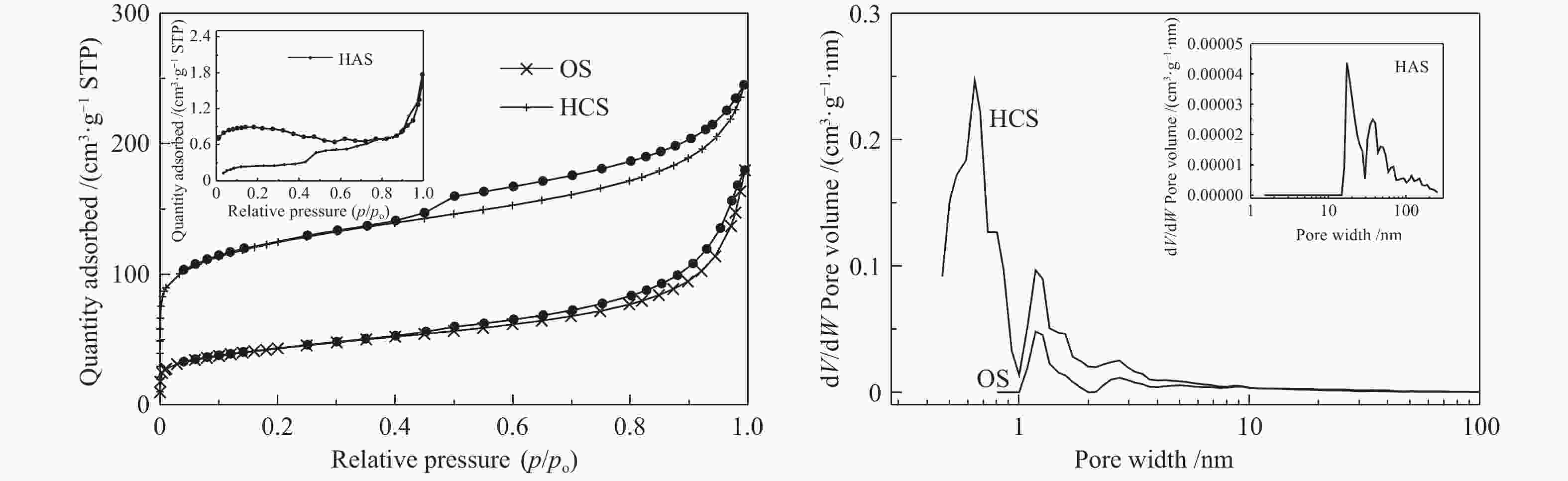
Table 2 Surface areas and pore structure parameters of samples
Sample BET surface area
/(m2·g−1)t-plot micropore area
/(m2·g−1)Total pore volume
/(cm3·g−1)t-plot micropore volume
/(cm3·g−1)Average pore diameter
/nmOS 223.1 79.9 0.3 0.1 4.5 HCS 787.8 439.9 0.9 0.2 5.5 HAS 3.5 2.2 − − 7.9 表 3 基于XPS光谱计算的吸附剂表面元素含量
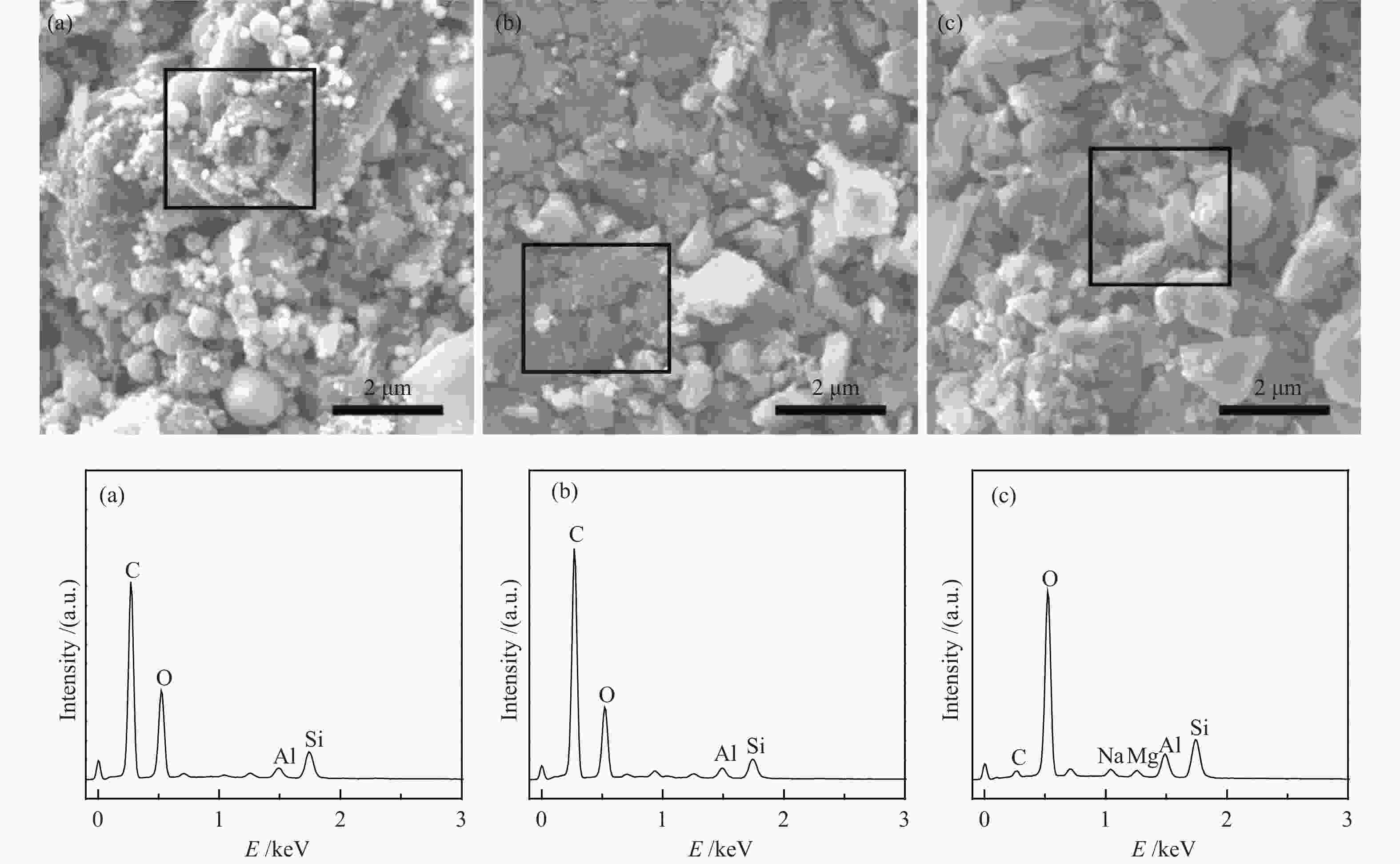
Table 3 Content of surface elements of sorbents calculated based on the XPS spectra
Sample Atomic content w/% Relative content w/% C O Fe S Hg Oα Oβ Oγ Fresh OS 28.32 69.89 1.31 0.48 − 13.73 64.33 21.94 Used OS 30.26 67.84 1.23 0.45 0.22 15.88 58.54 25.58 Fresh HCS 84.14 14.74 0.70 0.42 − 3.17 6.78 90.05 Used HCS 86.42 12.25 0.65 0.43 0.25 4.96 3.73 91.31 Fresh HAS 9.44 88.42 1.53 0.61 − 6.51 23.92 69.57 Used HAS 13.88 83.84 1.46 0.69 0.13 6.09 20.58 73.33 -
[1] LI P, FENG X B, QIU G L, SHANG L H, LI Z G. Mercury pollution in Asia: a review of the contaminated sites[J]. J Hazard Mater,2009,168(2):591−601. [2] GB 13223—2011, 火电厂大气污染物排放标准[S].GB 13223—2011, Emission standard of air pollutants for thermal power plants[S]. [3] CHI Y, YAN N Q, QU Z, QIAO S H, JIA J P. The performance of iodine on the removal of elemental mercury from the simulated coal-fired flue gas[J]. J Hazard Mater,2009,166(2/3):776−781. doi: 10.1016/j.jhazmat.2008.11.130 [4] YANG Z Q, LI H L, YANG J P, FENG S H, LIU X, ZHAO J X, QU W Q, LI P, FENG Y, LEE P H, SHIH K. Nanosized copper selenide functionalized zeolitic imidazolate framework-8 (CuSe/ZIF-8) for efficient immobilization of gas-phase elemental mercury[J]. Adv Funct Mater,2019,29(17):1807191. [5] 柏建华, 达胜富, 刘睿, 刘吉祥, 魏健鹏. 燃煤电厂烟气脱汞技术研究进展[J]. 甘肃科技,2017,33(15):46−52. doi: 10.3969/j.issn.1000-0952.2017.15.016BO Jian-hua, DA Sheng-fu, LIU Rui, LIU Ji-xiang, WEI Jian-peng. Research progress of flue gas mercury removal technology in coal-fired power plant[J]. Gansu Sci Technol,2017,33(15):46−52. doi: 10.3969/j.issn.1000-0952.2017.15.016 [6] 周强, 段钰锋, 冒咏秋, 朱纯. NH4Cl改性活性炭脱除气态Hg0的特性分析[J]. 化工进展,2018,37(10):4068−4073.ZHOU Qiang, DUAN Yu-feng, MAO Yong-qiu, ZHU Chun. Gaseous Hg0 removal by NH4Cl modified activated carbon[J]. Chem Ind Eng Prog,2018,37(10):4068−4073. [7] HONG D Y, ZHOU J S, HU C X, ZHOU Q X, MAO J Z, QIN Q W. Mercury removal mechanism of AC prepared by one-step activation with ZnCl2[J]. Fuel,2019,235:326−335. doi: 10.1016/j.fuel.2018.07.103 [8] WANG T, LIU J, ZHANG Y S, ZHANG H C, CHEN W Y, NORRIS P, PAM W P. Use of a non-thermal plasma technique to increase the number of chlorine active sites on biochar for improved mercury removal[J]. Chem Eng J,2018,331:536−544. doi: 10.1016/j.cej.2017.09.017 [9] 曲江山, 张建波, 孙志刚, 杨晨年, 史达, 李少鹏, 李会泉. 煤气化渣综合利用研究进展[J]. 洁净煤技术,2020,26(1):184−193.QU Jiang-shan, ZHANG Jian-bo, SUN Zhi-gang, YANG Chen-nian, SHI Da, LI Shao-peng, LI Hui-quan. Research progress on comprehensive utilization of coal gasification slag[J]. Clean Coal Technol,2020,26(1):184−193. [10] HAN F, GAO Y C, HUO Q H, HAN L. Characteristics of vanadium-based coal gasification slag and the NH3-selective catalytic reduction of NO[J]. Catalysts, 2018, 8(8): 327. [11] 高艳春, 韩芳, 韩丽娜, 常丽萍, 鲍卫仁, 王建成. V/CGS低温NH3-SCR催化剂的制备及性能研究[J]. 现代化工,2020,40(8):67−72.GAO Yan-chun, HAN Fang, HAN Li-na, CHANG Li-ping, BAO Wei-ren, WANG Jian-cheng. Preparation of V/CGS catalyst for low temperature NH3-SCR and study on its activities[J]. Mod Chem Ind,2020,40(8):67−72. [12] CHEN H J, HUO Q H, WANG Y H, HAN L N, LEI Z P, WANG J C, BAO W R, CHANG L P. Upcycling coal liquefaction residue into sulfur-rich activated carbon for efficient Hg0 removal from coal-fired flue gas[J]. Fuel Process Technol,2020,206:106467. doi: 10.1016/j.fuproc.2020.106467 [13] HUO Q H, WANG Y H, CHEN H J, HAN L N, WANG J C, BAO W R, CHANG L P, XIE K C. ZnS/AC sorbent derived from the high sulfur petroleum coke for mercury removal[J]. Fuel Process Technol,2019,191:36−43. doi: 10.1016/j.fuproc.2019.03.025 [14] 辛勤, 罗孟飞. 现代催化研究方法[M]. 北京: 科学出版社, 2009.XIN Qin, LUO Meng-fei. Morden Catalytic Research Methods[M]. Beijing: Science Press, 2009. [15] ZHAO X L, ZENG C, MAO Y Y, LI W H, PENG Y, WANG T, EITENEER B, ZAMANSKY V, FLETCHER T H. The surface characteristics and reactivity of residual carbon in coal gasification slag[J]. Energy Fuels,2010,24(1):91−94. doi: 10.1021/ef9005065 [16] 孟素丽, 段钰锋, 黄治军, 王运军, 杨立国. 燃煤飞灰吸附气态汞影响因素的试验研究[J]. 动力工程学报,2009,29(5):487−491. doi: 10.3321/j.issn:1000-6761.2009.05.016MENG Su-li, DUAN Yu-feng, HUANG Zhi-jun, WANG Yun-jun, YANG Li-guo. Experimental study on factors influencing adsorption of mercury vapor by coal-fired fly ash[J]. J Chin Soc Power Eng,2009,29(5):487−491. doi: 10.3321/j.issn:1000-6761.2009.05.016 [17] ZHU Y C, HAN X J, HUANG Z G, HOU Y Q, GUO Y P, WU M H. Superior activity of CeO2 modified V2O5/AC catalyst for mercury removal at low temperature[J]. Chem Eng J,2018,337:741−749. doi: 10.1016/j.cej.2017.10.115 [18] YANG S, WANG D L, LIU H, LIU C, XIE X F, XU Z F, LIU Z L. Highly stable activated carbon composite material to selectively capture gas-phase elemental mercury from smelting flue gas: Copper polysulfide modification[J]. Chem Eng J,2019,358:1235−1242. doi: 10.1016/j.cej.2018.10.134 [19] ZHAO L K, LI C T, ZHANG J, ZHANG X N, ZHAN F M, MA J F, XIE Y E, ZENG G M. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas[J]. Fuel,2015,153:361−369. doi: 10.1016/j.fuel.2015.03.001 [20] YU D Q, LIU Y, WU Z B. Low-temperature catalytic oxidation of toluene over mesoporous MnOx-CeO2/TiO2 prepared by sol-gel method[J]. Catal Commun,2010,11(8):788−791. doi: 10.1016/j.catcom.2010.02.016 [21] TAO S S, LI C T, FAN X P, ZENG G M, LU P, ZHANG X, WEN Q B, ZHAO W W, LUO D Q, FAN C Z. Activated coke impregnated with cerium chloride used for elemental mercury removal from simulated flue gas[J]. Chem Eng J,2012,210:547−556. doi: 10.1016/j.cej.2012.09.028 [22] LI H L, ZHU L, WANG J, LI L Q, SHIH K. Development of nano-sulfide sorbent for efficient removal of elemental mercury from coal combustion fuel gas[J]. Environ Sci Technol,2016,50(17):9551−9557. doi: 10.1021/acs.est.6b02115 [23] WU S J, UDDIN M A, NAGANO S, OZAKI M, SASAOKA E. Fundamental study on decomposition characteristics of mercury compounds over solid powder by temperature-programmed decomposition desorption mass spectrometry[J]. Energy Fuels,2011,25(1):144−153. doi: 10.1021/ef1009499 [24] HAN Z X, GUO Y X, YANG W, TANG R, WANG H, WU S J. Removal of mercury from flue gases over iron modified activated carbon made by in situ ion exchange method[J]. J Energy Inst,2020,93(4):1411−1418. doi: 10.1016/j.joei.2020.01.003 [25] 周强, 段钰锋, 洪亚光, 朱纯, 佘敏, 韦红旗. 模拟烟气活性炭喷射脱汞实验研究[J]. 中国电机工程学报,2013,33(35):36−43.ZHOU Qiang, DUAN Yu-feng, HONG Ya-guang, ZHU Chun, SHE Min, WEI Hong-qi. Experimential study on mercury capture using activated carbon injection in simulated flue gas[J]. Proc CSEE,2013,33(35):36−43. [26] 丁建东, 陈博, 刁永发, 沈恒银, 石健. 碳汞比对燃煤烟气中Hg脱除影响的实验研究[J]. 环境工程,2012,30(1):58−61.DING Jian-dong, CHEN Bo, DIAO Yong-fa, SHEN Heng-yin, SHI Jian. The experimental study on the influence of C/Hg ratio on removal of elemental mercury in simulated flue gas[J]. Environ Eng,2012,30(1):58−61. [27] CHEN Y, LIU H, GUO X, WU F, ZHAO Y C, ZHANG J Y. Performance of CuCl2-modified activated carbon on mercury capture after injection in an entrained flow reactor[J]. Ind Eng Chem Res,2020,59(13):5557−5565. doi: 10.1021/acs.iecr.9b06189 [28] 王永兴, 黄亚继, 董璐, 袁琦, 丁守一, 程好强, 王圣, 段钰锋. Co掺杂铁基氧化物吸附剂燃煤烟气脱汞实验研究[J]. 燃料化学学报,2020,48(7):785−794.WANG Yong-xing, HUANG Ya-ji, DONG Lu, YUAN Qi, DING Shou-yi, CHENG Hao-qiang, WANG Sheng, DUAN Yu-feng. Experimental study on mercury removal of coal-fired flue gas over Co-doped iron-based oxide sorbent[J]. J Fuel Chem Technol,2020,48(7):785−794. -




 下载:
下载: