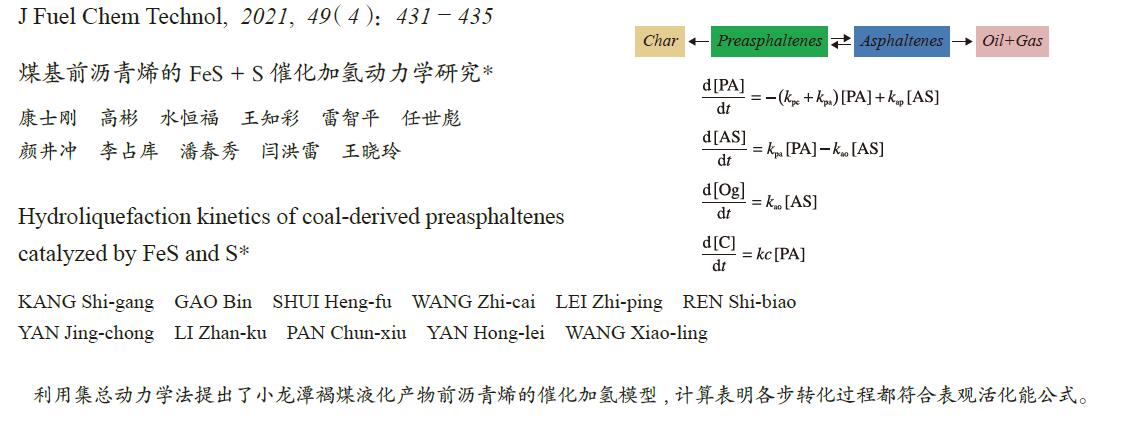
-
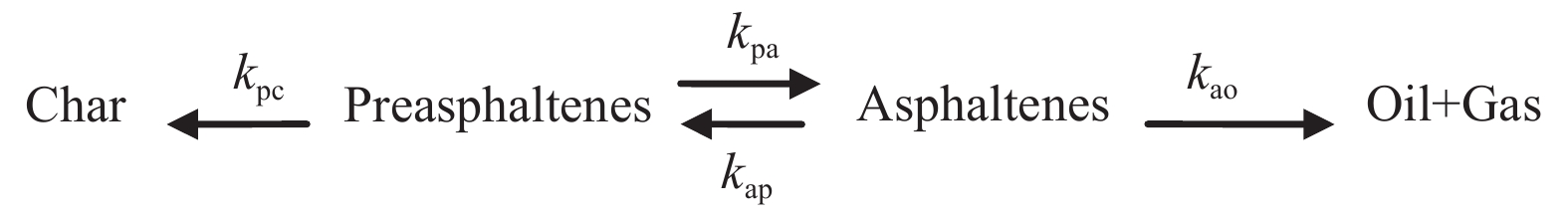
摘要: 为了优化煤直接液化工艺条件和提高油收率,本研究利用30 mL高压管式反应釜研究了煤直接液化重质产物前沥青烯加氢液化行为,考察了FeS + S催化剂下反应温度(380、400、420和440 ℃)、液化时间(0、5、10、20、30和60 min)、5.0 MPa氢初压和四氢萘溶剂条件下前沥青烯液化转化行为,同时考察了前沥青烯的催化加氢液化反应动力学。利用集总动力学法建立了FeS + S催化前沥青烯加氢的动力学模型。研究表明,前沥青烯加氢直接生成沥青烯和焦渣,而沥青烯进一步加氢裂解生成油和气,高温下发生明显的逆向缩合反应,即前沥青烯生成焦渣和沥青烯生成前沥青烯。温度和反应时间的增加有利于提高前沥青烯的转化率和油气收率,440 °C下反应60 min时,前沥青烯的转化率为79.45%,油气收率为34.7%。380−440 ℃温度下,动力学模型能够较好地描述小龙潭液化产物前沥青烯的加氢转化行为,各步转化均符合Arrhenius表观活化能公式,并且活化能变化为50−245 kJ/mol。Abstract: Hydroliquefaction behavior of preasphaltenes, derived from direct coal liquefaction, was carried out in a 30 mL autoclave with FeS + S catalyst and tetralin at initial hydrogen of 5.0 MPa, residence time of 0−60 min and reaction temperature of 380−440 °C in order to optimize the conditions of direct coal liquefaction and improve oil yield. The products distribution and kinetic parameters of preasphaltenes catalytic hydroliquefaction were investigated. A new kinetic model was established to simulate the preasphaltenes hydroliquefaction catalyzed by FeS + S catalyst using lump kinetic model. It was found that preasphaltenes were hydroliquefaction into asphaltenes and char directly, and then asphaltenes were hydrocracked into oil + gas products. Regressive reactions of preasphaltenes to char and asphaltenes to preasphaltenes occurred at higher temperatures. Higher temperature and longer time were favorable for increasing the conversion of preasphaltenes and the oil + gas yield. The hydroliquefaction of preasphaltenes under 440 °C and 60 min reached 79.45% with 34.7% of oil + gas yield. The hydroliquefaction conversions calculated from the model agreed well with the experimental data, and the activation energies ranged within 50−245 kJ/mol.
-
Key words:
- preasphaltenes /
- hydroliquefaction /
- kinetic /
- activation energies
-
Table 1 Proximate and ultimate analyses of samples
Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf C H N S ${\rm{O} }_{ {\rm{} } }^*$ XLT 14.60 10.83 62.76 62.91 5.01 2.11 3.63 26.34 PA − − − 80.42 5.03 3.06 1.36 10.13 M: moisture; A: ash; V: volatile matter; *: by difference Table 2 Conversions (%) and liquefaction product distributions (%) of PA liquefaction catalyzed by FeS + S at 380, 400, 420 and 440 °C
Reaction
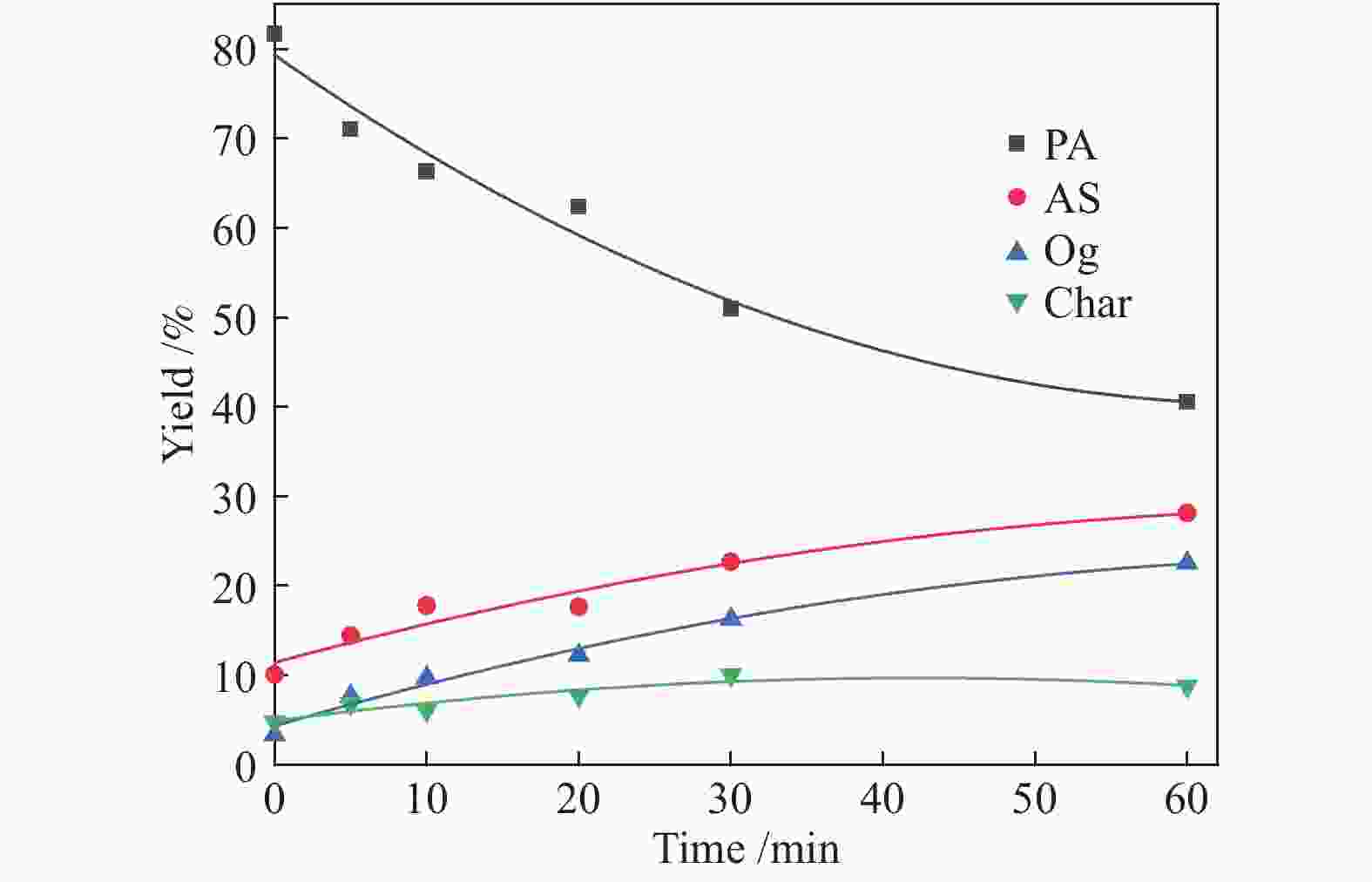
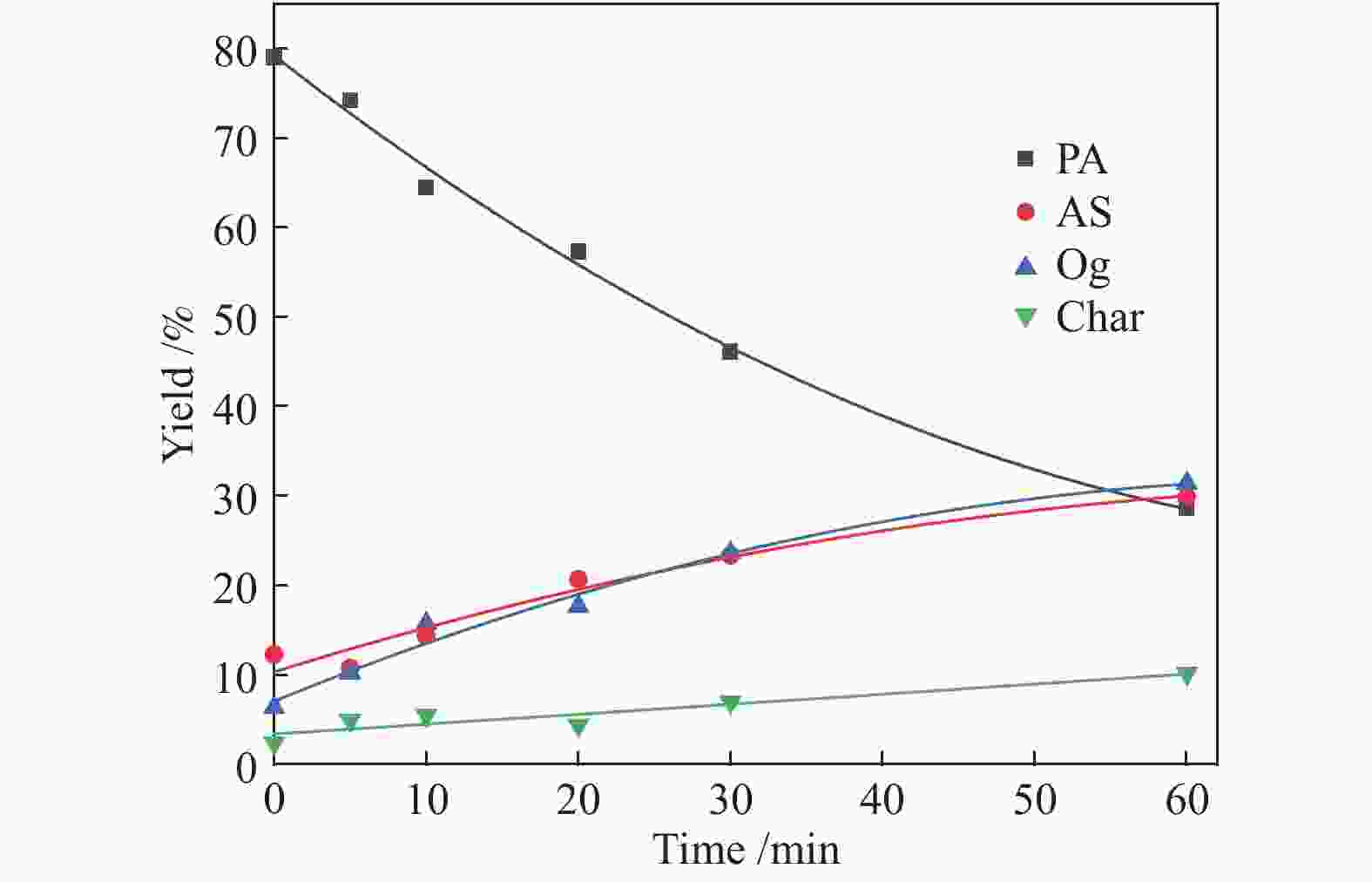
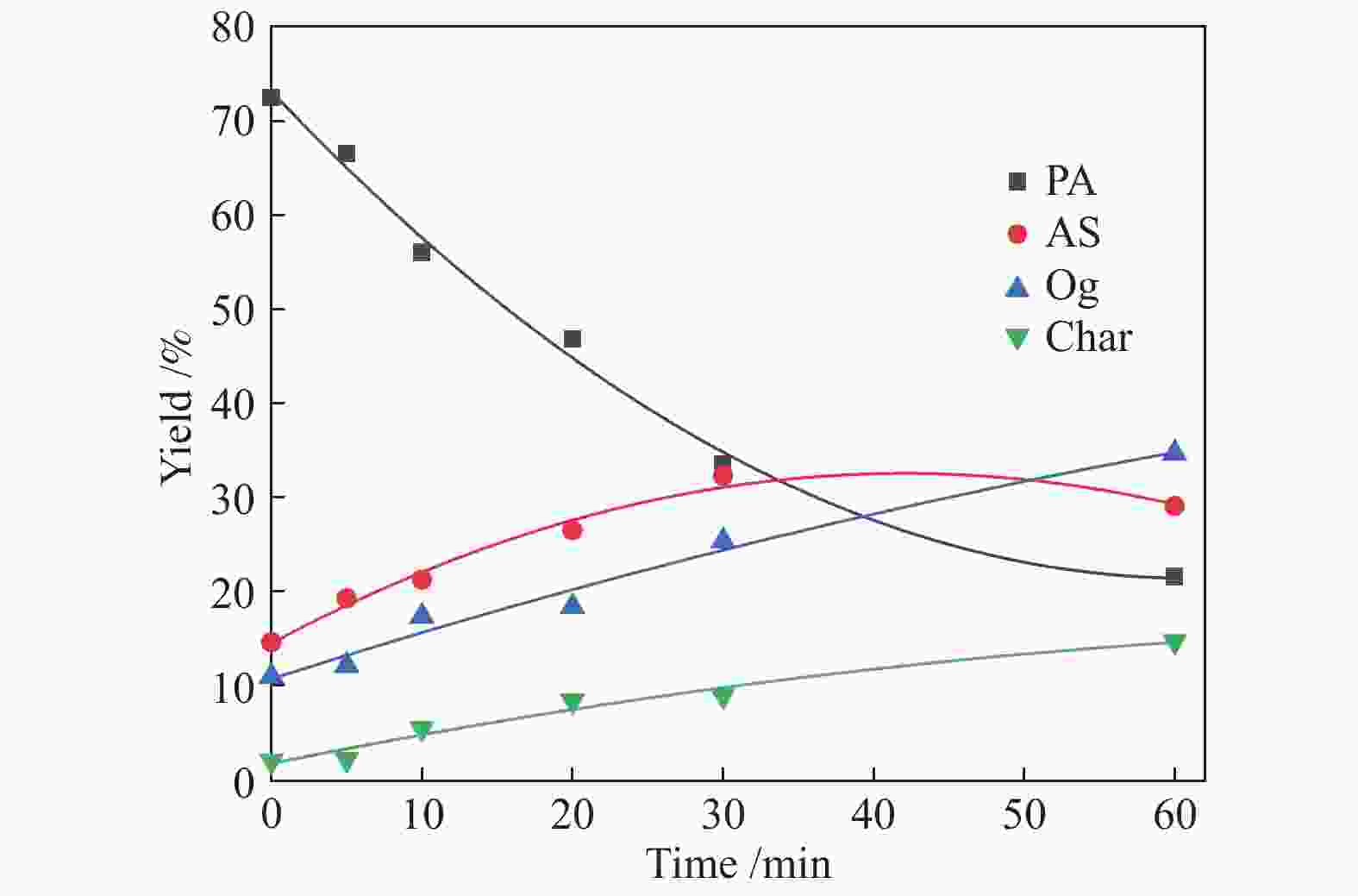
time/min380 °C 400 °C PA AS O char PA AS O char 0 81.68 10.11 3.38 4.83 79.01 12.25 6.33 2.41 5 71.06 14.44 7.69 6.81 74.17 10.75 10.19 4.89 10 66.31 17.8 9.77 6.12 64.46 14.36 15.71 5.47 20 62.33 17.65 12.25 7.77 57.3 20.66 17.64 4.4 30 50.94 22.72 16.31 10.03 46.08 23.33 23.63 6.96 60 40.54 28.13 22.55 8.78 28.55 29.88 31.42 10.15 Reaction
time/min420 °C 440 °C PA AS O char PA AS O char 0 76.96 12.54 8.02 2.48 72.43 14.6 10.96 2.01 5 69.03 14.86 12.75 3.36 66.51 19.26 12.12 2.11 10 63.65 20.54 10.01 5.8 55.99 21.22 17.36 5.43 20 49.97 23.21 16.8 10.0 46.77 26.47 18.37 8.39 30 39.68 25.84 26.25 8.23 33.47 32.26 25.36 8.91 60 25.34 32.48 31.72 10.4 21.55 29.08 34.7 14.67 Table 3 Kinetic parameters of PA hydroliquefaction calculated by the model
Rate
constant/min−1380 ℃ 400 ℃ 420 ℃ 440 ℃ Ea/(kJ·mol−1) kpa 0.3780 0.3797 0.4778 0.5616 108.28 kao 0.0203 0.0226 0.0284 0.0289 100.12 kap 1.0775 1.1032 1.1388 1.332 51.12 kpc 0.0021 0.0033 0.0041 0.0055 241.16 -
[1] KANDA N, ITOH H, YOKOYAMA S, OUCHI K. Mechanism of hydrogenation of coal-derived asphaltene[J]. Fuel,1978,57(11):676−680. doi: 10.1016/0016-2361(78)90020-0 [2] MARTINEZ M T, BENITO A M, CALLEJAS M A. Kinetics of asphaltene hydroconversion:1. Thermal hydrocracking of a coal residue[J]. Fuel,1997,76(10):899−905. doi: 10.1016/S0016-2361(97)00107-5 [3] BENITO A M, CALLEJAS M, MARTINEZ M T. Kinetics of asphaltene hydroconversion: 2.Catalytic hydrocracking of a coal residue[J]. Fuel,1997,76(10):907−911. doi: 10.1016/S0016-2361(97)00108-7 [4] BENITO A M, MARTINEZ M T, FERNANDEZ I, MIRANDA J L. Upgrading of an asphaltenic coal residue: thermal hydroprocessing[J]. Energy Fuels,1996,10(2):401−408. doi: 10.1021/ef9501556 [5] OUCHI K, KATOH T, ITOH H. Reaction mechanism for the hydrogenolysis of coal-derived preasphaltene[J]. Fuel,1981,60(8):689−693. doi: 10.1016/0016-2361(81)90220-9 [6] KEMP W, STEEDMAN W, THOMSON M A, SCOTT D A. Comparative reactivities of coal asphaltenes during hydropyrolysis[J]. Fuel,1985,64(10):1379−1382. doi: 10.1016/0016-2361(85)90338-2 [7] STEEDMAN W, KEMP W, JOHNSON P, SCOTT D A. Pyrolysis and hydropyrolysis of coal asphaltenes and preasphaltenes-relationships between reactivity and structural parameters derived from chemical and spectroscopic analyses[J]. Fuel,1989,68(8):1017−1022. doi: 10.1016/0016-2361(89)90068-9 [8] ZHANG C, LEE C W, KEOGH R A, DEMIREL B, DAVIS B H. Thermal and catalytic conversion of asphaltenes[J]. Fuel,2001,80(8):1131−1146. doi: 10.1016/S0016-2361(00)00178-2 [9] WANG Z C, SHUI H F, LEI Z P, REN S B, KANG S G, ZHOU H, GU X P, GAO J S. Study of the preasphaltenes of coal liquefaction and its hydro-conversion kinetics catalyzed by SO42-/ZrO2[J]. Fuel Process Technol,2011,92(10):1830−1835. doi: 10.1016/j.fuproc.2011.04.039 [10] KANG S G, ZONG Z M, SHUI H F, WANG Z C, WEI X Y. Comparison of catalytic hydroliquefaction of Xiaolongtan lignite over FeS, FeS + S and $ {\rm{SO}}_4^{2 - }/{\rm{Zr}}{{\rm{O}}_2} $ [J]. Energy,2011,36(1):41−45. doi: 10.1016/j.energy.2010.10.025[11] WANG Z C, SHUI H F, ZHANG D X, GAO J S. A comparison of FeS, FeS+S and solid superacid catalytic properties for coal hydro-liquefaction[J]. Fuel,2007,86(5-6):835−842. doi: 10.1016/j.fuel.2006.09.018 [12] DING W B, LIANG J, ANDERSON L L. Kinetics of thermal and catalytic coal liquefaction with plastic-derived liquids as solvent[J]. Ind Eng Chem Res,1997,36(5):1444−1452. doi: 10.1021/ie960568+ [13] RAMDOSS P K, TARRER A R. Modeling of two-stage coal coprocessing process[J]. Energy Fuels,1997,11(1):194−201. doi: 10.1021/ef960068t [14] WELLER S, CLARK E L, PELIPETZ M G. Mechanism of coal hydrogenation[J]. Ind Eng Chem,1950,42(2):334−336. doi: 10.1021/ie50482a033 [15] WELLER S, PELIPETZ M G, FRIEDMAN S. Kinetics of coal hydrogenation conversion of asphalt[J]. Ind Eng Chem,1951,43(7):1572−1575. doi: 10.1021/ie50499a030 [16] CRONAUER D C, SHAH Y T, RUBERTO R G. Kinetics of thermal liquefaction of Belle Ayr subbituminous coal[J]. Ind Eng Chem Process Des Dev,1978,17(3):281−288. doi: 10.1021/i260067a013 [17] MOHAN G, SILLA H. Kinetics of donor-solvent liquefaction of bituminous coals in nonisothermal experiments[J]. Ind Eng Chem Process Des Dev,1981,20(2):349−358. doi: 10.1021/i200013a026 [18] GERTENBACH D D, BALDWIN R M, BAIN R L. Modeling of bench-scale coal liquefaction systems[J]. Ind Eng Chem Process Des Dev,1982,21(3):490−500. doi: 10.1021/i200018a024 [19] ABICHANDANI J S, SHAH Y T, CRONAUER D C, RUBERTO R G. Kinetics of thermal liquefaction of coal[J]. Fuel,1982,61(3):276−282. doi: 10.1016/0016-2361(82)90125-9 [20] SUZUKI T, ANDO T, WATANABE Y. Kinetic studies on the hydroliquefaction of coals using organometallic complexes[J]. Energy Fuels,1987,1(3):294−300. doi: 10.1021/ef00003a013 [21] SIMSEK E H, KARADUMAN A, OLCAY A. Investigation of dissolution mechanism of six Turkish coals in tetralin with microwave energy[J]. Fuel,2001,80(15):2181−2187. doi: 10.1016/S0016-2361(01)00102-8 [22] SHUI H F, CHEN Z X, WANG Z C, ZHANG D X. Kinetics of Shenhua coal liquefaction catalyzed by $ {\rm{SO}}_4^{2 - }/{\rm{Zr}}{{\rm{O}}_2} $ solid acid[J]. Fuel,2010,89(1):67−72. doi: 10.1016/j.fuel.2009.02.019[23] WANG Z C, SHUI H F, ZHANG D X, GAO J S. Catalysis of $ {{\rm{SO}}_{4}^{2-}}/{\rm{ZrO}}_{2} $ solid acid for the liquefaction of coal[J]. Fuel,2009,88(5):885−889. doi: 10.1016/j.fuel.2008.10.040[24] YADAY G D, NAIR J J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes[J]. Microporous Mesoporous Mater,1999,33(1/3):1−48. doi: 10.1016/S1387-1811(99)00147-X -




 下载:
下载: