Highly selective synthesis of LPG from CO2 hydrogenation over In2O3/SSZ-13 binfunctional catalyst
-
摘要: 通过In2O3/SSZ-13双功能催化剂实现了二氧化碳(CO2)加氢高选择性合成液化石油气(LPG,
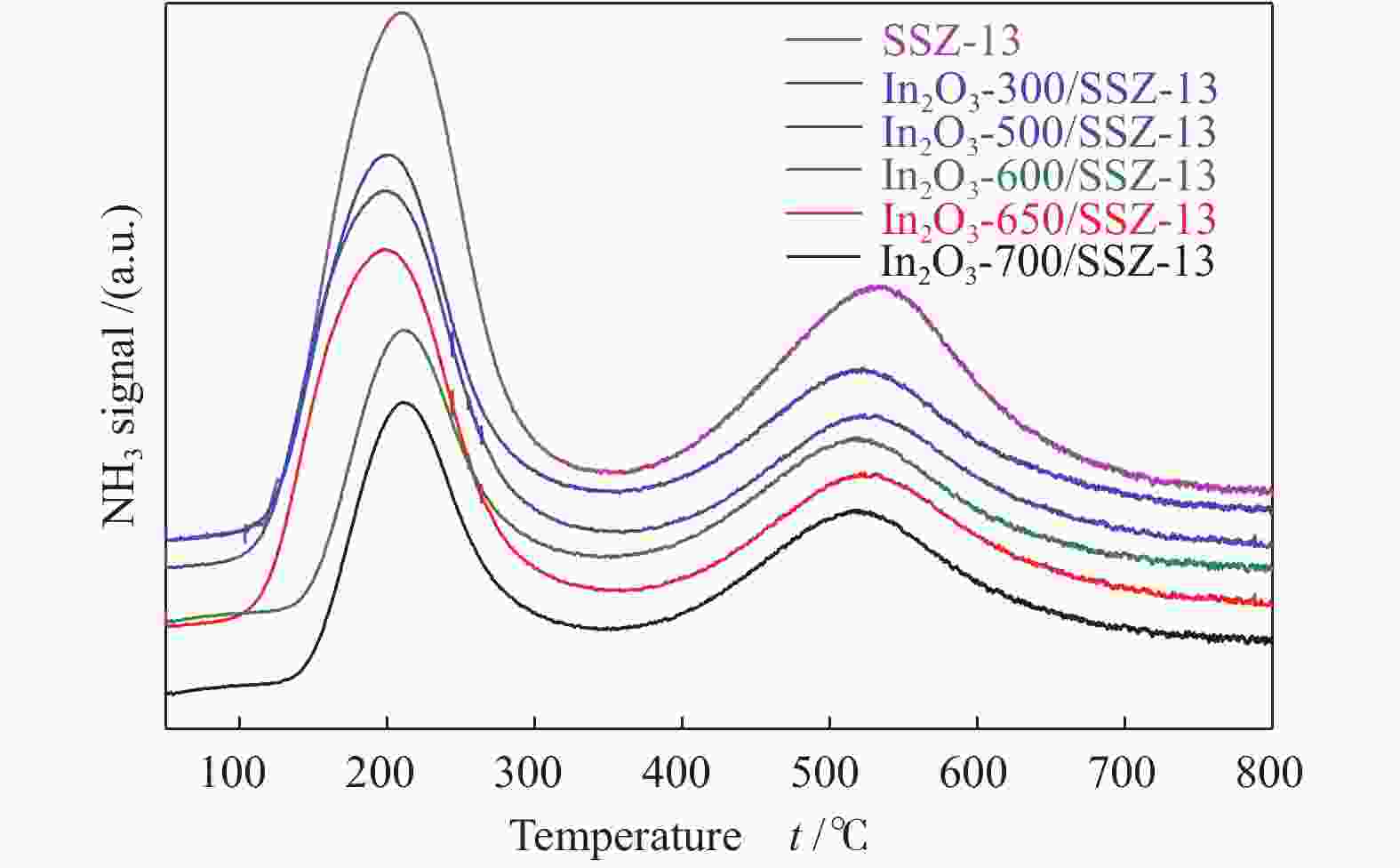
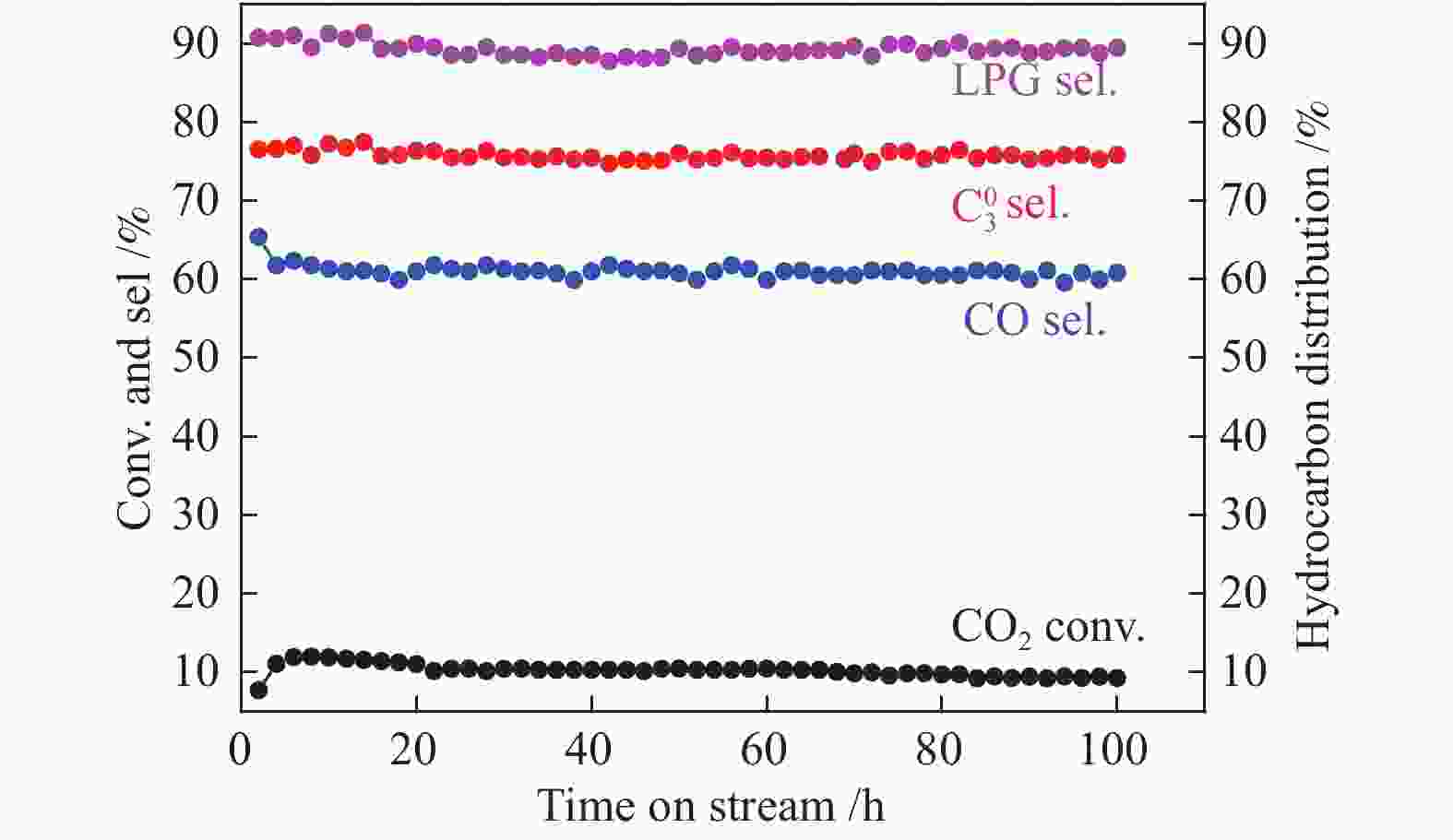
${\rm{C}}_3^0 $ 和${\rm{C}}_4^0 $ )。利用X射线衍射(XRD)、N2吸附-脱附、扫描电镜(SEM)、透射电镜(TEM)、NH3程序升温脱附(NH3-TPD)等表征手段对双功能催化剂的物化性质进行了表征。在固定床反应器上研究了氧化铟的晶粒尺寸、反应条件对In2O3/SSZ-13催化二氧化碳加氢制液化石油气性能的影响。结果表明,SSZ-13分子筛的八元环结构和强酸性位点有利于丙烷的选择性生成,初始晶粒尺寸为5 nm的氧化铟具有最高的CO2转化率(11.7%)和CO选择性(61.0%),而烃类产物分布受In2O3晶粒尺寸影响较小,其中,烃类产物中LPG的选择性基本维持在90%左右,丙烷选择性约为75%。增加反应压力、降低反应空速均有利于LPG收率的提高,在350 ℃,3 MPa,9000 mL/(gcat·h)的反应条件下,In2O3/SSZ-13双功能催化剂反应100 h未观察到显著失活现象。本研究为CO2加氢高选择合成液化石油气提供了新的探索途径。-
关键词:
- CO2加氢 /
- LPG合成 /
- In2O3/SSZ-13双功能催化剂 /
- 尺寸影响 /
- 反应条件
Abstract: Highly selective synthesis of liquefied petroleum gas (LPG,${\rm{C}}_3^0 $ and${\rm{C}}_4^0 $ ) from CO2 hydrogenation have realized over the In2O3/SSZ-13 bifunctional catalyst. The physicochemical properties of the bifunctional catalyst were characterized by X-ray diffraction spectroscopy (XRD), N2 physical adsorption, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and NH3 temperature-programmed desorption (NH3-TPD). The particle size effect of In2O3 and reaction conditions were investigated for CO2 hydrogenation to LPG over the In2O3/SSZ-13 bifunctional catalyst. Results indicate that CO2 conversion and CO selectivity are related to the particle size of In2O3, and fresh 5 nm In2O3 shows the highest CO2 conversion (11.7%) and the highest CO selectivity (61.0%), since it is more prone to reverse water gas reaction (RWGS). However, the hydrocarbon distribution does not exhibit a dependence of In2O3 size changes, and the selectivity of LPG maintains at 90% and the selectivity of propane reaches up to 76.8% due to the 8-MR micropores and strong acid sites of SSZ-13 zeolite. Additionally, the yield of LPG shows a volcano type with increasing reaction temperature, and the optimal reaction temperature is 370 ℃. Low space velocity is more favorable to the CO2 conversion, and LPG selectivity in hydrocarbon products still maintains about 90%. High reaction pressure is beneficial to improving the yield of LPG via promoting the secondary hydrogenation reaction over the SSZ-13 zeolite and inhibiting CO formation. Furthermore, no obvious deactivation is observed after a time on stream (TOS) of 100 h over the In2O3/SSZ-13 bifunctional catalyst at 350 ℃, 3 MPa and 9000 mL/(gcat·h). The research provides a new strategy for highly selective synthesis of LPG from CO2 hydrogenation.-
Key words:
- CO2 hydrogenation /
- LPG synthesis /
- In2O3/SSZ-13 composite catalyst /
- size effect /
- reaction condition
-
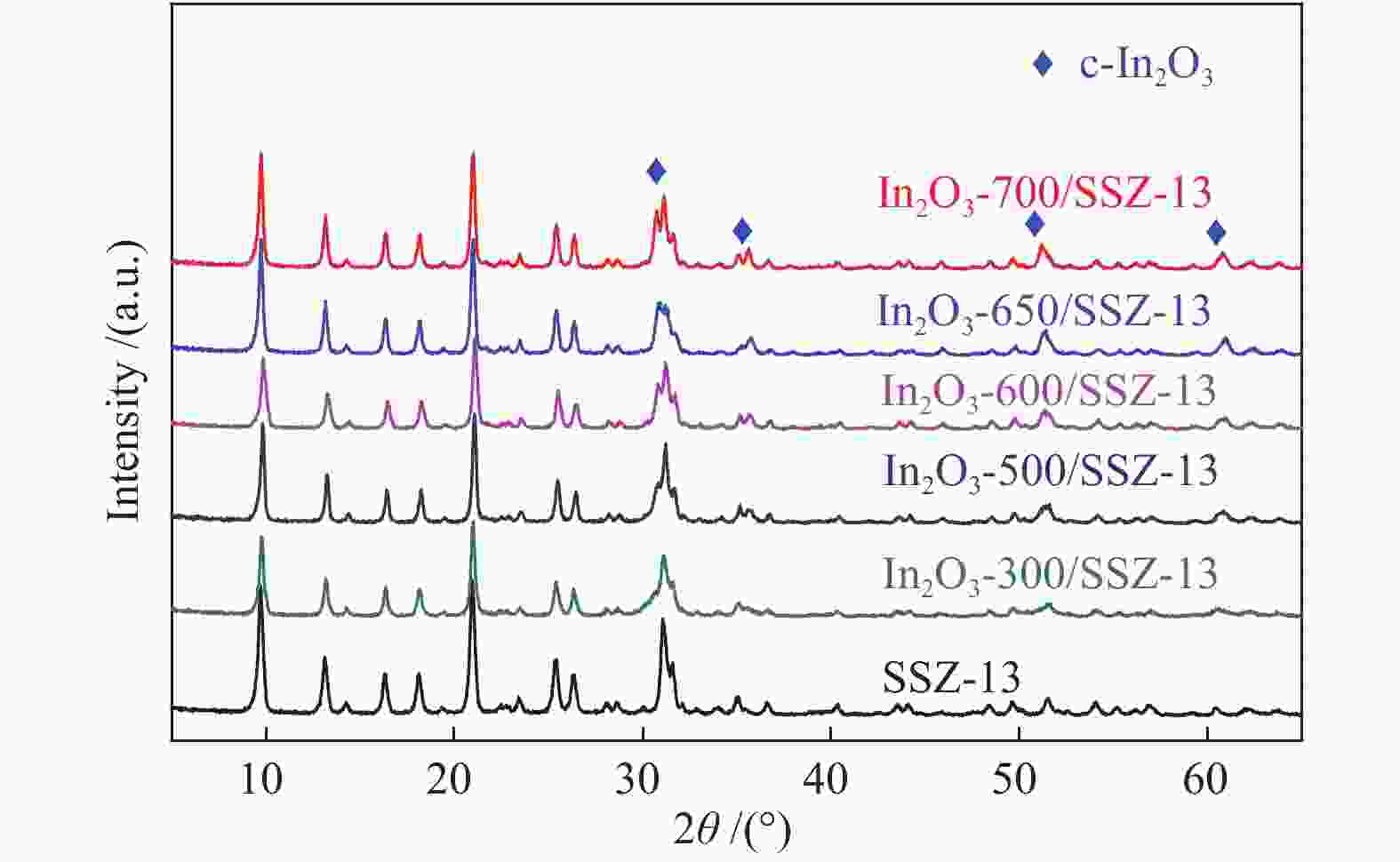
表 1 In2O3/SSZ-13双功能催化剂的结构性质
Table 1 Texture properties of the In2O3–x/SSZ-13 composite catalysts
Sample SBET/
(m2·g–1)Smicro/
(m2·g–1)vmeso/
(cm3·g–1)vmicro/
(cm3·g–1)SSZ-13 487 460 0.35 0.23 In2O3-300/SSZ-13 447 380 0.30 0.19 In2O3-500/SSZ-13 410 371 0.28 0.18 In2O3-600/SSZ-13 394 363 0.28 0.17 In2O3-650/SSZ-13 373 344 0.26 0.17 In2O3-700/SSZ-13 350 318 0.25 0.16 表 2 双功能催化剂In2O3-x/SSZ-13上的CO2加氢制LPG催化性能
Table 2 Catalytic performance for CO2 hydrogenation to LPG over bifunctional catalysts containing In2O3 oxides with different crystal sizes and SSZ-13 zeolites
Sample CO2 conv. /% CO sel. /% Hydrocarbon distribution/% STYLPG/
(mmol·gcat–1·h–1)CH4 ${\rm{C}}_2^0 $ LPG (${\rm{C}}_3^0 $) ${\rm{C}}_2^= $– ${\rm{C}}_4^= $ C5+ In2O3-300/SSZ13 11.7 61.0 3.4 2.2 90.6 (76.8) 2.3 1.5 3.99 In2O3-500/SSZ13 11.3 57.8 3.2 2.4 89.2 (75.7) 3.1 2.2 4.10 In2O3-600/SSZ13 9.8 56.7 3.5 2.3 89.9 (76.7) 2.1 2.2 3.68 In2O3-650/SSZ13 9.0 54.2 3.5 2.3 89.8 (76.4) 2.3 2.1 3.57 In2O3-700/SSZ13 7.6 53.8 3.6 2.4 88.6 (75.5) 3.0 2.4 3.00 standard reaction conditions: In2O3 (0.4 g) + SSZ-13 (0.8 g), 350 ℃, 3.0 MPa, H2/CO2 = 3, WHSV = 9000 mL/(gcat·h) results from 12 h time on stream 表 3 In2O3-300/SSZ-13在不同温度下催化CO2加氢制液化石油气的反应性能
Table 3 Catalytic performance for CO2 hydrogenation to LPG over In2O3-300/SSZ-13 catalysts under different reaction temperatures
Temperature/℃ CO2 conv. /% CO sel. /% Hydrocarbon distribution/% STYLPG/
(mmol·gcat–1·h–1)CH4 ${\rm{C}}_2^0 $ LPG (${\rm{C}}_3^0 $) ${\rm{C}}_2^= $– ${\rm{C}}_4^= $ C5+ 310 5.8 50.5 4.2 2.2 89.5 (74.3) 1.9 2.2 2.48 330 9.7 58.8 3.5 2.5 90.8 (77.0) 1.9 1.3 3.50 350 11.7 61.0 3.4 2.2 90.6 (76.8) 2.3 1.5 3.99 370 22.9 72.2 3.9 3.0 88.5 (75.8) 2.5 2.1 5.43 390 29.4 78.3 6.1 3.6 84.8 (72.7) 3.5 2.0 5.22 standard reaction conditions: In2O3 (0.4 g)+SSZ-13 (0.8 g), 3 MPa, WHSV = 9000 mL/(gcat·h) results from 12 h time on stream 表 4 In2O3-300/SSZ-13在不同空速下催化CO2加氢制液化石油气的反应性能
Table 4 Catalytic performance for CO2 hydrogenation to LPG over In2O3-300/SSZ-13 catalysts under different reaction space velocity
Space velocity/
(mL·gcat–1·h–1)CO2 conv. /% CO sel. /% Hydrocarbon distribution/% STYLPG/
(mmol·gcat–1·h–1)CH4 ${\rm{C}}_2^0 $ LPG (${\rm{C}}_3^0 $) ${\rm{C}}_2^= $– ${\rm{C}}_4^= $ C5+ 3000 14.9 67.1 3.6 2.4 90.7 (77.8) 1.8 1.5 4.29 6000 11.9 62.8 3.6 2.3 91.1 (77.7) 2.3 0.7 3.89 9000 11.7 61.0 3.4 2.2 90.6 (76.8) 2.3 1.5 3.99 12000 10.0 55.4 3.6 2.2 89.1 (75.4) 2.9 2.2 3.83 15000 8.9 55.0 3.7 2.1 89.0 (75.2) 3.4 1.8 3.44 standard reaction conditions: In2O3 (0.4 g)+SSZ-13 (0.8 g), 350 ℃, 3 MPa results from 12 h time on stream 表 5 In2O3-300/SSZ-13在不同压力下催化CO2加氢制液化石油气的反应性能
Table 5 Catalytic performance for CO2 hydrogenation to LPG over In2O3-300/SSZ-13 catalysts under different reaction pressure
Pressure/MPa CO2 conv. /% CO sel. /% Hydrocarbon distribution/% STYLPG/
(mmol·gcat–1·h–1)CH4 ${\rm{C}}_2^0 $ LPG (${\rm{C}}_3^0 $) ${\rm{C}}_2^= $– ${\rm{C}}_4^= $ C5+ 1.0 8.1 70.9 2.6 3.3 81.8 (70.8) 11.1 1.2 1.86 2.0 9.7 65.3 3.1 2.3 88.1 (75.5) 4.5 2.0 2.86 3.0 11.7 61.0 3.4 2.2 90.6 (76.8) 2.3 1.5 3.99 4.0 13.4 57.6 3.7 2.4 89.4 (75.6) 2.0 2.5 4.90 5.0 15.9 57.1 3.8 2.5 90.2 (75.9) 1.4 2.1 5.93 standard reaction conditions: In2O3 (0.4 g)+SSZ-13 (0.8 g), 350 ℃, WHSV = 9000 mL/(gcat·h) results from 12 h time on stream -
[1] ALVAREZ A, BANSODE A, URAKAWA A, BAVYKINA A V, WEZENDONK T A, MAKKEE M, GASCON J, KAPTEIJN F. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chem Rev,2017,117(14):9804−9838. doi: 10.1021/acs.chemrev.6b00816 [2] LIU X L, WANG M H, ZHOU C, ZHOU W, CHENG K, KANG J C, ZHANG Q H, DENG W P, WANG Y. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34[J]. ChemComm,2018,54:140−143. [3] JIANG X, NIE X, GUO X, SONG C, CHEN J G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chem Rev,2020,120(15):7984−8034. doi: 10.1021/acs.chemrev.9b00723 [4] DAS S, PEREZ RAMIREZ J, GONG J, DEWANGAN N, HIDAJAT K, GATES B. C, KAWI S. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2[J]. Chem Soc Rev,2000,49:2937−3004. [5] GOEPPERT A, CZAUN M, JONES J P, SURYA PRAKASH G K, OLAH G A. Recycling of carbon dioxide to methanol and derived products - closing the loop[J]. Chem Soc Rev,2014,43(23):7995−8048. doi: 10.1039/C4CS00122B [6] LI C, YUAN X, FUJIMOTO K. Direct synthesis of LPG from carbon dioxide over hybrid catalysts comprising modified methanol synthesis catalyst and β-type zeolite[J]. Appl Catal A: Gen,2014,475:155−160. doi: 10.1016/j.apcata.2014.01.025 [7] LI H, ZHANG P, GUO L, HE Y, ZENG Y, THONGKAM M, NATAKARANAKUL J, KOJIMA T, REUBROYCHAROEN P, VITIDSANT T, YANG G, TSUBAKI N. A well-defined core-shell-structured capsule catalyst for direct conversion of CO2 into liquefied petroleum gas[J]. Chem Sus Chem,2020,13(8):2060−2065. doi: 10.1002/cssc.201903576 [8] GAO P, DANG S, LI S, BU X, LIU Z, QIU M, YANG C, WANG H, ZHONG L, HAN Y, LIU Q, WEI W, SUN Y. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2017,8:571−578. [9] LI Z L, WANG J J, QU Y Z, LIU H L, TANG C Z, MIAO S, FENG Z C, AN H Y, LI C. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catal,2017,7(12):8544−8548. doi: 10.1021/acscatal.7b03251 [10] LIU X, WANG M, YIN H, HU J, CHENG K, KANG J, ZHANG Q, WANG Y. Tandem catalysis for hydrogenation of CO and CO2 to lower olefins with bifunctional catalysts composed of spinel oxide and SAPO-34[J]. ACS Catal,2020,10:8303−8314. [11] MA Z, POROSOFF M D. Development of tandem catalysts for CO2 hydrogenation to olefins[J]. ACS Catal,2019,9(3):2639−2656. doi: 10.1021/acscatal.8b05060 [12] SONG G, LI M, YAN P, NAWAZ M A, LIU D. High conversion to aromatics via CO2-FT over a CO-reduced Cu-Fe2O3 catalyst integrated with HZSM-5[J]. ACS Catal,2020,10(19):11268−11279. doi: 10.1021/acscatal.0c02722 [13] WANG Y, TAN L, TAN M H, ZHANG P P, FANG Y, YONEYAMA Y, YANG G H, TSUBAKI N. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics[J]. ACS Catal,2019,9(2):895−901. doi: 10.1021/acscatal.8b01344 [14] ZHOU C, SHI J, ZHOU W, CHENG K, ZHANG Q, KANG J, WANG Y. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catal,2019,10:302−310. [15] GAO P, ZHANG L, LI S, ZHOU Z, SUN Y. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Cent Sci,2020,6(10):1657−1670. doi: 10.1021/acscentsci.0c00976 [16] GAO P, LI S, BU X, DANG S, LIU Z, WANG H, ZHONG L, QIU M, YANG C, CAI J, WEI W, SUN Y. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem,2017,9(10):1019−1024. doi: 10.1038/nchem.2794 [17] WEI J, YAO R, GE Q, XU D, FANG C, ZHANG J, XU H, SUN J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation[J]. Appl Catal B: Environ,2021,283:119648. [18] LIU Z, NI Y, SUN T, ZHU W, LIU Z. Conversion of CO2 and H2 into propane over InZrO and SSZ-13 composite catalyst[J]. J. Energy Chem,2021,54:111−117. doi: 10.1016/j.jechem.2020.04.069 [19] XU Z, MA H, HUANG Y, QIAN W, ZHANG H, YING W. Synthesis of submicron SSZ-13 with tunable acidity by the seed-assisted method and its performance and coking behavior in the MTO reaction[J]. ACS Omega,2020,5(38):24574−24583. doi: 10.1021/acsomega.0c03075 [20] YU H F, ZHANG G P, HAN LN, CHANG L P, BAO W R, WANG J C. Cu-SSZ-13 catalyst synthesized under microwave irradiation and its performance in catalytic removal of NOx from vehicle exhaust[J]. Acta Phys-Chim Sin,2015,31(11):2165−2173. doi: 10.3866/PKU.WHXB201509184 [21] XU Z, LI J, HUANG Y, MA H, QIAN W, ZHANG H, YING W. Size control of SSZ-13 crystals with APAM and its influence on the coking behaviour during MTO reaction[J]. Catal Sci Technol,2019,9(11):2888−2897. doi: 10.1039/C9CY00412B [22] WU L, HENSEN E J M. Comparison of mesoporous SSZ-13 and SAPO-34 zeolite catalysts for the methanol-to-olefins reaction[J]. Catalysis Today,2014,235:160−168. doi: 10.1016/j.cattod.2014.02.057 [23] DANG S S, GAO P, LIU Z Y, CHEN X Q, YANG C G, WANG H, ZHONG L S, LI S G, SUN Y H. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts[J]. J Catal,2018,364:382−393. doi: 10.1016/j.jcat.2018.06.010 [24] JIA X, SUN K, WANG J, SHEN C, LIU C J. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst[J]. J. Energy Chem,2020,50:409−415. doi: 10.1016/j.jechem.2020.03.083 [25] YU Z, HONG N W, RUO YU C. In situ synthesis of Cu-SSZ-13/cordierite monolithic catalyst for the selective catalytic reduction of NO with NH3[J]. Acta Phys-Chim Sin,2015,31(2):329−336. doi: 10.3866/PKU.WHXB201412082 [26] LI Z, QU Y, WANG J, LIU H, LI M, MIAO S, LI C. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts[J]. Joule,2019,3(2):570−583. doi: 10.1016/j.joule.2018.10.027 [27] YE J, LIU C, M EI, D, G E, Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study[J]. ACS Catal,2013,3(6):1296−1306. doi: 10.1021/cs400132a [28] MARTIN O, MARTIN A J, MONDELLI C, MITCHELL S, SEGAWA T F, HAUERT R, DROUILLY C, CURULLA-FERRE D, PEREZ-RAMIREZ J. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angew Chem Int Ed Eng,2016,55(21):6261−6265. doi: 10.1002/anie.201600943 [29] LI Y, Wang W T, ZHAO F, XU Y T. H - SSZ - 13 molecular sieve synthesized by introducing accelerant and its effect on reactivity of MTO[J]. J Tiangong Univ,2015,34(6):35−40. [30] NUMPILAI T, WATTANAKIT C, CHAREONPANICH M, LIMTRAKUL J, WITOON T. Optimization of synthesis condition for CO2 hydrogenation to light olefins over In2O3 admixed with SAPO-34[J]. Energy Convers Manage,2019,180:511−523. doi: 10.1016/j.enconman.2018.11.011 -








 下载:
下载: