Effect of Si content on the performance of direct synthesis of dimethyl ether over slurry CuZnAl catalyst prepared by complete liquid phase technology
-
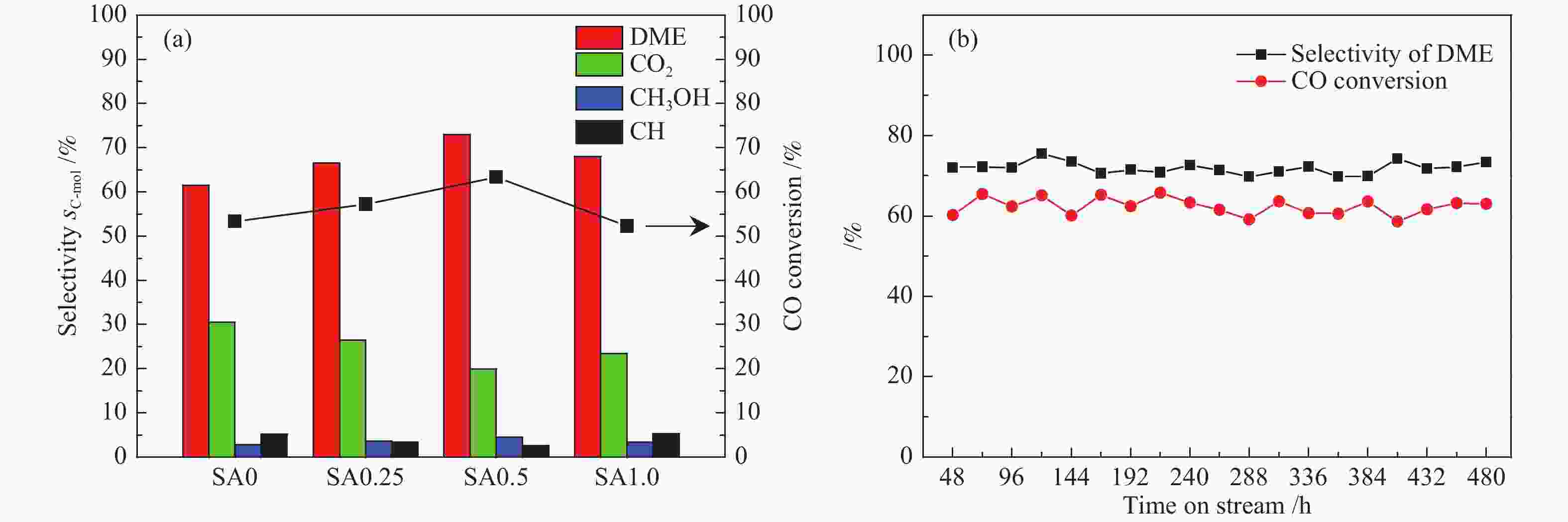
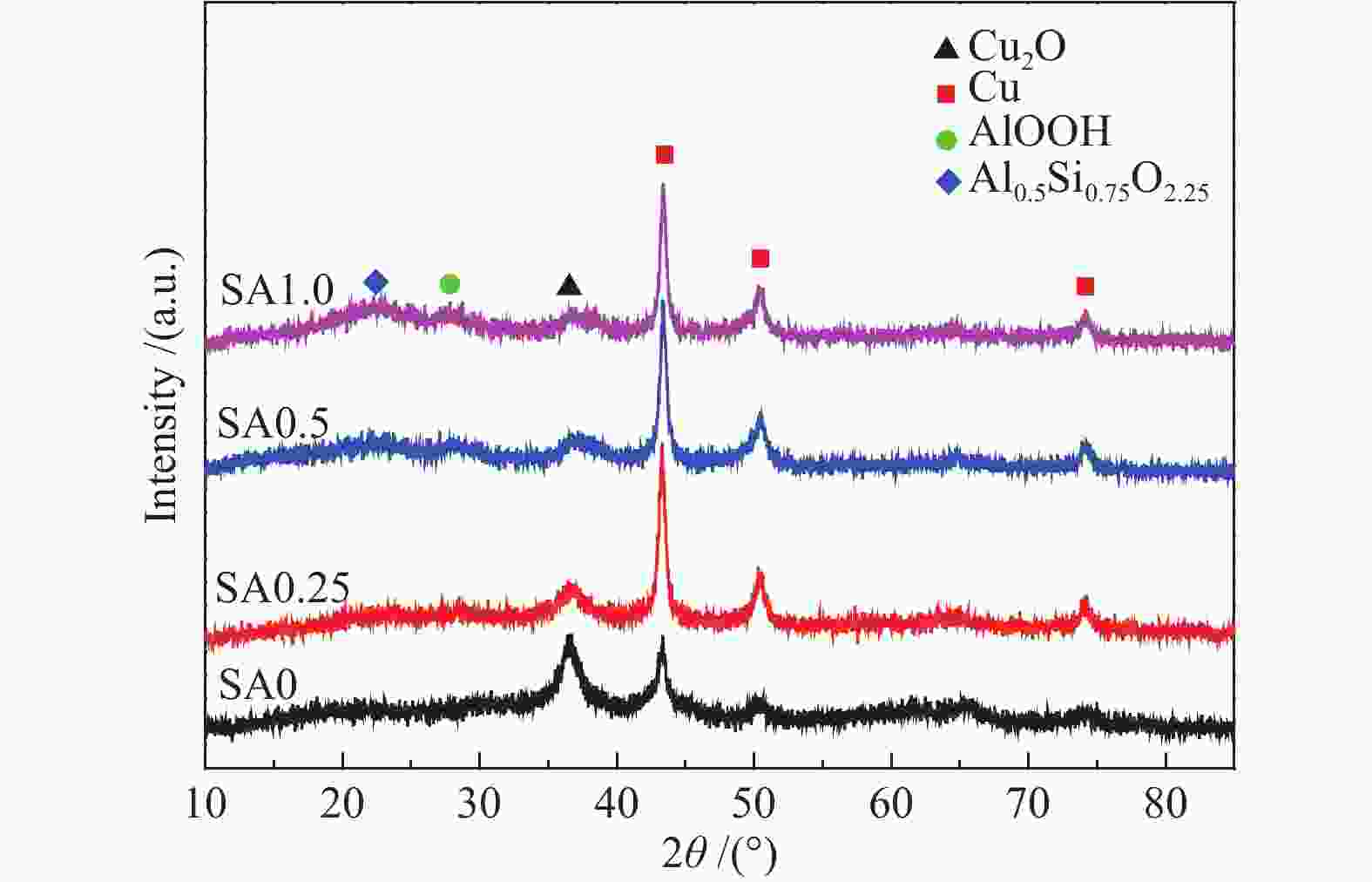
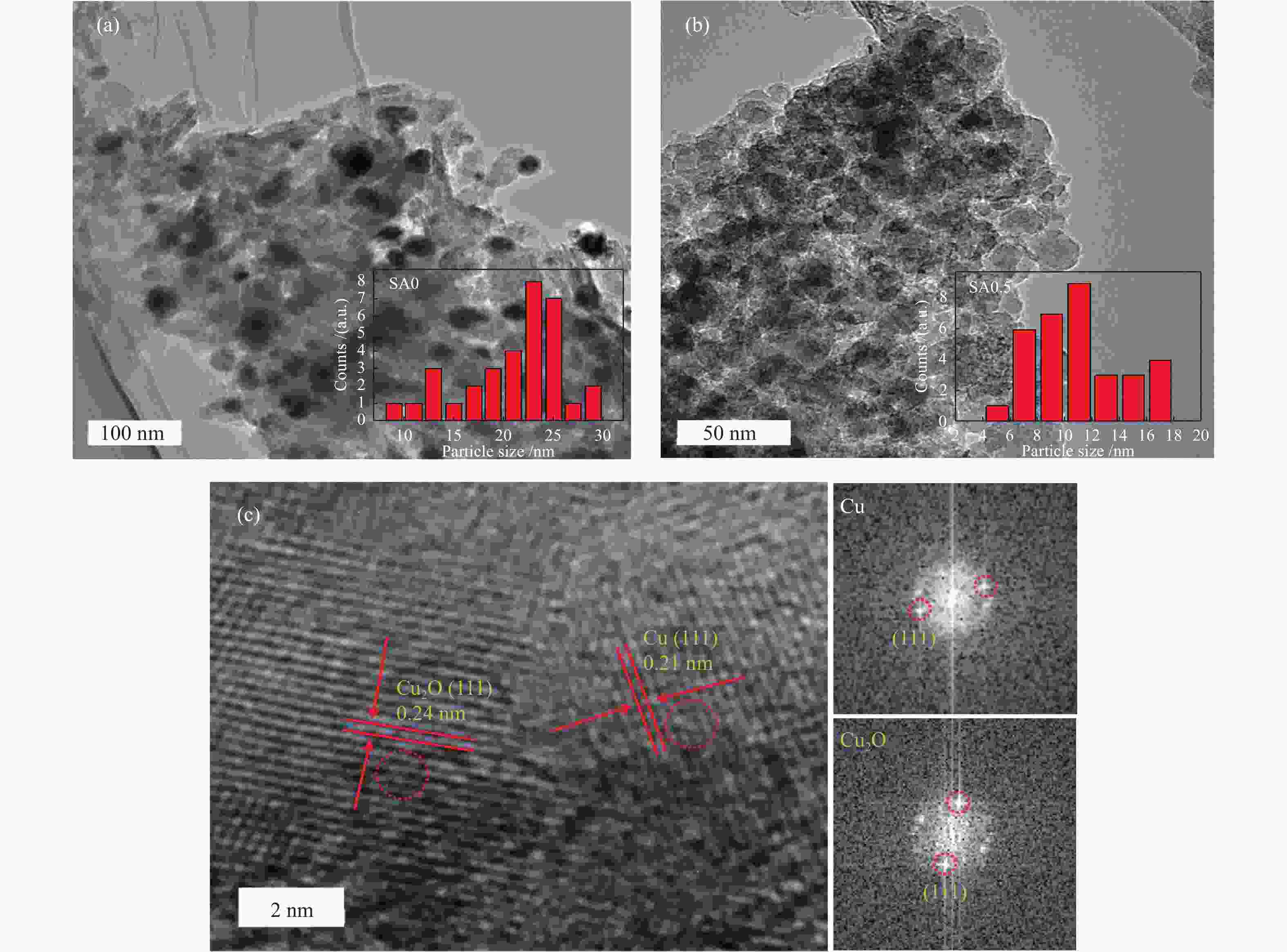
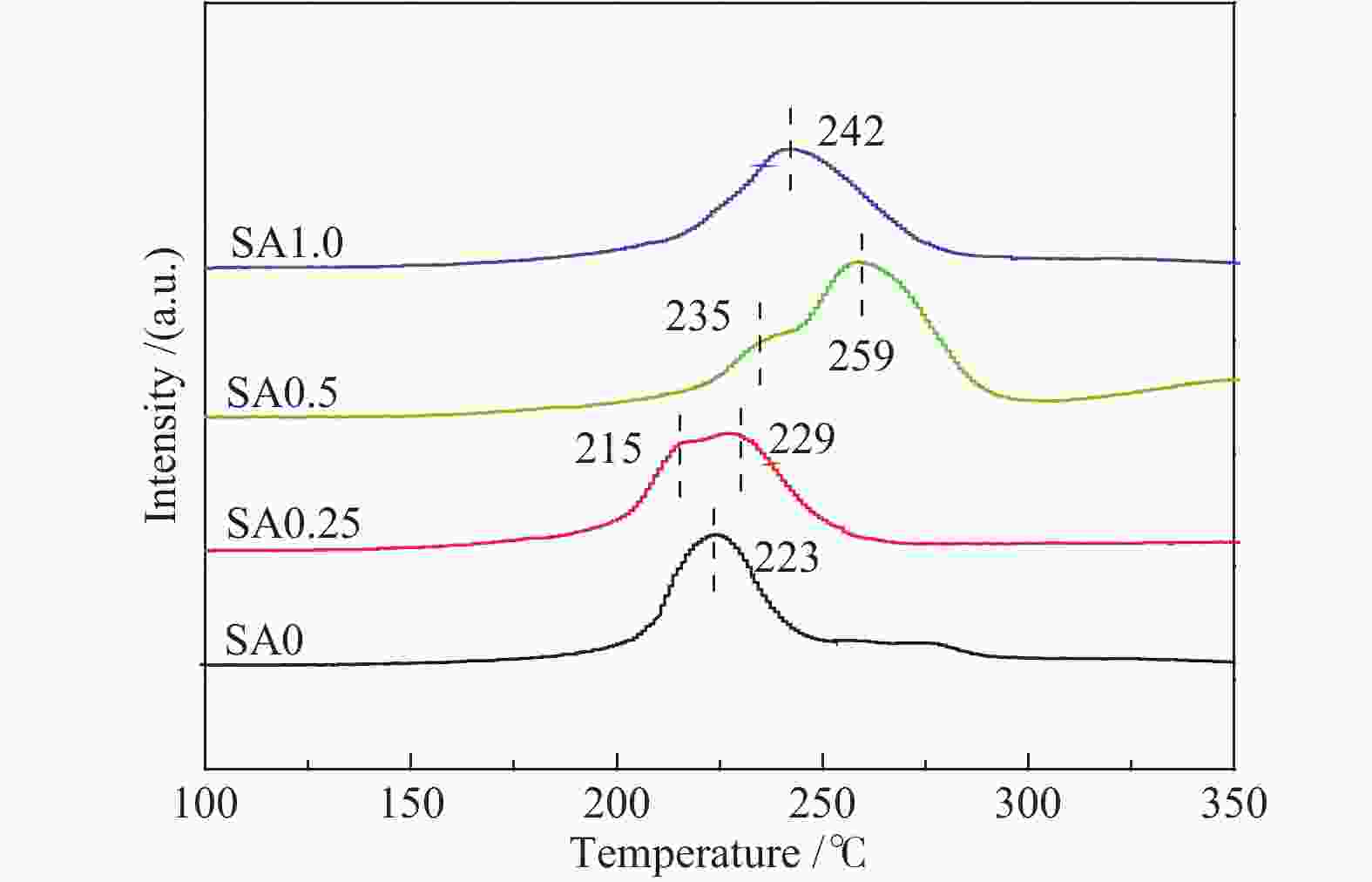
摘要: 在完全液相法制备工艺中,考察不同Si含量对浆状CuZnAl催化剂上合成气直接制备二甲醚性能的影响。其中,SA0.5催化剂(Si/Al=0.5)显示了最优异的催化性能,CO转化率为63.31%,二甲醚选择性为72.96%,在反应480 h过程中催化剂催化性能稳定。通过X射线衍射(XRD)、透射电子显微镜(TEM)和氮气吸附-脱附表征发现,Si的引入促进了催化剂Cu物种颗粒的分散及比表面积的增大,提高了CO转化率。此外,氢气程序升温还原(H2-TPR)和X射线光电子能谱(XPS)表征揭示了Cu物种与催化剂其他组分(Si物种)之间存在电子相互作用,抑制了Cu物种还原,催化剂表面富集更多Cu+物种,有利于甲醇合成,同时有效地抑制了水煤气副反应产物CO2的生成。再者,SA0.5催化剂表面富集了大量的Al物种(AlOOH),有利于甲醇脱水,促进二甲醚的生成。总之,浆状CuZnAlSi体系中Cu+和AlOOH协同催化作用,提高了催化剂活性及二甲醚选择性。
-
关键词:
- Si含量 /
- Cu+-AlOOH协同催化 /
- 二甲醚 /
- 完全液相法 /
- 合成气
Abstract: The effect of Si content on the performance of slurry CuZnAl catalyst prepared by complete liquid phase technology for direct synthesis of dimethyl ether from syngas was investigated. Among them, catalyst with Si/Al ratio of 0.5 showed the best catalytic performance with the CO conversion of 63.31% and the dimethyl ether selectivity of 72.96%. The catalyst was stable after 480 h reaction. As revealed by the X-ray diffraction (XRD), transmission electron microscopy (TEM) and nitrogen adsorption and desorption characterizations, the introduction of Si promoted the dispersion of Cu species nanoparticles and led to increased specific surface area, which was beneficial for improving the CO conversion. Besides, temperature programmed reduction (H2-TPR) and X-ray photoelectron spectroscopy (XPS) characterizations showed that an electronic interaction between Cu species and other components of the catalyst (especially, Si species) could inhibit the reduction of Cu species, resulting in the abundant Cu+ species on the catalyst surface. This was conducive to the synthesis of methanol and could effectively inhibit the formation of CO2, which was a by-product of the water-gas shift reaction. Moreover, a large amount of Al species (AlOOH) was enriched on the SA0.5 catalyst surface, which might contribute to the dehydration of methanol to produce dimethyl ether. In conclusion, the synergetic catalysis of Cu+ and AlOOH in slurry CuZnAlSi system improved the catalytic activity and dimethyl ether selectivity. -
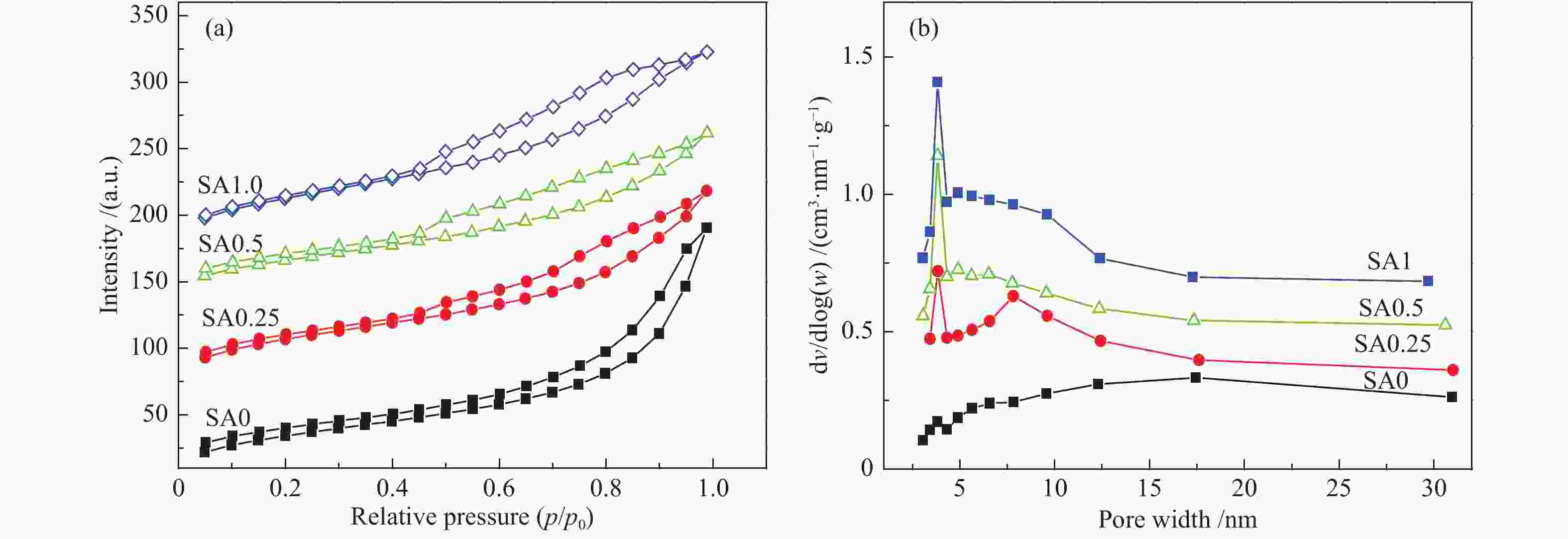
表 1 不同催化剂试样比表面积及孔结构参数
Table 1 Specific surface area and pore structure parameters of different catalysts
Sample BET surface area A/(m2·g−1) Pore volume v/(cm3·g−1) Average pore diameter d/nm SA0 128.0 0.29 9.2 SA0.25 133.5 0.24 6.8 SA0.5 162.7 0.22 5.8 SA1 151.4 0.23 6.1 表 2 不同催化剂反应前Cu物种结合能及俄歇动能数据
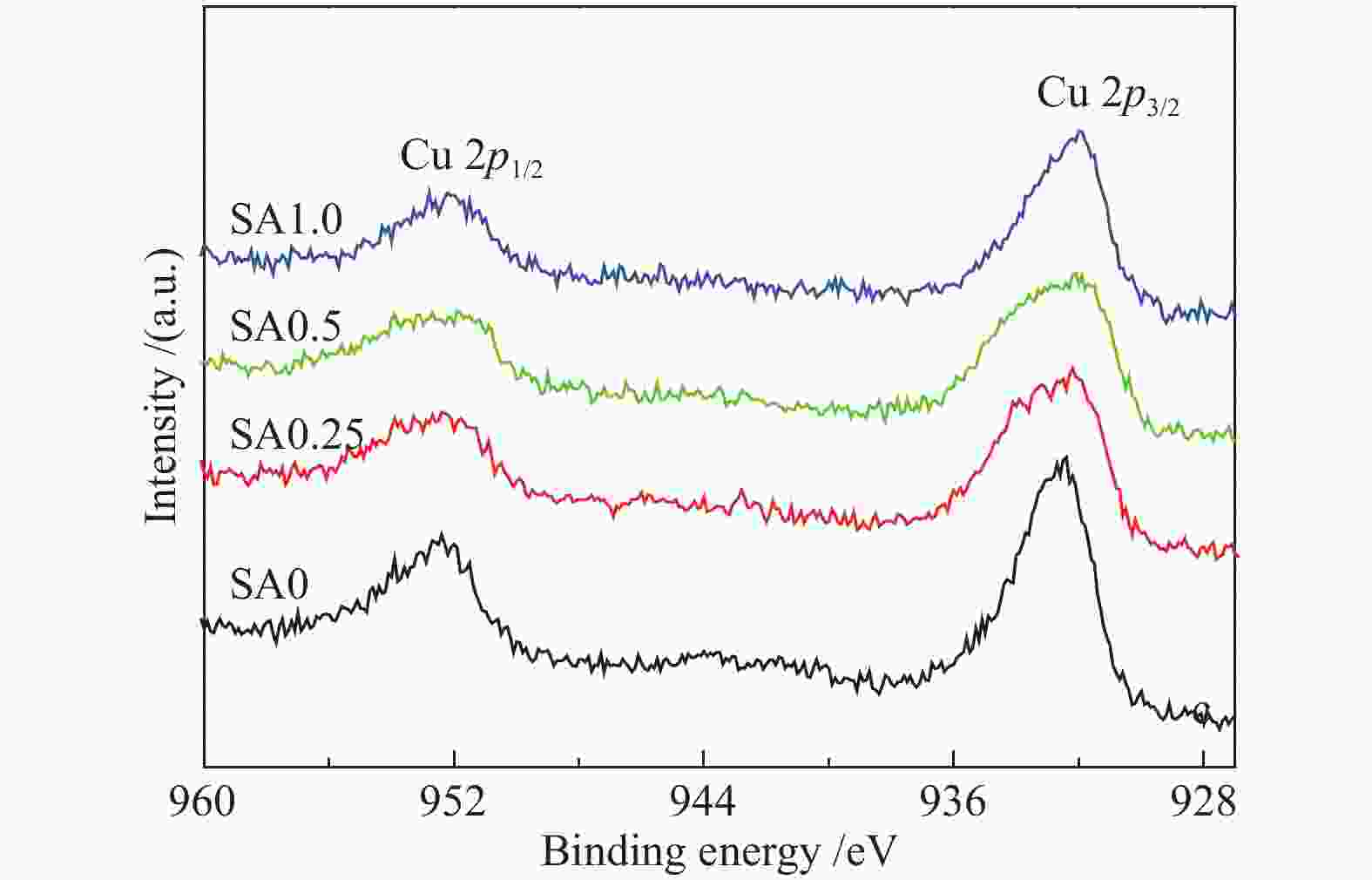
Table 2 Binding energy (BE) and Kinetic energy (KE) of Cu species over fresh catalysts
Catalyst BE/eV KE/eV α′Cu SA0 932.5 916.3 1848.8 SA0.25 932.4 916.5 1848.9 SA0.5 932.2 916.4 1849.0 SA1.0 932.0 916.8 1848.8 表 3 不同催化剂反应前表面元素物质的量比
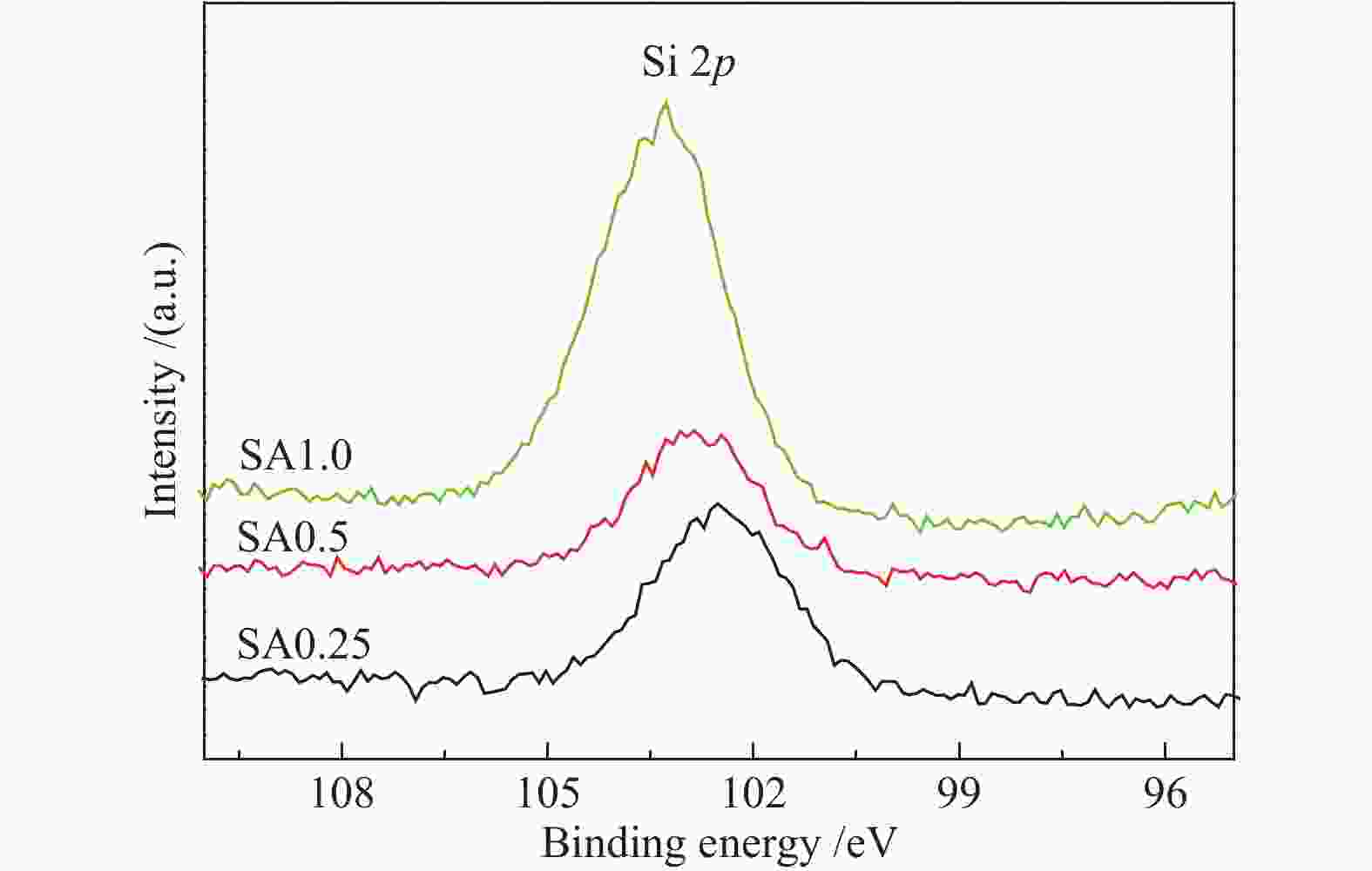
Table 3 Molar ratios between relevant elements on fresh catalysts
Catalyst Si/Al Cu/Zn Zn/Al Al/(Cu+Zn) SA0.25 0.32 0.58 0.13 4.86 SA0.5 0.24 0.67 0.11 5.27 SA1.0 0.50 0.36 0.17 4.46 -
[1] FARRELL A E, PLEVIN R J, TURNER B T, JONES A D, HARE M, KAMMEN D M. Ethanol can contribute to energy and environmental goals[J]. Science,2006,311(5760):506−508. doi: 10.1126/science.1121416 [2] SARAVANAN K, HAM H, TSUBAKI N, BAE J W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts[J]. Appl Catal B: Environ,2017,217:494−522. doi: 10.1016/j.apcatb.2017.05.085 [3] CAI M, PALČIĆ A, SUBRAMANIAN V, MOLDOVAN S, ERSEN O, VALTCHEV V, ORDOMSKY V V, KHODAKOV A Y. Direct dimethyl ether synthesis from syngas on copper-zeolite hybrid catalysts with a wide range of zeolite particle sizes[J]. J Catal,2016,338:227−238. doi: 10.1016/j.jcat.2016.02.025 [4] SUN J, YANG G, YONEYAMA Y, TSUBAKI N. Catalysis chemistry of dimethyl ether synthesis[J]. ACS Catal,2014,4(10):3346−3356. doi: 10.1021/cs500967j [5] ARCOUMANIS C, BAE C, CROOKES R, KINOSHITA E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review[J]. Fuel,2008,87(7):1014−1030. doi: 10.1016/j.fuel.2007.06.007 [6] JEONG J, AHN C, LEE D, UM S, BAE J. Effects of Cu-ZnO content on reaction rate for direct synthesis of DME from syngas with bifunctional Cu–ZnO/γ-Al2O3 Catalyst[J]. Catal Lett,2013,143(7):666−672. doi: 10.1007/s10562-013-1022-6 [7] BAE J, POTDAR H S, KANG S, JUN K. Coproduction of methanol and dimethyl ether from biomass-derived syngas on a Cu-ZnO-Al2O3/γ-Al2O3 hybrid catalyst[J]. Energy Fuels,2008,22(1):223−230. [8] VENUGOPAL A, PALGUNADI J, DEOG J K, JOO O, SHIN C. Dimethyl ether synthesis on the admixed catalysts of Cu-Zn-Al-M (M=Ga, La, Y, Zr) and γ-Al2O3: The role of modifier[J]. J Mol Catal A: Chem,2009,302(1):20−27. [9] PALOMO J, RODRÍGUEZ M Á, RODRÍGUEZ J, CORDERO T. ZSM-5-decorated CuO/ZnO/ZrO2 fibers as efficient bifunctional catalysts for the direct synthesis of DME from syngas[J]. Appl Catal B: Environ,2020,270:118893. doi: 10.1016/j.apcatb.2020.118893 [10] AGUAYO A T, EREÑA J, MIER D, ARANDES J M, OLAZAR M, BILBAO J. Kinetic modeling of dimethyl ether synthesis in a single step on a CuO-ZnO-Al2O3/γ-Al2O3 catalyst[J]. Ind Eng Chem Res,2007,46(17):5522−5530. doi: 10.1021/ie070269s [11] FUJIMOTO K, ASAMI K, SHIKADA T, TOMINAGA H. Selective synthesis of dimethyl ether from synthesis gas[J]. Chem Lett,1984,13(12):2051−2054. doi: 10.1246/cl.1984.2051 [12] YANG G, TSUBAKI N, SHAMOTO J, YONEYAMA Y, ZHANG Y. Confinement effect and synergistic function of H-ZSM-5/Cu-ZnO-Al2O3 capsule catalyst for one-step controlled synthesis[J]. J Am Chem Soc,2010,132(23):8129−8136. doi: 10.1021/ja101882a [13] GARCÍA T A, MARTÍNEZ A. Direct synthesis of DME from syngas on hybrid CuZnAl/ZSM-5 catalysts: New insights into the role of zeolite acidity[J]. Appl Catal A: Gen,2012,411-412:170−179. doi: 10.1016/j.apcata.2011.10.036 [14] TAN Y, XIE H, CUI H, HAN Y, ZHONG B. Modification of Cu-based methanol synthesis catalyst for dimethyl ether synthesis from syngas in slurry phase[J]. Catal Today,2005,104(1):25−29. doi: 10.1016/j.cattod.2005.03.033 [15] SONG F, TAN Y, XIE H, ZHANG Q, HAN Y. Direct synthesis of dimethyl ether from biomass-derived syngas over Cu-ZnO-Al2O3-ZrO2(x)/γ-Al2O3 bifunctional catalysts: Effect of Zr-loading[J]. Fuel Process Technol,2014,126:88−94. doi: 10.1016/j.fuproc.2014.04.021 [16] TIAN S, TAN M, MA Q, WU X, LUAN C, FANG Y, LI H, YANG G, TSUBAKI N, TAN Y. LDH-Derived (CuZn)xAly bifunctional catalyst for direct synthesis of dimethyl ether from syngas[J]. Ind Eng Chem Res,2020,59(23):11087−11097. doi: 10.1021/acs.iecr.0c01508 [17] 黄伟, 高志华, 郝利峰, 阴丽华, 谢克昌. 浆态床催化剂及制备方法: 中国, 1314491C[P]. 2007-05-09.HUANG Wei, GAO Zhi-hua, HAO Li-fen, Yin Li-hua, XIE Ke-chang. The liquid phase preparation technology of catalyst used in slurry reactor: CN, 1314491C[P]. 2007-05-09. [18] GAO Z, HUANG W, YIN L, XIE K. Liquid-phase preparation of catalysts used in slurry reactors to synthesize dimethyl ether from syngas: Effect of heat-treatment atmosphere[J]. Fuel Process Technol,2009,90(12):1442−1446. doi: 10.1016/j.fuproc.2009.06.022 [19] FAN J, CHEN C, ZHAO J, HUANG W, XIE K. Effect of surfactant on structure and performance of catalysts for DME synthesis in slurry bed[J]. Fuel Process Technol,2010,91(4):414−418. doi: 10.1016/j.fuproc.2009.05.005 [20] WANG P, HUANG W, ZHANG G, GAO Z, TANG Y, SUN K, ZHANG X. The facile preparation of Cu-Zn-Al oxide composite catalysts with high stability and performance for the production of dimethyl ether using modified aluminum alkoxide[J]. J Ind Eng Chem,2015,26:243−250. doi: 10.1016/j.jiec.2014.12.001 [21] SUN K, WANG P, BIAN Z, HUANG W. An investigation into the effects of different existing states of aluminum isopropoxide on copper-based catalysts for direct synthesis of dimethyl ether from syngas[J]. Appl Surf Sci,2018,428:534−540. doi: 10.1016/j.apsusc.2017.09.159 [22] 孙凯, 张小雨, 张琳, 边仲凯, 黄伟, 赵志换. 酸碱性硅溶胶对浆状Cu/Zn/Al催化剂性能的影响[J]. 燃料化学学报,2015,43(10):1221−1229. doi: 10.1016/S1872-5813(15)30037-2SUN Kai, ZHANG Xiao-yu, ZHANG Lin, BIAN Zhong-kai, HUANG Wei, ZHAO Zhi-huan. Influence of acid and alkaline silica sol on the performance of Cu/Zn/Al slurry catalysts[J]. J Fuel Chem Technol,2015,43(10):1221−1229. doi: 10.1016/S1872-5813(15)30037-2 [23] SUN K, WU Y, TAN M, WANG L, YANG G, ZHANG M, ZHANG W, TAN Y. Ethanol and higher alcohols synthesis from syngas over CuCoM (M=Fe, Cr, Ga and Al) nanoplates derived from hydrotalcite-like precursors[J]. ChemCatChem,2019,11(11):2695−2706. doi: 10.1002/cctc.201900096 [24] CHEN W, FAN Z, LAI Z. Synthesis of core-shell heterostructured Cu/Cu2O nanowires monitored by in situ XRD as efficient visible-light photocatalysts[J]. J Mater Chem A,2013,1(44):13862−13868. doi: 10.1039/c3ta13413j [25] LI Z, ZUO Z, HUANG W, XIE K. Research on Si-Al based catalysts prepared by complete liquid-phase method for DME synthesis in a slurry reactor[J]. Appl Surf Sci,2011,257(6):2180−2183. doi: 10.1016/j.apsusc.2010.09.069 [26] HUANG W C, LYU L M, YANG Y C, HUANG M H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity[J]. J Am Chem Soc,2012,134(2):1261−1267. doi: 10.1021/ja209662v [27] SUI Y, FU W, ZENG Y, YANG H, ZHANG Y, CHEN H, LI Y, LI M, ZOU G. Synthesis of Cu2O nanoframes and nanocages by selective oxidative etching at room temperature[J]. Angew Chem Int Ed,2010,49(25):4282−4285. doi: 10.1002/anie.200907117 [28] WITOON T, CHALORNGTHAM J, DUMRONGBUNDITKUL P, CHAREONPANICH M, LIMTRAKUL J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases[J]. Chem Eng J,2016,293:327−336. doi: 10.1016/j.cej.2016.02.069 [29] SUN K, GAO X, BAI Y, TAN M, YANG G, TAN Y. Synergetic catalysis of bimetallic copper-cobalt nanosheets for direct synthesis of ethanol and higher alcohols from syngas[J]. Catal Sci Technol,2018,8(15):3936−3947. doi: 10.1039/C8CY01074A [30] WU J, GAO G, SUN P, LONG X, LI F. Synergetic catalysis of bimetallic cuco nanocomposites for selective hydrogenation of bioderived esters[J]. ACS Catal,2017,7(11):7890−7901. doi: 10.1021/acscatal.7b02837 [31] YAO X, TANG C, GAO F, DONG L. Research progress on the catalytic elimination of atmospheric molecular contaminants over supported metal-oxide catalysts[J]. Catal Sci Technol,2014,4(9):2814−2829. doi: 10.1039/C4CY00397G [32] SUN K, TAN M, BAI Y, GAO X, WANG P, GONG N, ZHANG T, YANG G, TAN Y. Design and synthesis of spherical-platelike ternary copper-cobalt-manganese catalysts for direct conversion of syngas to ethanol and higher alcohols[J]. J Catal,2019,378:1−16. doi: 10.1016/j.jcat.2019.08.013 [33] CHEN L F, GUO P J, QIAO M H, YAN S R, LI H X, SHEN W, XU H L, FAN K N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal,2008,257(1):172−180. doi: 10.1016/j.jcat.2008.04.021 -




 下载:
下载: