Preparation of WS2/C composite material and its electrocatalytic hydrogen evolution performance
-
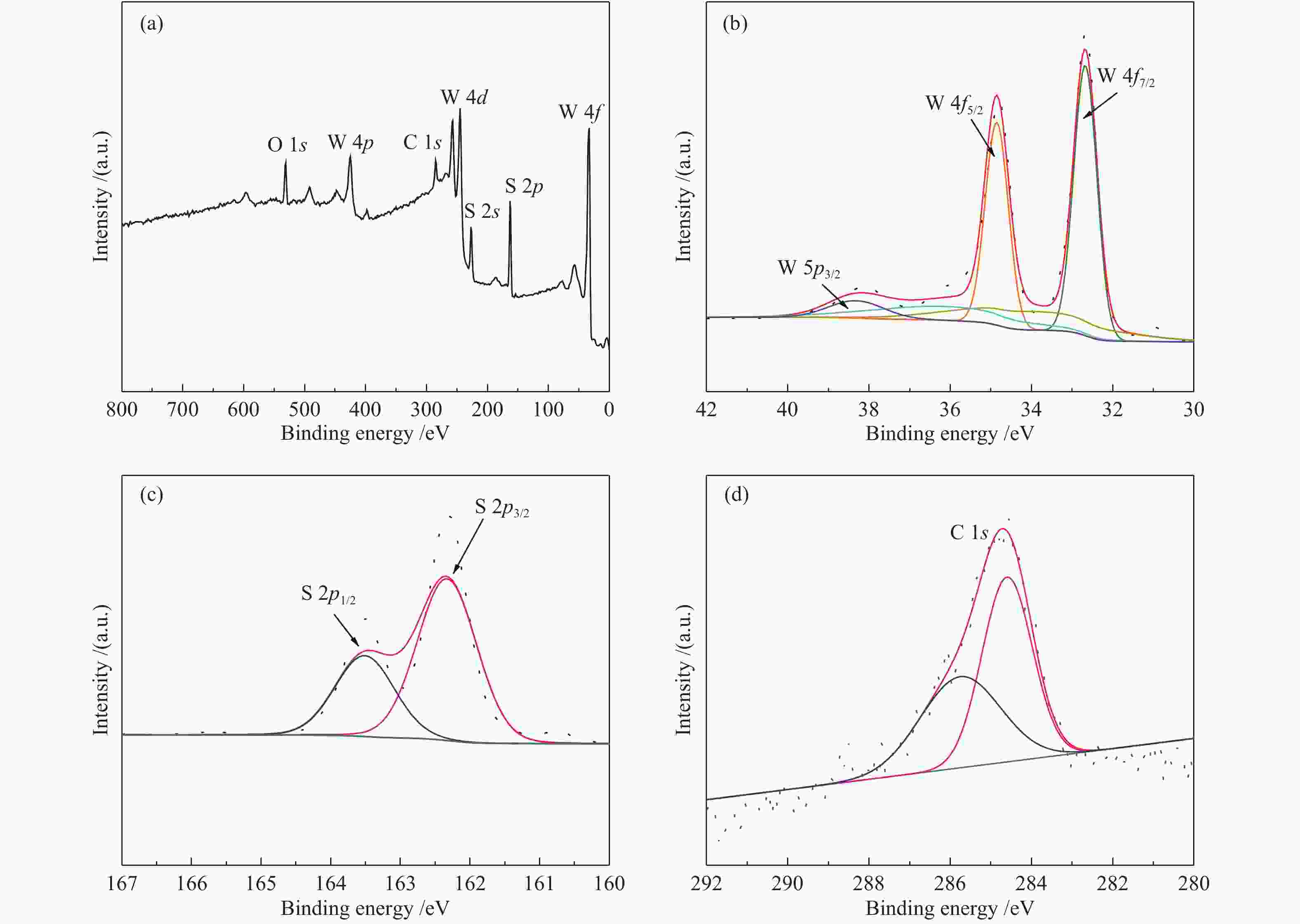
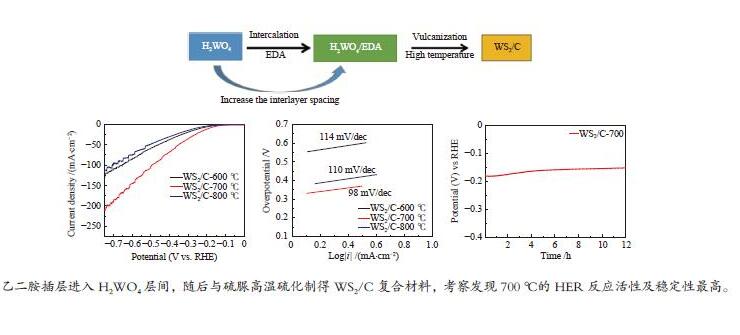
摘要: 以H2WO4和EDA为前驱体,通过机械搅拌与原位固相热解,得到WO3/C中间体,通过高温硫化得到WS2/C复合材料。通过X射线衍射(XRD)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)等仪器分析方法对制备的WS2/C复合材料结构和形貌等进行表征。同时对材料进行了电催化稳态极化曲线(LSV)、塔菲尔斜率(Tafel)、循环稳定性(CP)和电化学阻抗(PEIS)和电化学活性表面积(ECSA)测试,分析了催化剂的电催化性能。结果表明,当WS2/C复合材料的电流密度为10 mA/cm2时,过电位为179 mV,Tafel斜率为98 mV/dec。Abstract: With H2WO4 and EDA as precursors, WO3/C intermediate was obtained by mechanical stirring and in-situ solid-phase pyrolysis, then WS2/C composite material was obtained by high temperature vulcanization. The WS2/C composite was characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS) and other instrumental analysis methods. At the same time, the electrocatalytic performance of the catalyst was analyzed by the electrocatalytic steady-state polarization curve (LSV), Tafel slope (Tafel), cycle stability (CP), electrochemical impedance (PEIS) and electrochemically active surface area (ECSA) tests of the material. The results show that when the current density of the WS2/C composite is 10 mA/cm2, overpotential is 179 mV, and Tafel slope is 98 mV/dec.
-
Key words:
- WS2 /
- electrocatalysis /
- hydrogen evolution reaction
-
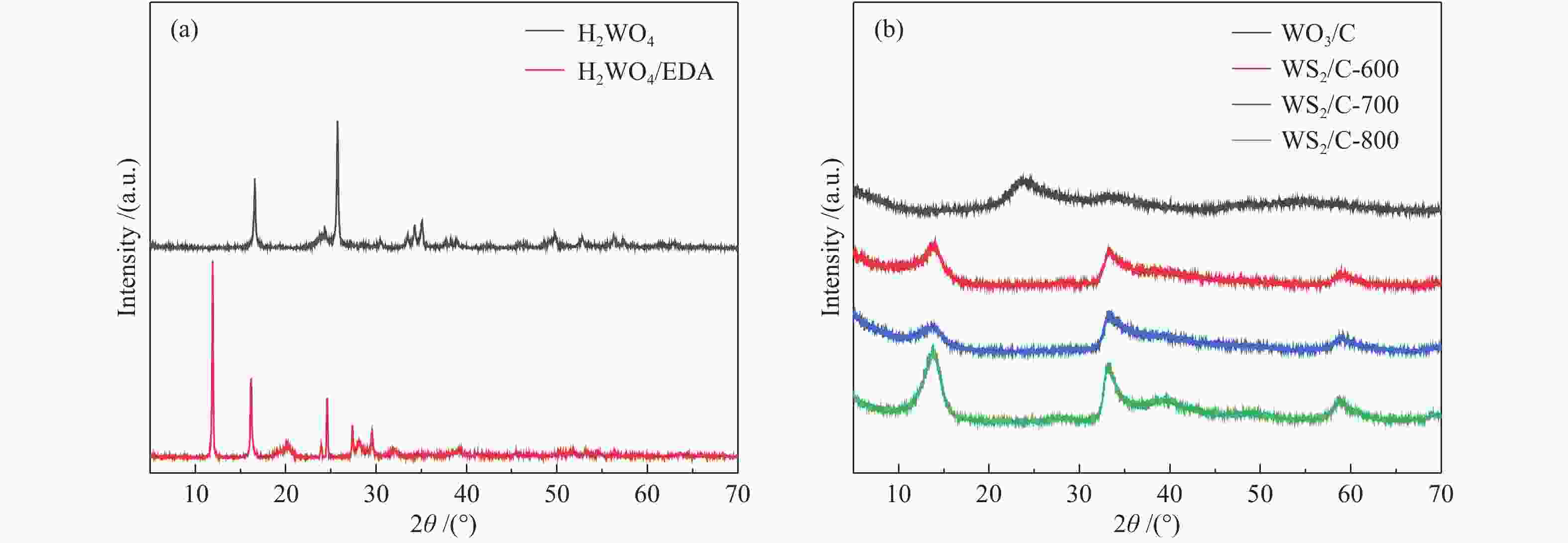
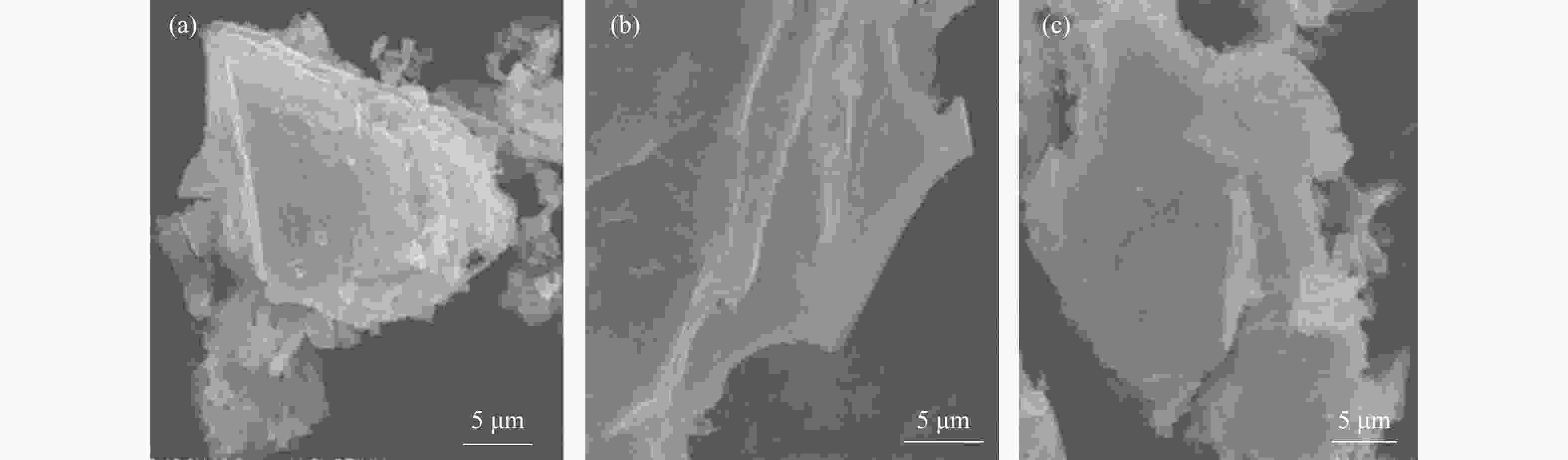
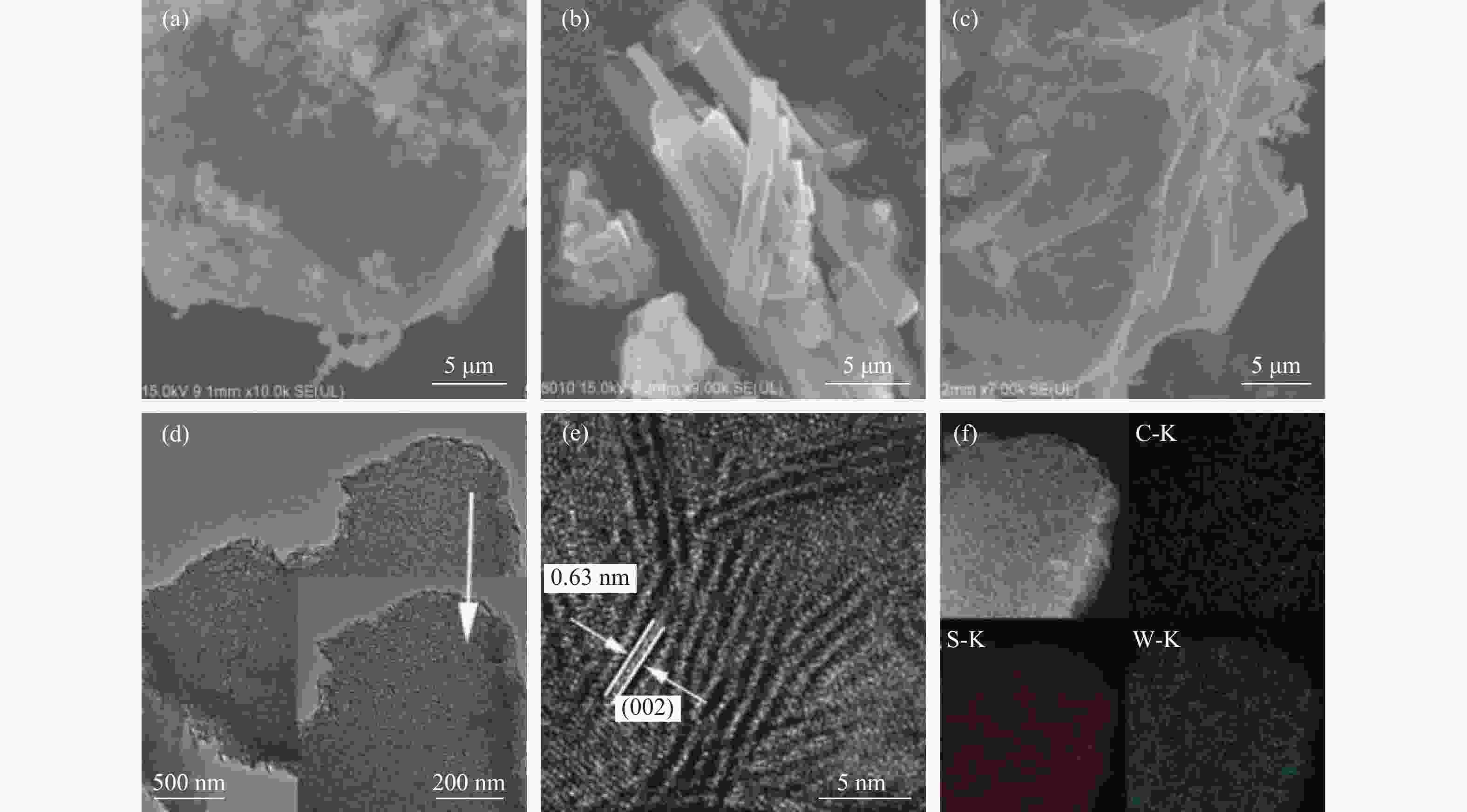
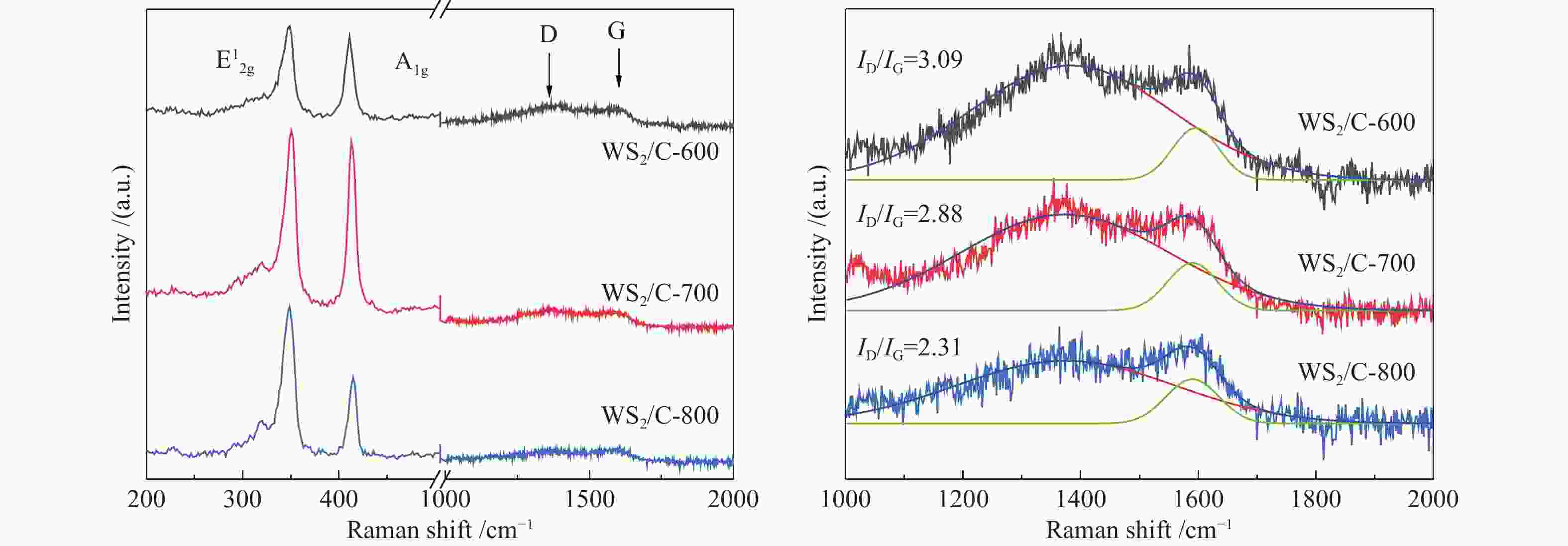
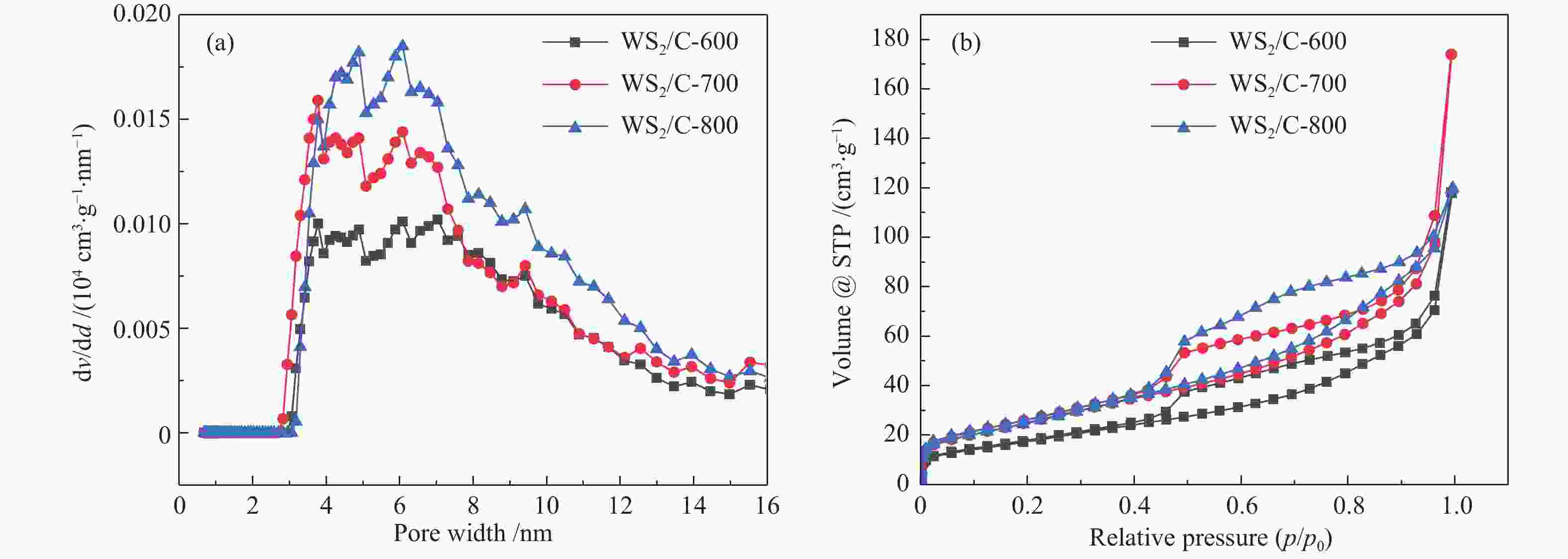
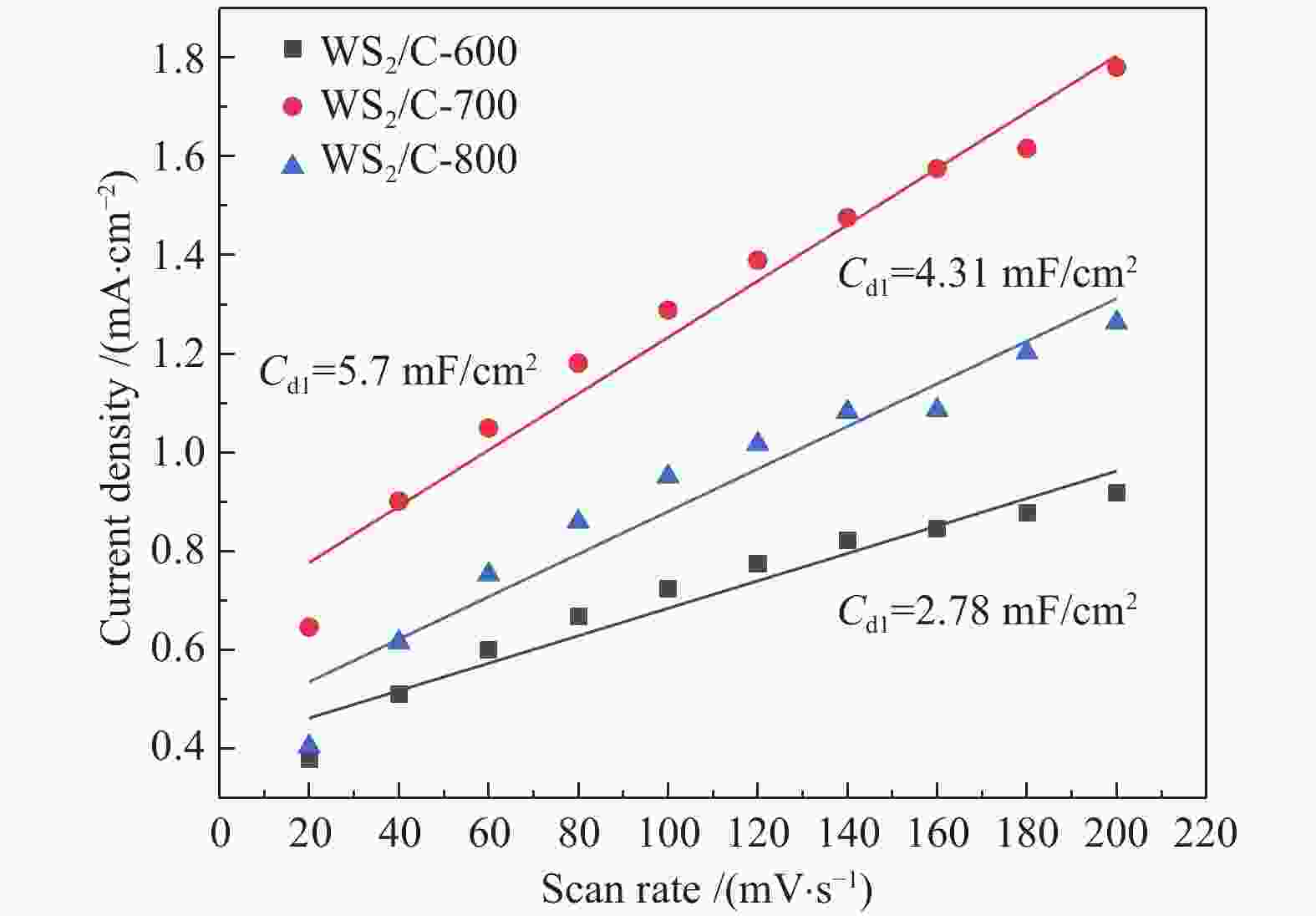
图 4 ((a)−(c)):H2WO4材料、H2WO4/EDA和WS2/C-700复合材料的SEM照片;(d) :WS2/C-700复合材料的TEM照片;(e):WS2/C-700复合材料的高分辨率TEM照片;(f):WS2/C-700复合材料的元素EDX mapping分布
Figure 4 ((a)−(c)) SEM images of H2WO4 materials, H2WO4/EDA and WS2/C-700 composites; (d) TEM images of WS2/C-700 composites; (e) High resolution TEM images of WS2/C-700 composites; (f) EDX mapping distribution of elements of WS2/C-700 composite
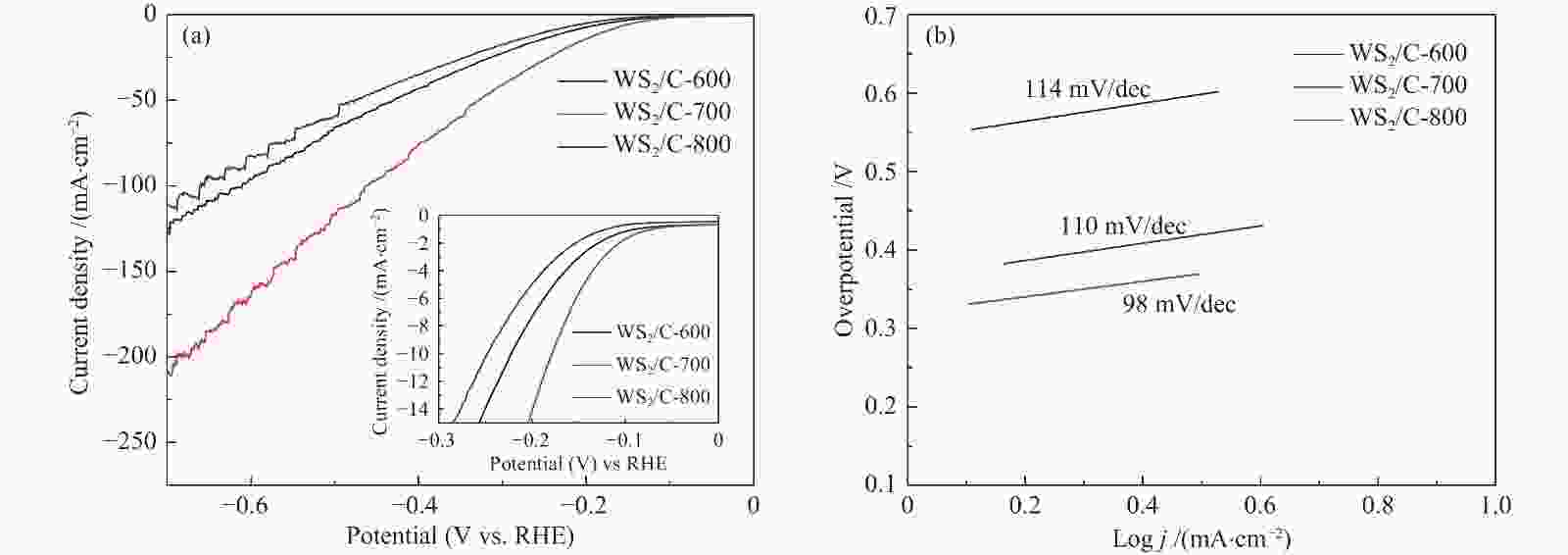
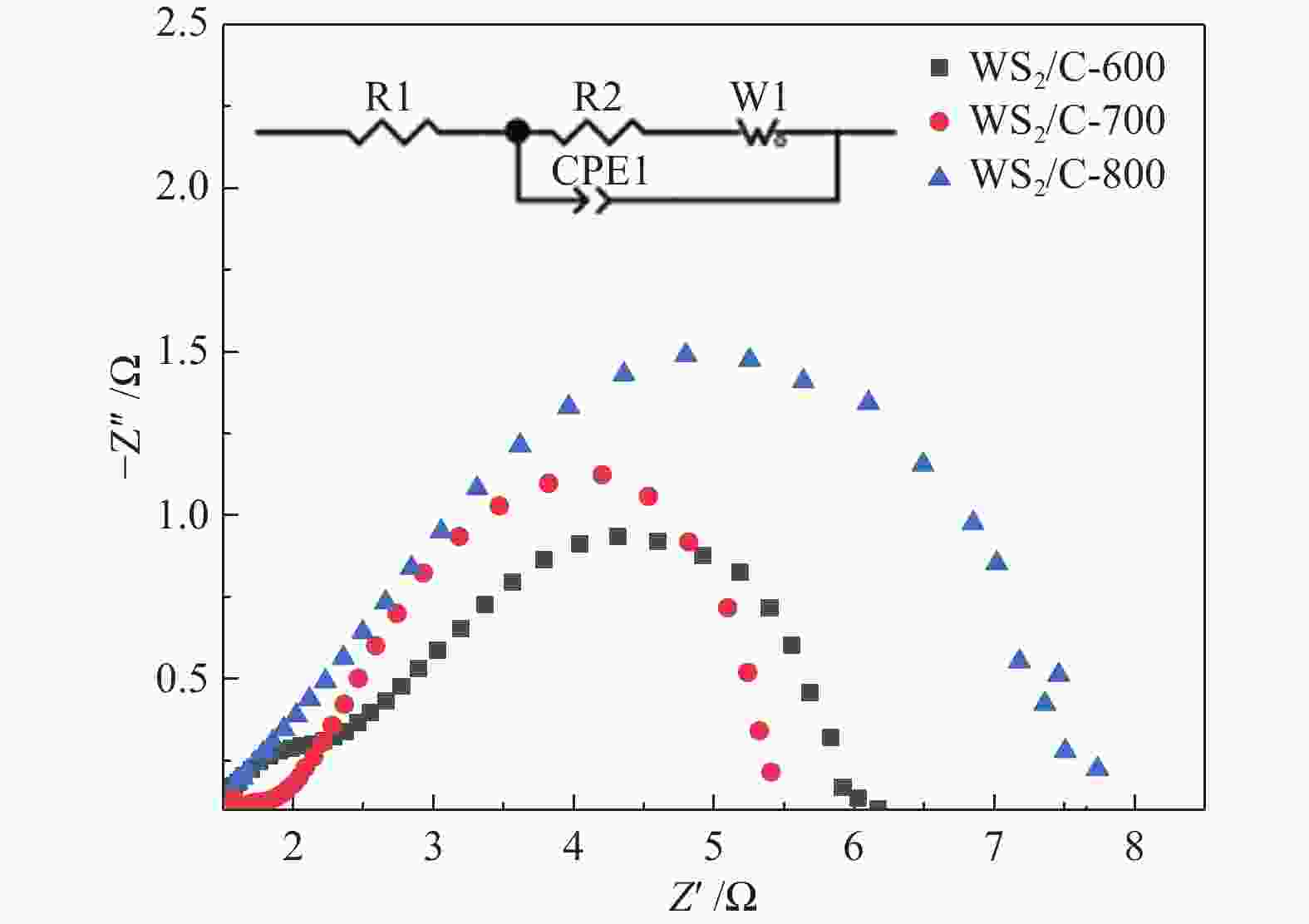
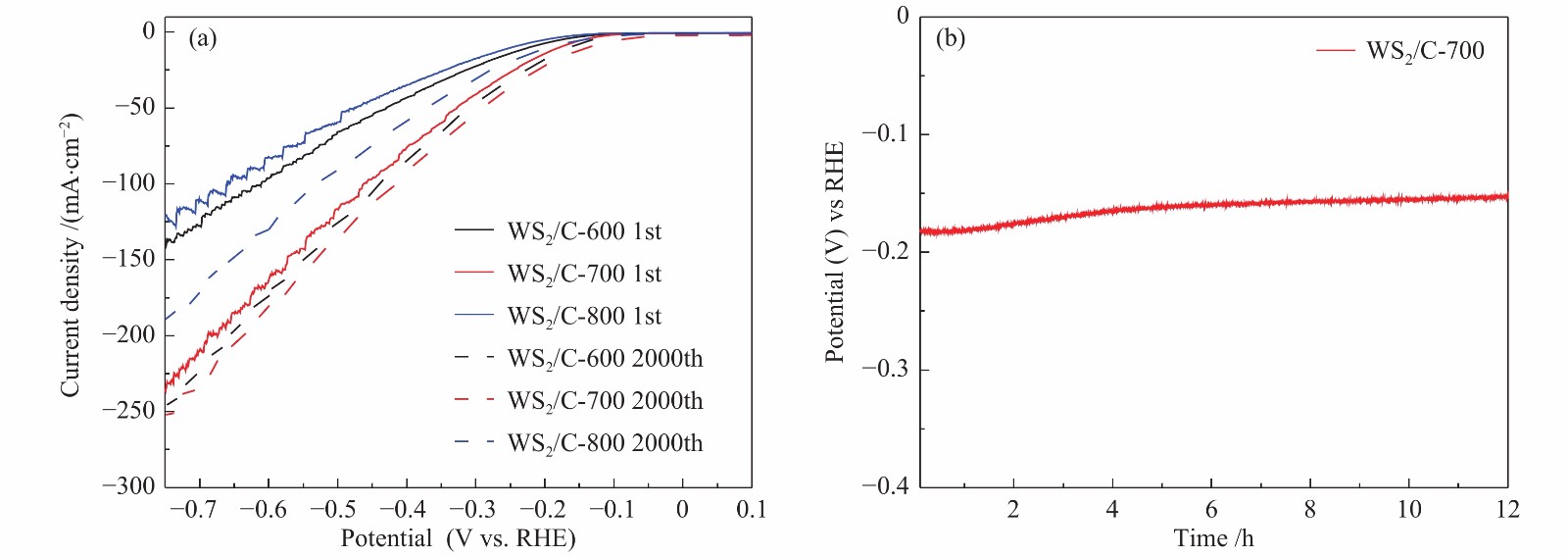
图 10 (a): WS2/C-600、WS2/C-700和WS2/C-800复合材料在2000圈循环伏安测试前后的极化曲线;(b): WS2/C-700复合材料在0.5 mol/L H2SO4电解液中的恒电流曲线
Figure 10 (a): polarization curves of WS2/C-600, WS2/C-700 and WS2/C-800 composites before and after 2000 cycles of cyclic voltammetry; (b): constant current curve of WS2/C-700 composite in 0.5 M H2SO4 electrolyte
-
[1] LING Y, YANG Z H, ZHANG Q, ZHANG Y F, CAI W W, CHENG H S. A self-template synthesis of defect-rich WS2 as a highly efficient electrocatalyst for the hydrogen evolution reaction[J]. Chem Commun,2018,54(21):2631−2634. doi: 10.1039/C7CC08962G [2] REDDY K G, DEEPAK T G, ANJUSREE G S. On global energy scenario, dye-sensitized solar cells and the promise of nanotechnology[J]. Phys Chem Chem Phys,2014,16(15):6838−6858. doi: 10.1039/c3cp55448a [3] PARK S K, LEE S W, SUNG S J, LEE S H, LEE C H, BAE K, KIM H M, HAN Y S. Effects of TiO2: MgO-mixed overlayer on the performance of dye-sensitized solar cells[J]. J Nanosci Nanotechnol,2016,16(8):8575−8579. doi: 10.1166/jnn.2016.12494 [4] ZHANG Y, XIAO J, LV Q, WANG S. Self-supported transition metal phosphide based electrodes as high-efficient water splitting cathodes[J]. Front Chem Sci Eng,2018,12(3):494−508. doi: 10.1007/s11705-018-1732-9 [5] LI Y, LUO K. Flexible cupric oxide photocathode with enhanced stability for renewable hydrogen energy production from solar water splitting[J]. RSC Adv,2019,9(15):8350−8354. doi: 10.1039/C9RA00865A [6] NOJAVAN S, ZARE K, MOHAMMADI-IVATLOO B. Application of fuel cell and electrolyzer as hydrogen energy storage system in energy management of electricity energy retailer in the presence of the renewable energy sources and plug-in electric vehicles[J]. Energy Conv Manag,2017,136(1):404−417. [7] ALAM M, KUMAR K, DUTTA V. Design and analysis of fuel cell and photovoltaic based 110 V DC microgrid using hydrogen energy storage[J]. Energy Storage,2019,1(3):e60. [8] 刘坚, 钟财富. 我国氢能发展现状与前景展望[J]. 中国能源,2019,41(2):32−36.LIU Jian, ZHONG Cai-fu. Chinese hydrogen energy development status and prospects[J]. China Energy,2019,41(2):32−36. [9] LIN Q C, LI Z S, LIN T J, LI B, LIAO X C, YU H Q, YU C L. Controlled preparation of P-doped g-C3N4 nanosheets for efficient photocatalytic hydrogen production[J]. Chin J Chem Eng,2020,28(10):2677−2688. doi: 10.1016/j.cjche.2020.06.037 [10] LI Z, MA X Z, WU L, YE H. Synergistic effect of cocatalytic NiSe2 on stable 1T-MoS2 for hydrogen evolution[J]. RSC Adv,2021,11(12):6842−6849. doi: 10.1039/D1RA00506E [11] ANANTHARAJ S, KARTHIK E, SUBRAMANIAN B, KUNDU S. Pt nanoparticles anchored molecular self-assemblies of DNA: An extremely stable and efficient HER electrocatalyst with ultra-low Pt content[J]. ACS Catal,2016,6(7):4660−4672. doi: 10.1021/acscatal.6b00965 [12] HOU D M, ZHOU W J, LIU X J, ZHOU K, XIE J, LI G Q, CHEN X W. Pt nanoparticles/MoS2 nanosheets/carbon fibers as efficient catalyst for the hydrogen evolution reaction[J]. Electrochim Acta,2015,166:26−31. doi: 10.1016/j.electacta.2015.03.067 [13] REN X P, YANG F, CHEN R, REN P Y, WANG Y H. Improvement of HER activity for MoS2: Insight into the effect and mechanism of phosphorus post-doping[J]. New J Chem,2020,44(4):1493−1499. doi: 10.1039/C9NJ05229A [14] LI Y W, YIN X L, HUANG X H, LIU X L, WU W. Efficient and scalable preparation of MoS2 nanosheet/carbon nanotube composites for hydrogen evolution reaction[J]. Int J Hydrogen Energy,2020,45(33):16489−16499. doi: 10.1016/j.ijhydene.2020.04.085 [15] VOIRY D, YAMAGUCHI H, LI J, SILVA R, ALVES D C B, FUJITA T, CHEN M, ASEFA T, SHENOY V B, EDA G, CHHOWALLA M. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution[J]. Nat Mater,2013,12(9):850−855. doi: 10.1038/nmat3700 [16] PAN Y P, ZHENG F W, WANG X X, QIN H Y, LIU E Z, SHA J W, ZHAO N Q, ZHANG P, MA L Y. Enhanced electrochemical hydrogen evolution performance of WS2 nanosheets by Te doping[J]. J Catal,2020,382:204−211. doi: 10.1016/j.jcat.2019.12.031 [17] TIAN L, QIAO H, HUANG Z Y, QI X. Li-ion intercalated exfoliated WS2 nanosheets with enhanced electrocatalytic hydrogen evolution performance[J]. Cryst Res Technol,2021,3:2000165. [18] CHENG L, HUANG W, GONG Q, LIU C, DAI H. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction[J]. Angew Chem Int Ed,2014,53(30):7860−7863. doi: 10.1002/anie.201402315 [19] YAO Y, JIN Z W, CHEN Y H, GAO Z F, YAN J Q, LIU H B, WANG J Z, LI Y L, LIU S Z. Graphdiyne-WS2 2D-Nanohybrid electrocatalysts for high-performance hydrogen evolution reaction[J]. Carbon,2018,129:228−235. [20] LONKAR S P, PILLAI V V, ALHASSAN S M. Three dimensional (3D) nanostructured assembly of MoS2-WS2/Graphene as high performance electrocatalysts[J]. Int J Hydrogen Energy,2019,45(17):361−369. [21] LI W, XIA F, QU J, LI P, CHEN D H, CHEN Z, YU Y, LU Y, CARUSO R A, SONG W G. Versatile inorganic-organic hybrid WOx-ethylenediamine nanowires: Synthesis, mechanism and application in heavy metal ion adsorption and catalysis[J]. Nano Res,2014,7(6):903−916. doi: 10.1007/s12274-014-0452-9 [22] YANG J Z, YU Z B, SUN W, LI Y, WU H D, GENG Z X, YANG Z X. Efficient electrocatalytic performance of WP nanorods propagated on WS2/C for Hydrogen evolution reduction[J]. ChemElectroChem,2020,7(14):3082−3088. doi: 10.1002/celc.202000649 [23] YUAN Z Y, JIANG Q, FENG C Q, CHEN X, GUO Z P. Synthesis and performance of tungsten disulfide/carbon (WS2/C) composite as anode material[J]. J Electron Mater,2018,47:251−260. doi: 10.1007/s11664-017-5740-1 [24] CHOI S H, KANG Y C. Sodium ion storage properties of WS2-decorated three-dimensional reduced graphene oxide microspheres[J]. Nanoscale,2015,7(9):3965−3970. doi: 10.1039/C4NR06880G [25] YU S, JUNG J W, KIM I D. Single layers of WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as anode materials for lithium-ion batteries[J]. Nanoscale,2015,7(28):11945−11950. doi: 10.1039/C5NR02425K [26] YANG Z L, Gao D Q, ZHANG J, SHI S P, XU Q, XUE D S. Realization of high Curie temperature ferromagnetism in atomically thin MoS2 and WS2 nanosheets with uniform and flower-like morphology[J]. Nanoscale,2015,7(2):650−658. doi: 10.1039/C4NR06141A [27] LIU M M, GENG A F, YAN J H. Construction of WS2 triangular nanoplates array for hydrogen evolution reaction over a wide pH range[J]. Int J Hydrog Energy,2020,45(4):2909−2916. doi: 10.1016/j.ijhydene.2019.11.053 [28] HUANG H D, ZHANG X F, ZHANG Y, HUANG B H, CAI J N, LIN S. Facile synthesis of laminated porous WS2/C composite and its electrocatalysis for oxygen reduction reaction[J]. Int J Hydrogen Energy,2018,43(17):8290−8297. [29] SAHOO M, SREENA K P, VIANYAN B P, RAMAPRABHU S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery[J]. Mater Res Bull,2015,61:383−390. doi: 10.1016/j.materresbull.2014.10.049 [30] SIRAGHI CA C, HARTSCHUH A, QIAN H, PISCANEC S, FERRARI A C. Raman spectroscopy of graphene edges[J]. Nano Letters,2009,9(4):1433−1441. doi: 10.1021/nl8032697 [31] 王新红. 硫脲的热分析研究[J]. 应用化工,2008,37(6):692−693.WANG Xin-hong. Thermal analysis of thiourea[J]. Appl Chem Ind,2008,37(6):692−693. [32] QI K, YU S S, WANG Q Y, ZHANG W, FAN J C, ZHENG W T, CUI X Q. Decoration of the inert basal plane of defect-rich MoS2 with Pd atoms for achieving Pt-similar HER activity[J]. J Mater Chem A,2016,4(4):4025−4031. [33] HU T S, BIAN K, Tai G A, ZENG T, WANG X F, HUANG X H, XIONG K, ZHU K J. Oxidation-sulfidation approach for vertically growing MoS2 nanofilms catalysts on molybdenum foils as efficient HER catalysts[J]. J Phys Chem C,2016,120(50):25843−25850. -




 下载:
下载: