Study on the performance of platinum and tungsten bifunctional catalyst supported on Al2O3 in the hydrogenolysis of glycerol to 1,3-propanediol
-
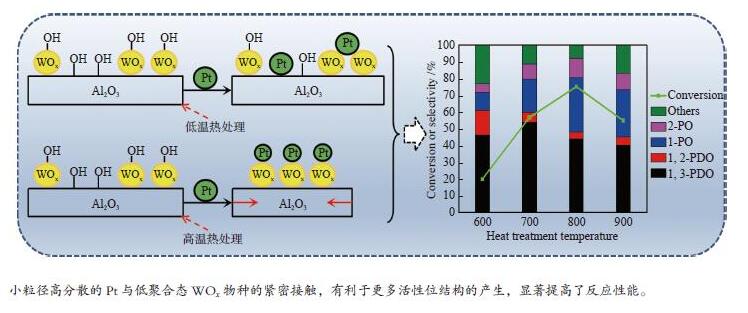
摘要: 采用水热晶化法合成了一种高比表面积且富含不饱和五配位铝位点的棒状Al2O3,并利用等体积顺序浸渍法使钨物种先以低聚合态纳米簇的形式锚定在Al2O3载体表面。通过高温热处理方式使铂物种以小粒径高分散的形式与钨物种紧密接触,极大地加强了铂和钨物种之间的相互作用程度,有利于更多活性位结构的产生,显著地提高了甘油氢解制1,3-丙二醇(1,3-PDO)的催化活性。其催化反应性能评价结果表明,在固定床反应器中,反应温度160 ℃,压力5.0 MPa,10%甘油水溶液连续进液时,Pt-WOx/Al2O3催化剂的甘油转化率为75.2%,1,3-PDO的收率达到了33.1%。Abstract: In this work, a rod-shaped Al2O3 with high specific surface area and rich in unsaturated pentahedral coordination Al3+ sites was synthesized by hydrothermal crystallization method, and the tungsten species was anchored on the surface of the Al2O3 support in the form of oligomeric nanoclusters using the incipient-wetness impregnation method. Then the platinum species were in close contact with the tungsten species in the form of small particle size and high dispersion through high temperature heat treatment. It greatly enhances the degree of interaction between platinum and tungsten species, is conducive to the generation of more active site structures, and significantly improves the catalytic activity of glycerol hydrogenolysis to 1,3-propanediol (1,3-PDO). In a fixed-bed reactor, when the reaction temperature is 160 ℃, the pressure is 5.0 MPa, and the 10% glycerol aqueous solution is continuously added, the catalytic reaction performance evaluation results show that the glycerol conversion of the Pt-WOx/Al2O3 catalyst is 75.2%, and the yield of 1,3-PDO reach 33.1%.
-
Key words:
- Al2O3 /
- glycerol hydrogenolysis /
- 1,3-propanediol /
- Pt /
- W
-
表 1 载体和催化剂的物化性质
Table 1 Physico-chemical properties of the supports and the catalysts
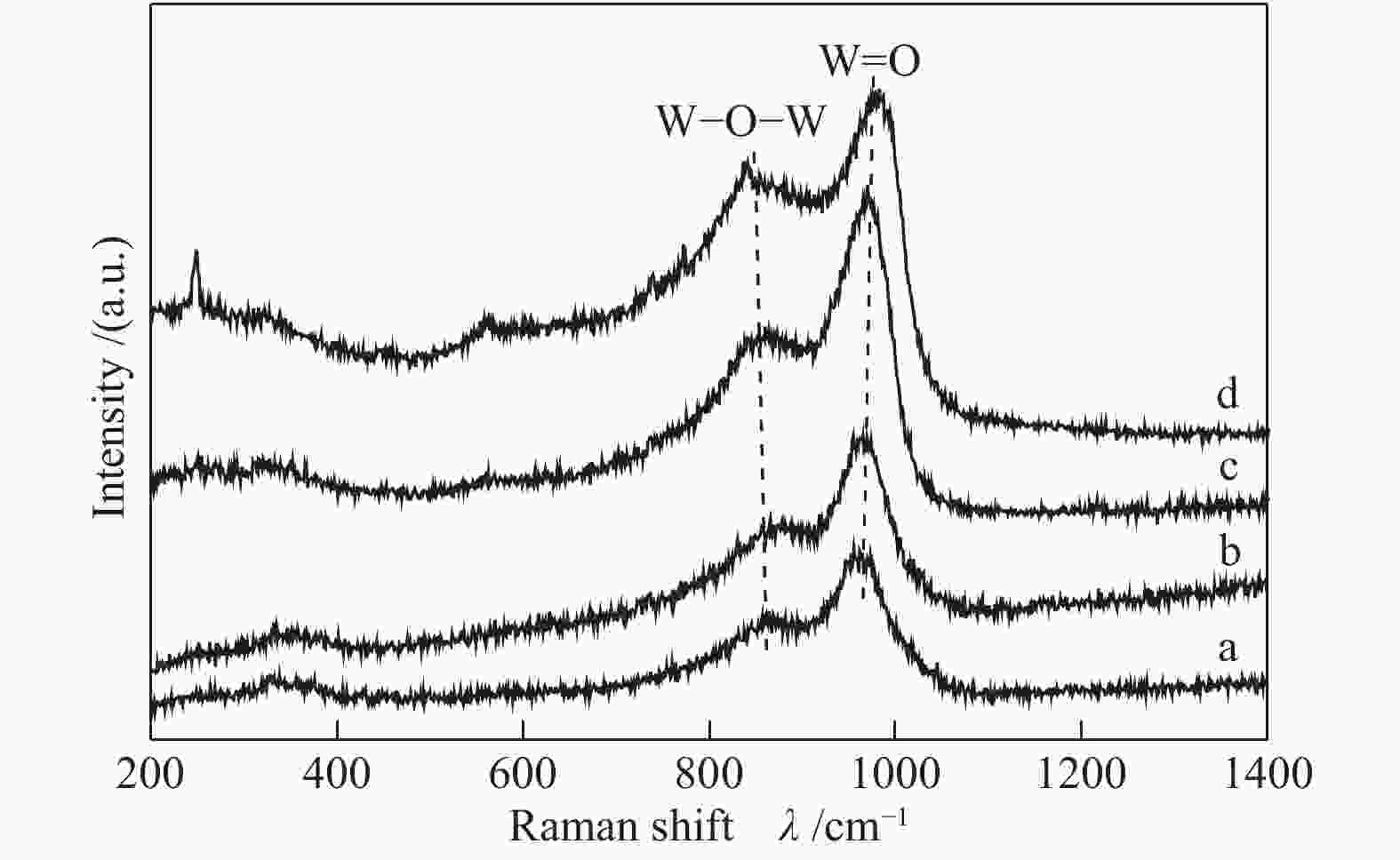
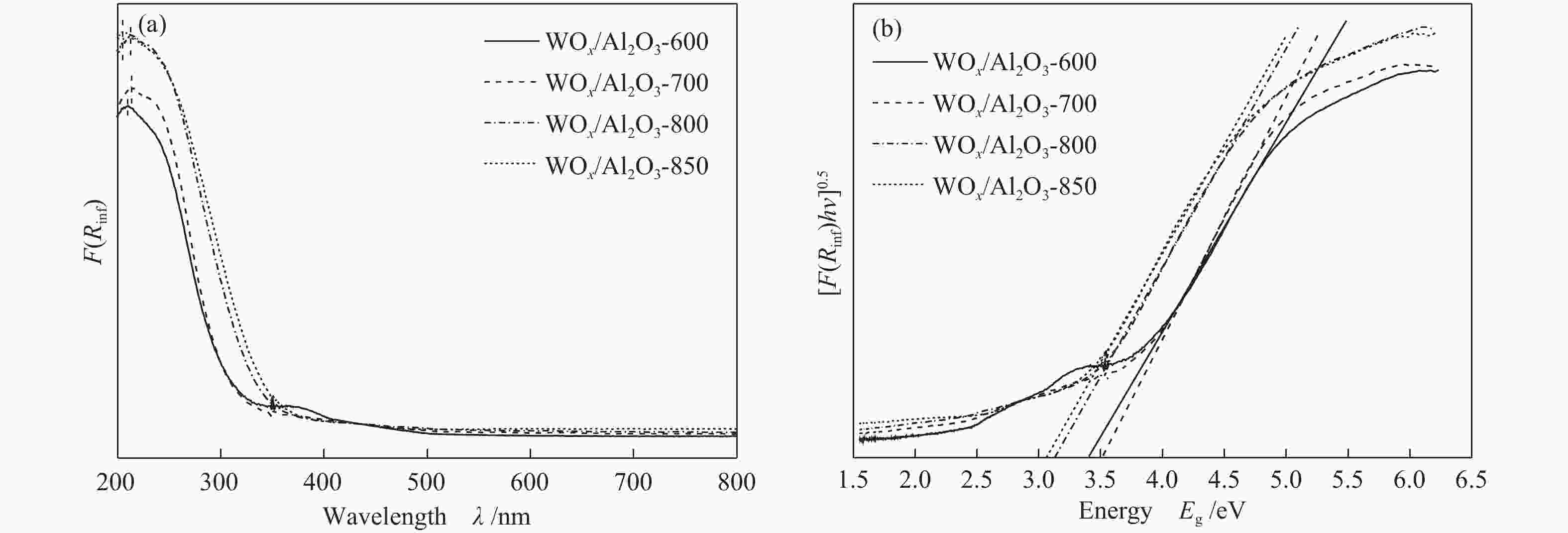
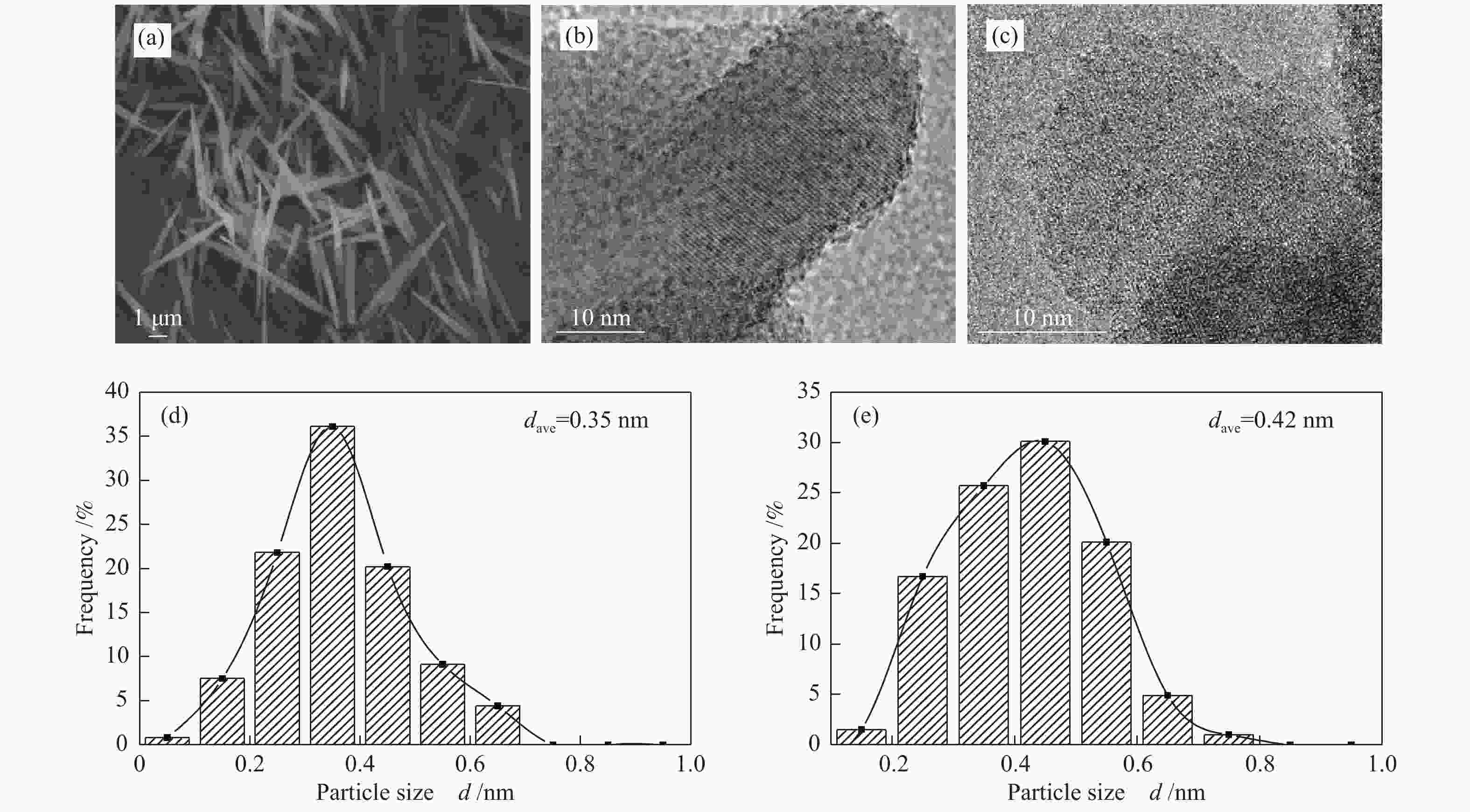
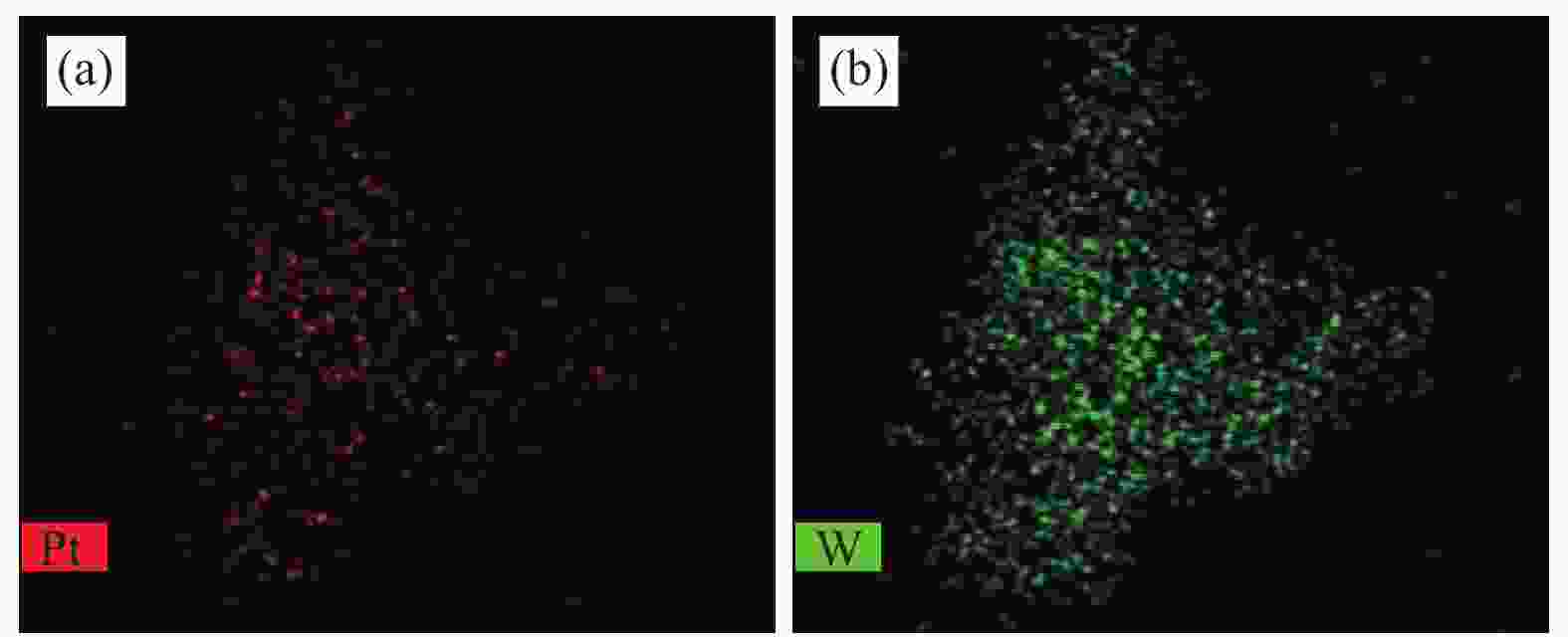
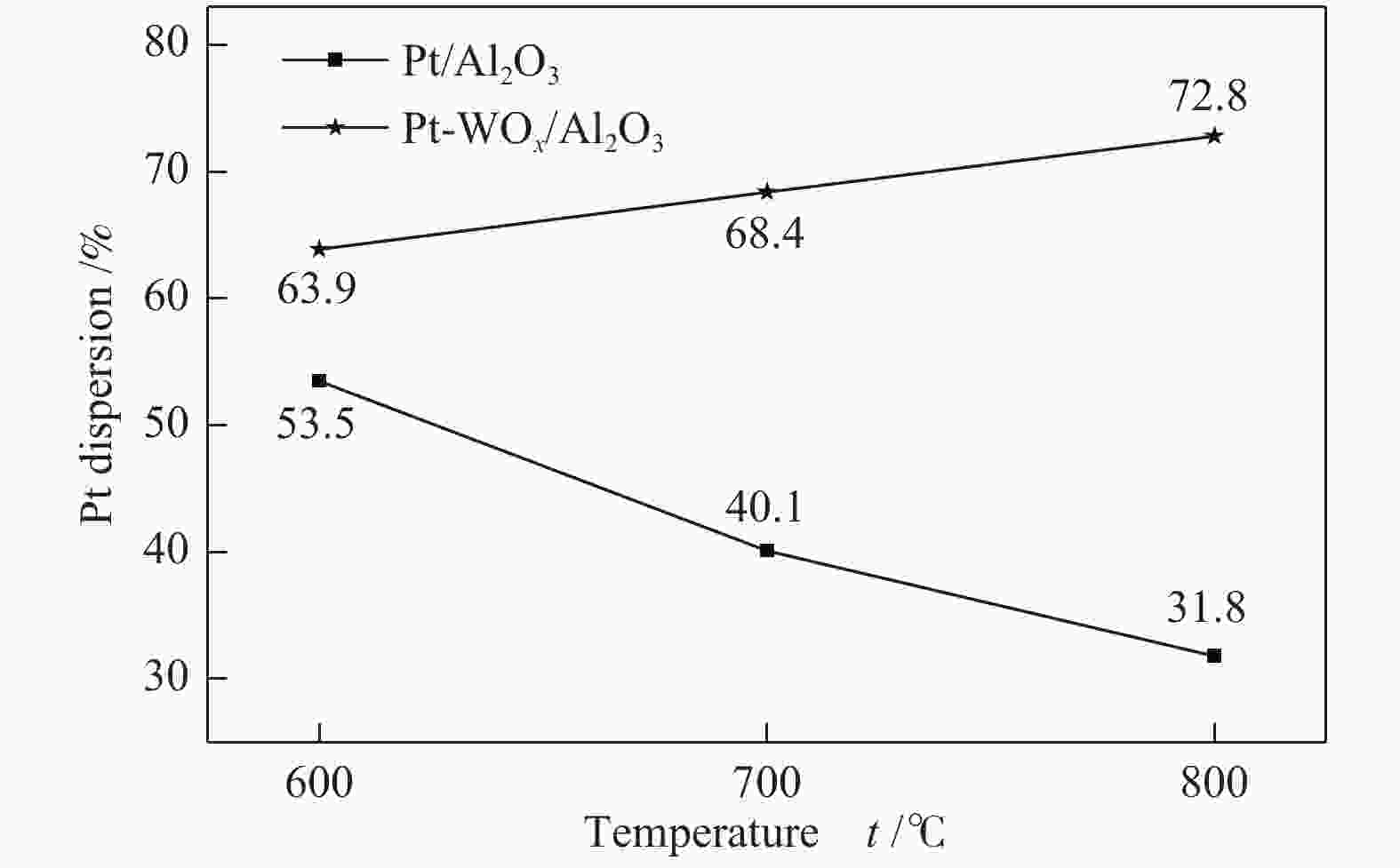
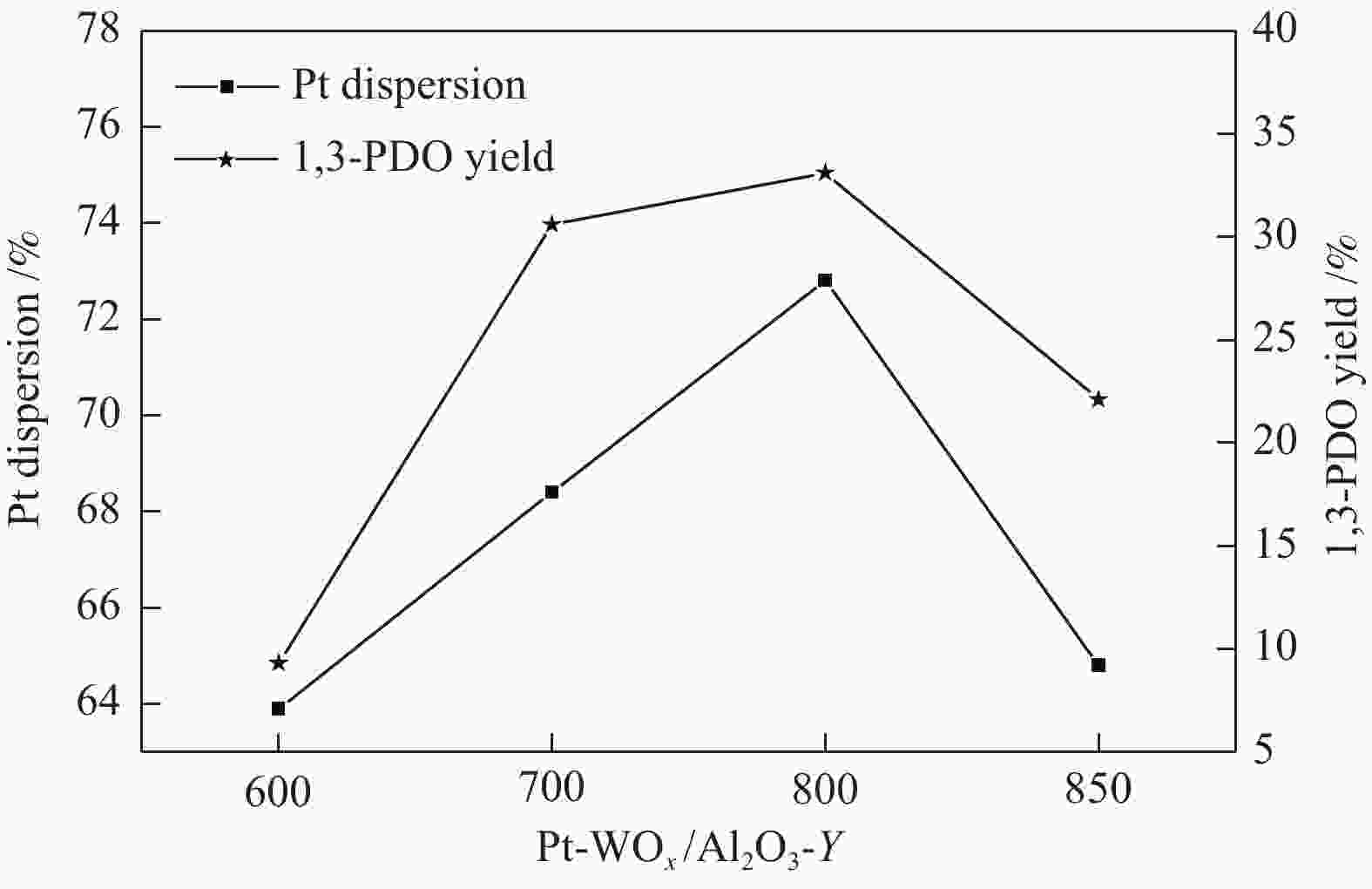
Sample ABET/(m2·g−1) vp/(cm3·g−1) dp/nm Pt contenta w/% W contentb w/% Al2O3 260 0.24 3.6 − − WOx/Al2O3-800 82 0.45 19.3 − − Pt-WOx/Al2O3-600 225 0.45 6.8 1.9 7.8 Pt-WOx/Al2O3-700 176 0.50 9.4 2.0 8.1 Pt-WOx/Al2O3-800 62 0.28 18.7 1.9 8.0 Pt-WOx/Al2O3-850 103 0.38 14.1 2.1 7.4 ABET: BET surface area; vp: BJH pore volume; dp: average pore diameter; a,b: Pt and W content is detected by ICP 表 2 不同载体和催化剂中Al3+的配位态
Table 2 Coordination states of Al3+ in different supports and catalysts
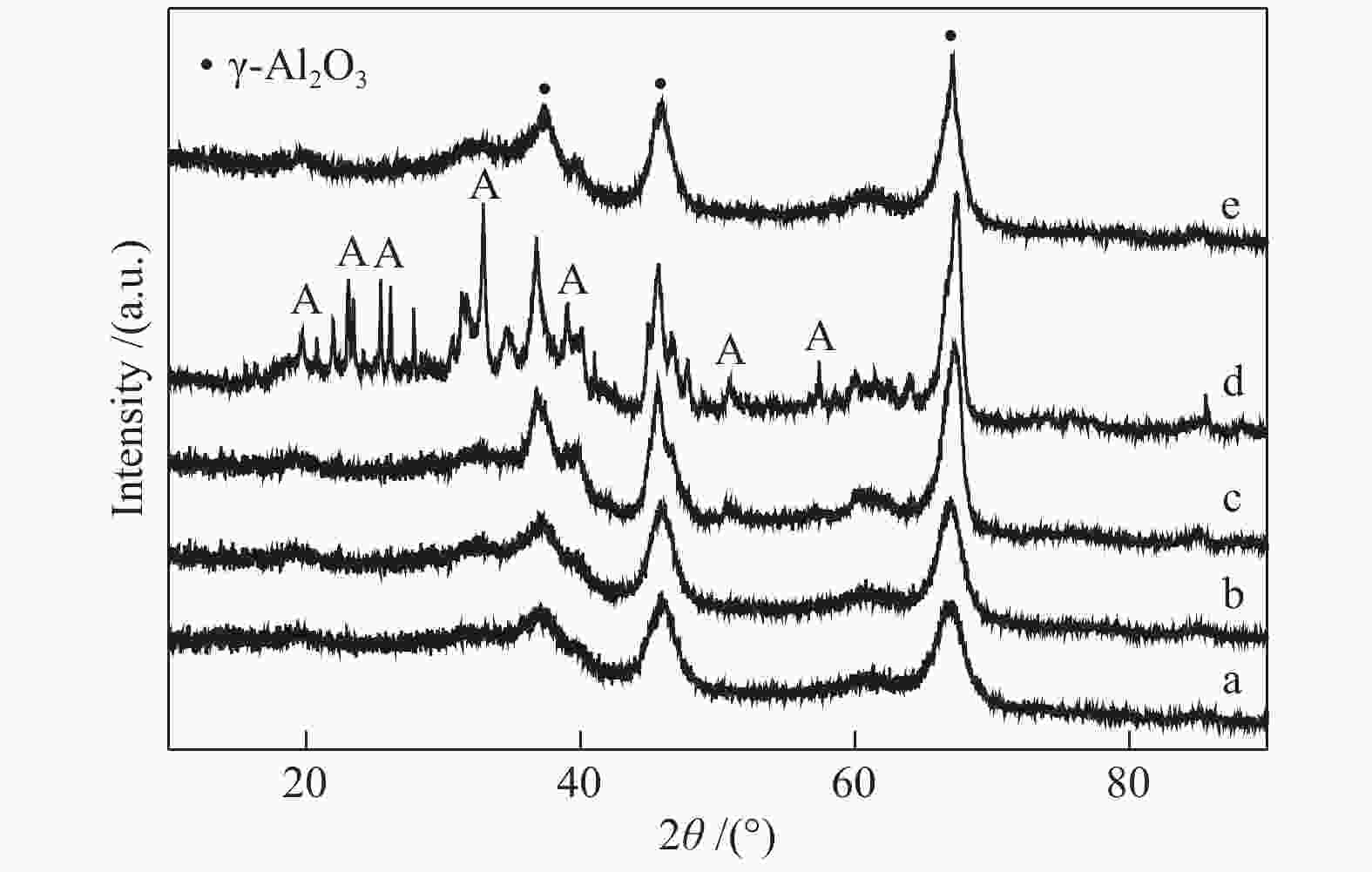
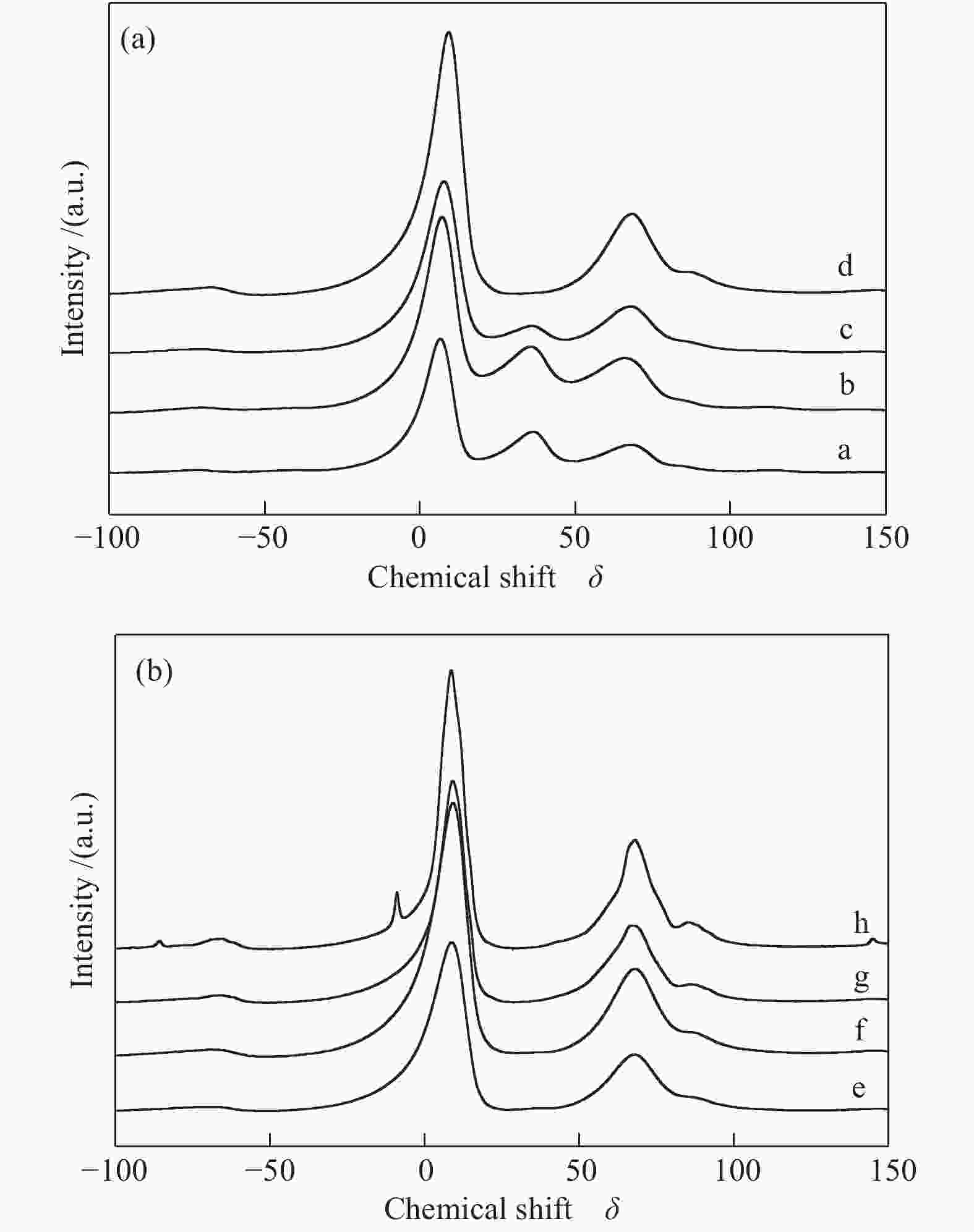
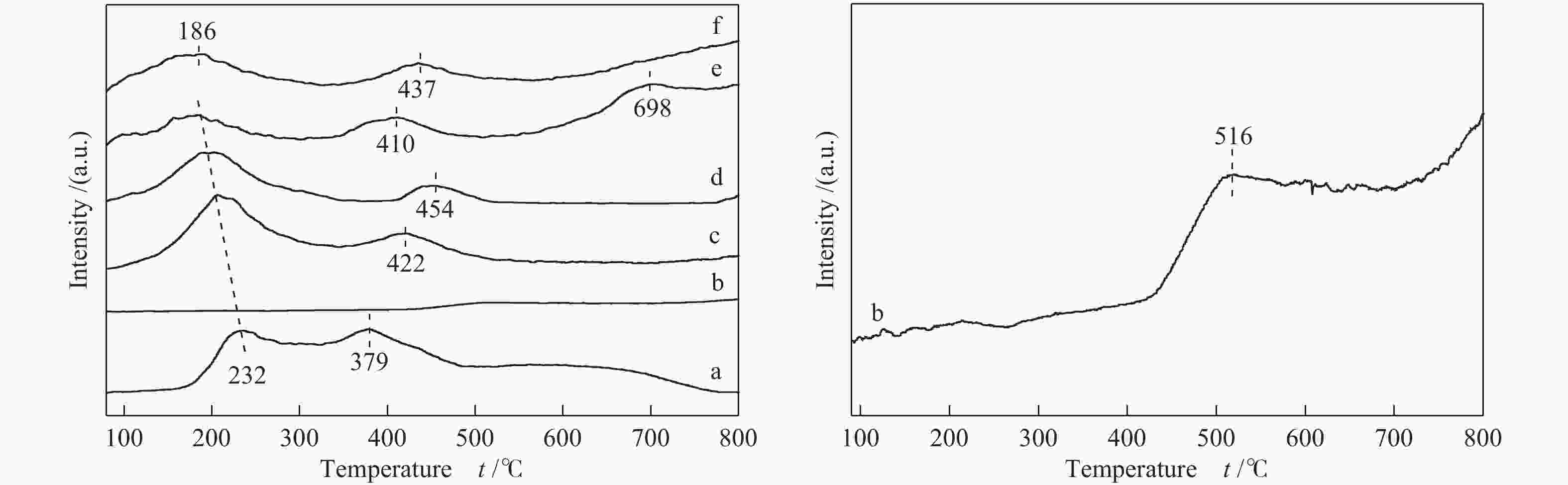
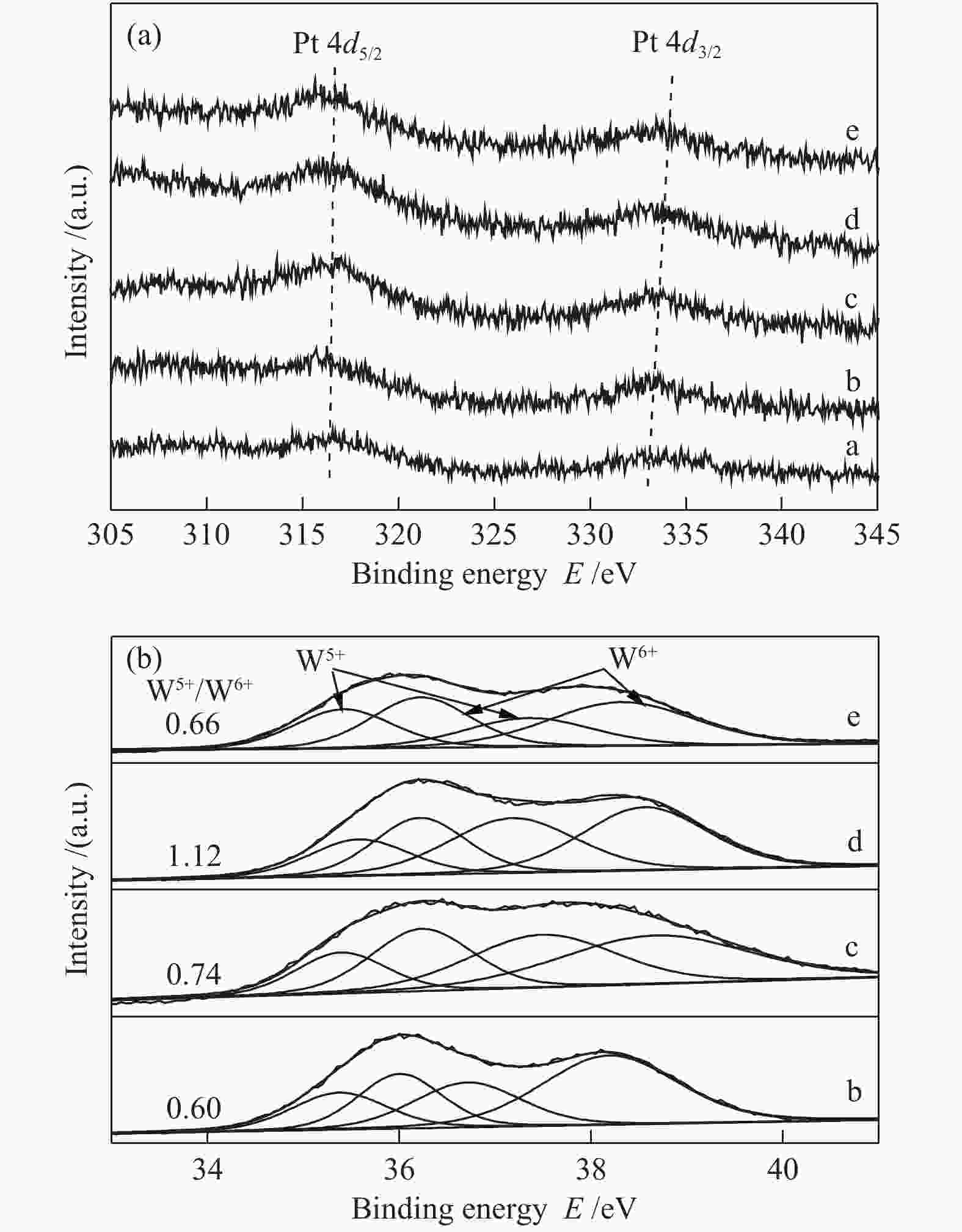
Sample Percentage of different coordinated Al3+ sites/%a ${\rm{Al}}_{{\rm{octa}}}^{3 + } $ ${\rm{Al}}_{{\rm{penta}}}^{3 + } $ ${\rm{Al}}_{{\rm{tetra}}}^{3 + } $ Al2O3-300 59 22 17 Al2O3-600 53 24 23 Al2O3-700 65 10 25 Al2O3-800 69 0 31 WOx/Al2O3-600 70 0 30 WOx/Al2O3-700 68 0 32 WOx/Al2O3-800 65 0 35 WOx/Al2O3-850 63 0 37 a: percentages of various coordination states are calculated according to the peak areas of different coordination Al3+ sites 表 3 催化剂的甘油氢解制1,3-PDO反应性能
Table 3 Catalysis performance of catalysts in hydrogenolysis of glycerol to 1,3-PDO
Catalyst Conversion/% Selectivity/% Selectivity ratio(1,3/ 1,2) 1,3-PDO 1,2-PDO 1-PO 2-PO Pt/Al2O3 5.5 3.4 50.0 2.1 0.2 0.07 Pt-WOx/Al2O3-600 18.9 49.8 15.8 11.5 5.6 3.15 Pt-WOx/Al2O3-700 56.8 53.8 6.1 19.8 9.1 8.82 Pt-WOx/Al2O3-800 75.2 44.0 3.9 32.8 11.5 11.28 Pt-WOx/Al2O3-850 54.9 40.2 4.9 28.4 9.6 8.20 reaction conditions: 160 ℃, 5.0 MPa, 10% GLY, glycerol aqueous solution (0.05 mL/min), 1,3-PDO: 1,3-propanediol, 1,2-PDO: 1,2-propanediol, 1-PO: 1-propanol, 2-PO: 2-propanol -
[1] COWIE A L, GARDNER W D. Competition for the biomass resource: Greenhouse impacts and implications for renewable energy incentive schemes[J]. Biomass Bioenergy,2007,31(9):601−607. doi: 10.1016/j.biombioe.2007.06.019 [2] BEHR A, EILTING J, IRAWADI K, LESCHINSKI J, LINDNER F. Improved utilisation of renewable resources: New important derivatives of glycerol[J]. Green Chem,2008,10(1):13−30. doi: 10.1039/B710561D [3] 成诗婕, 曾杨, 裴燕, 范康年, 乔明华, 宗保宁. 短孔道Pt/W-s-SBA-15催化剂的合成及甘油催化氢解制1, 3-丙二醇性能的研究[J]. 化学学报,2019,77(10):1054−1062. doi: 10.6023/A19060219CHENG Shi-jie, ZENG Yang, PEI Yan, FAN Kang-nian, QIAO Ming-hua, ZONG Bao-ning. Synthesis and catalysis of Pt/W-s-SBA-15 catalysts with short channel for glycerol hydrogenolysis to 1, 3-propanediol[J]. Acta Chim Sin,2019,77(10):1054−1062. doi: 10.6023/A19060219 [4] ZHOU C H, BELTRAMINI J N, FAN Y X, LU G Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals[J]. Chem Soc Rev,2008,37(3):527−549. doi: 10.1039/B707343G [5] CORMA A, IBORRA S, VELTY A. Chemical routes for the transformation of biomass into chemicals[J]. Chem Rev,2007,107(6):2411−2502. doi: 10.1021/cr050989d [6] 方伟国, 姚小兰, 杨继东, 崔芳. 生物基甘油氢解合成1,3-丙二醇催化剂的研究进展[J]. 分子催化,2018,32(6):581−593.FANG Wei-guo, YAO Xiao-lan, YANG Ji-dong, CUI Fang. Research progress of catalysts in hydrogenolysis of bioglycerol to 1,3-propanediol[J]. J Mol Catal,2018,32(6):581−593. [7] GARCÍA-FERNÁNDEZ S, GANDARIAS I, REQUIES J, SOULIMANI F, ARIAS P L, WECKHUYSEN B M. The role of tungsten oxide in the selective hydrogenolysis of glycerol to 1,3-propanediol over Pt/WOx/Al2O3[J]. Appl Catal B: Environ,2017,204:260−272. doi: 10.1016/j.apcatb.2016.11.016 [8] LEI N, ZHAO X, HOU B, YANG M, ZHOU M, LIU F, WANG A, ZHANG T. Effective hydrogenolysis of glycerol to 1,3-propanediol over metal-acid concerted Pt/WOx/Al2O3 catalysts[J]. ChemCatChem,2019,11(16):3903−3912. doi: 10.1002/cctc.201900689 [9] ZHOU W, LI Y, WANG X, YAO D, WANG Y, HUANG S, LI W, ZHAO Y, WANG S, MA X. Insight into the nature of Brönsted acidity of Pt-(WOx)n-H model catalysts in glycerol hydrogenolysis[J]. J Catal,2020,388:154−163. doi: 10.1016/j.jcat.2020.05.019 [10] MIAO G, SHI L, ZHOU Z, ZHU L, ZHANG Y, ZHAO X, LUO H, LI S, KONG L, SUN Y. Catalyst design for selective hydrodeoxygenation of glycerol to 1,3-propanediol[J]. ACS Catal,2020,10(24):15217−15226. doi: 10.1021/acscatal.0c04167 [11] FAN Y, CHENG S, WANG H, TIAN J, XIE S, PEI Y, QIAO M, ZONG B. Pt-WOx on monoclinic or tetrahedral ZrO2: Crystal phase effect of zirconia on glycerol hydrogenolysis to 1,3-propanediol[J]. Appl Catal B: Environ,2017,217:331−341. doi: 10.1016/j.apcatb.2017.06.011 [12] GARCÍA-FERNÁNDEZ S, GANDARIAS I, TEJIDO-NÚÑEZ Y, REQUIES J, ARIAS P L. Influence of the support of bimetallic platinum tungstate catalysts on 1,3-propanediol formation from glycerol[J]. ChemCatChem,2017,9(24):4508−4519. doi: 10.1002/cctc.201701067 [13] ZHU S, GAO X, ZHU Y, LI Y. Promoting effect of WOx on selective hydrogenolysis of glycerol to 1,3-propanediol over bifunctional Pt-WOx/Al2O3 catalysts[J]. J Mol Catal A: Chem,2015,398:391−398. doi: 10.1016/j.molcata.2014.12.021 [14] ZHOU W, LUO J, WANG Y, LIU J, ZHAO Y, WANG S, MA X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2[J]. Appl Catal B: Environ,2019,242:410−421. doi: 10.1016/j.apcatb.2018.10.006 [15] GARCÍA-FERNÁNDEZ S, GANDARIAS I, REQUIES J, GÜEMEZ M B, BENNICI S, AUROUX A, ARIAS P L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1,3-propanediol[J]. J Catal,2015,323:65−75. doi: 10.1016/j.jcat.2014.12.028 [16] WAN C, HU M Y, JAEGERS N R, SHI D, WANG H, GAO F, QIN Z, WANG Y, HU J Z. Investigating the surface structure of γ-Al2O3 supported WOx catalysts by high field 27Al MAS NMR and electronic structure calculations[J]. J Phys Chem C,2016,120(40):23093−23103. doi: 10.1021/acs.jpcc.6b09060 [17] SHI D, WANG H, KOVARIK L, GAO F, WAN C, HU J Z, WANG Y. WOx supported on γ-Al2O3 with different morphologies as model catalysts for alkanol dehydration[J]. J Catal,2018,363:1−8. doi: 10.1016/j.jcat.2018.04.004 [18] ZHAO B, LIANG Y, LIU L, HE Q, DONG J. Discovering positively charged Pt for enhanced hydrogenolysis of glycerol to 1,3-propanediol[J]. Green Chem,2020,22(23):8254−8259. doi: 10.1039/D0GC03364B [19] ZHU S, QIU Y, ZHU Y, HAO S, ZHENG H, LI Y. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids[J]. Catal Today,2013,212:120−126. doi: 10.1016/j.cattod.2012.09.011 [20] SALAZAR J B, FALCONE D D, PHAM H N, DATYE A K, PASSOS F B, DAVIS R J. Selective production of 1,2-propanediol by hydrogenolysis of glycerol over bimetallic Ru-Cu nanoparticles supported on TiO2[J]. Appl Catal A: Gen,2014,482:137−144. doi: 10.1016/j.apcata.2014.06.002 [21] SONG K, ZHANG H, ZHANG Y, TANG Y, TANG K. Preparation and characterization of WOx/ZrO2 nanosized catalysts with high WOx dispersion threshold and acidity[J]. J Catal,2013,299:119−128. doi: 10.1016/j.jcat.2012.11.011 [22] KITANO T, HAYASHI T, UESAKA T, SHISHIDO T, TERAMURA K, TANAKA T. Effect of high-temperature calcination on the generation of Brønsted acid sites on WO3/Al2O3[J]. ChemCatChem,2014,6(7):2011−2020. doi: 10.1002/cctc.201400053 [23] KWAK J, HU J, KIM D, SZANYI J, PEDEN C. Penta-coordinated Al3+ ions as preferential nucleation sites for BaO on γ-Al2O3: An ultra-high-magnetic field 27Al MAS NMR study[J]. J Catal,2007,251(1):189−194. doi: 10.1016/j.jcat.2007.06.029 [24] MEI J H K J H D. Coordinatively unsaturated centers as binding sites for active catalyst phases of platinum on gamma-Al2O3[J]. Science,2009,325:1670−1673. doi: 10.1126/science.1176745 [25] TAYLOR M N, ZHOU W, GARCIA T, SOLSONA B, CARLEY A F, KIELY C J, TAYLOR S H. Synergy between tungsten and palladium supported on titania for the catalytic total oxidation of propane[J]. J Catal,2012,285(1):103−114. doi: 10.1016/j.jcat.2011.09.019 [26] ROSS-MEDGAARDEN E I, WACHS I E. Structural determination of bulk and surface tungsten oxides with UV-vis diffuse reflectance spectroscopy and Raman spectroscopy[J]. J Phys Chem C,2007,111(41):15089−15099. doi: 10.1021/jp074219c [27] MARTINEZ A, PRIETO G, ARRIBAS M, CONCEPCION P, SANCHEZROYO J. Influence of the preparative route on the properties of WOx-ZrO2 catalysts: A detailed structural, spectroscopic, and catalytic study[J]. J Catal,2007,248(2):288−302. doi: 10.1016/j.jcat.2007.03.022 [28] LEI N, MIAO Z, LIU F, WANG H, PAN X, WANG A, ZHANG T. Understanding the deactivation behavior of Pt/WO3/Al2O3 catalyst in the glycerol hydrogenolysis reaction[J]. Chin J Catal,2020,41(8):1261−1267. doi: 10.1016/S1872-2067(20)63549-5 [29] PRINS R. Hydrogen spillover. Facts and fiction[J]. Chem Rev,2012,112(5):2714−2738. doi: 10.1021/cr200346z [30] ZHANG Q, QIN X X, MU D, PENG F, JI H M, SHEN Z R, HAN X P, HU W B. Isolated platinum atoms stabilized by amorphous tungstenic acid: Metal-support interaction for synergistic oxygen activation[J]. Angew Chem Int Ed Eng,2018,57(30):9351−9356. doi: 10.1002/anie.201804319 [31] AIHARA T, MIURA H, SHISHIDO T. Effect of perimeter interface length between 2D WO3 monolayer domain and γ-Al2O3 on selective hydrogenolysis of glycerol to 1,3-propanediol[J]. Catal Sci Technol,2019,9(19):5359−5367. doi: 10.1039/C9CY01385G [32] CONTRERAS J L, FUENTES G A, ZEIFERT B, SALMONES J. Stabilization of supported platinum nanoparticles on γ-alumina catalysts by addition of tungsten[J]. J Alloy Compd,2009,483(1/2):371−373. doi: 10.1016/j.jallcom.2008.08.144 [33] FAN Y, CHENG S, WANG H, YE D, XIE S, PEI Y, HU H, HUA W, LI Z H, QIAO M, ZONG B. Nanoparticulate Pt on mesoporous SBA-15 doped with extremely low amount of W as a highly selective catalyst for glycerol hydrogenolysis to 1,3-propanediol[J]. Green Chem,2017,19(9):2174−2183. doi: 10.1039/C7GC00317J [34] DENG T, LIU H. Promoting effect of SnOx on selective conversion of cellulose to polyols over bimetallic Pt-SnOx/Al2O3 catalysts[J]. Green Chem,2013,15(1):116−124. doi: 10.1039/C2GC36088H [35] CHAMINAND J, DJAKOVITCH L A, GALLEZOT P, MARION P, PINEL C, ROSIER C C. Glycerol hydrogenolysis on heterogeneous catalysts[J]. Green Chem,2004,6(8):359−361. doi: 10.1039/b407378a [36] KIM T Y, PARK D S, CHOI Y, BAEK J, PARK J R, YI J. Preparation and characterization of mesoporous Zr-WOx/SiO2 catalysts for the esterification of 1-butanol with acetic acid[J]. J Mater Chem,2012,22(19):10021−10028. doi: 10.1039/c2jm30904a -





 下载:
下载: