Interface effect of C3N4-Ti4O7-MoS2 composite toward enhanced electrocatalytic hydrogen evolution reaction
-
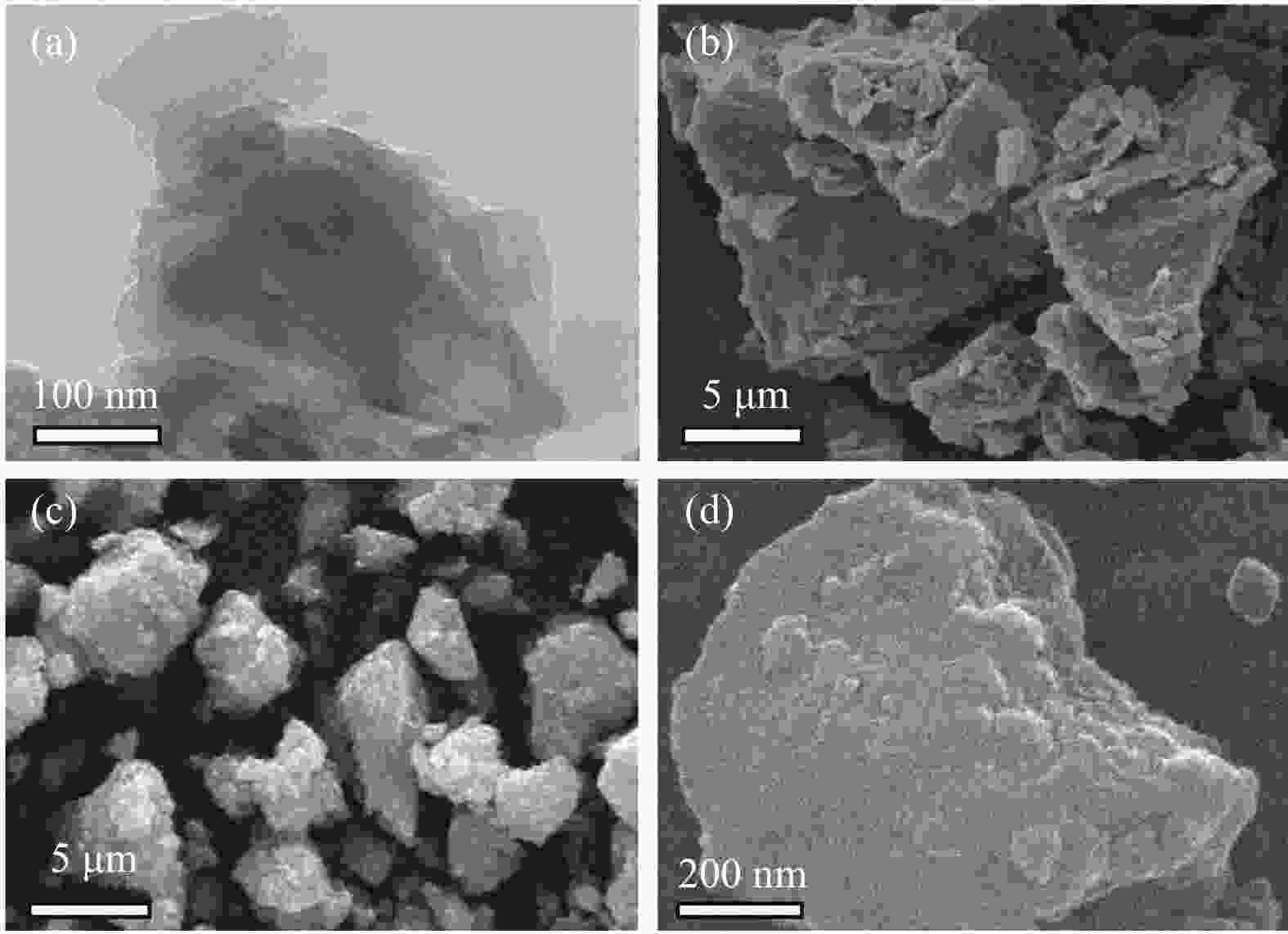
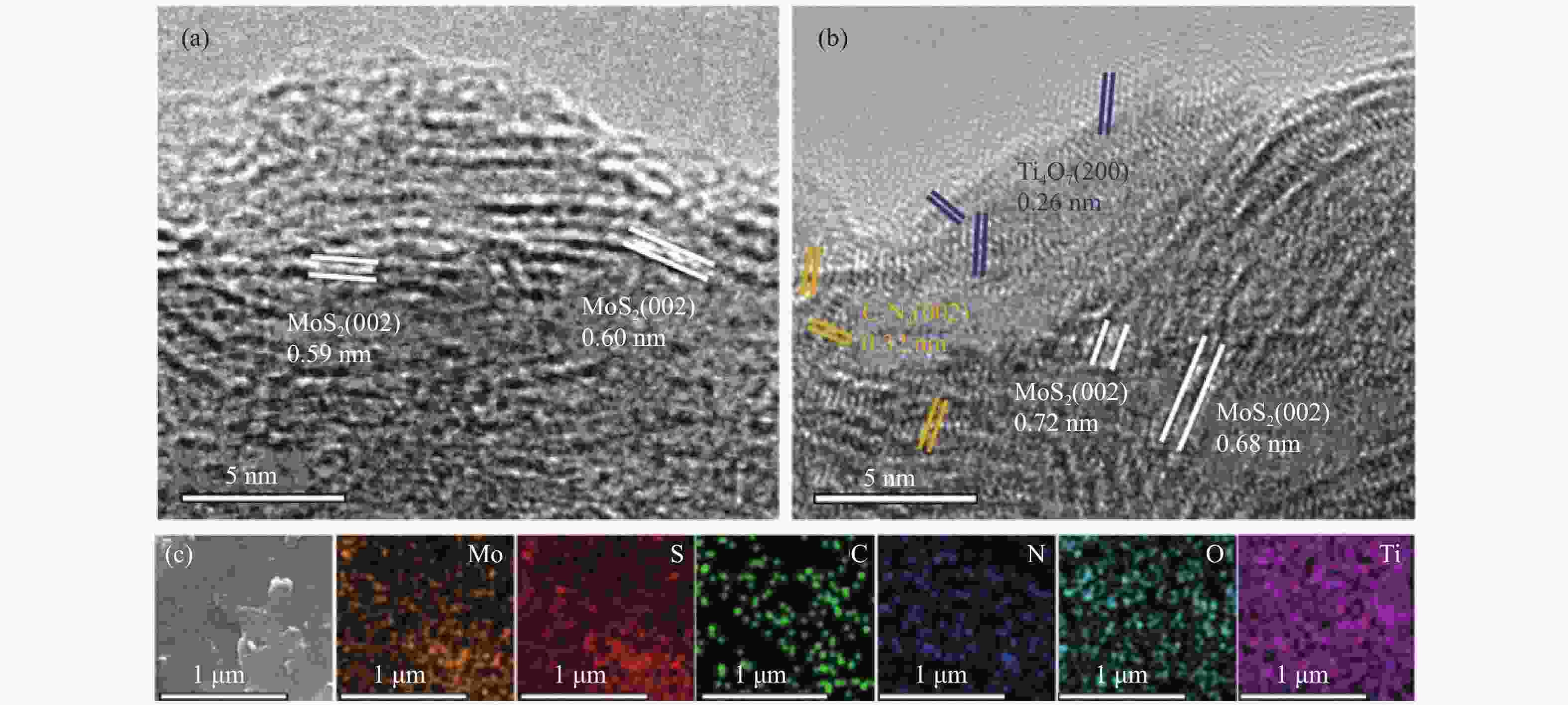
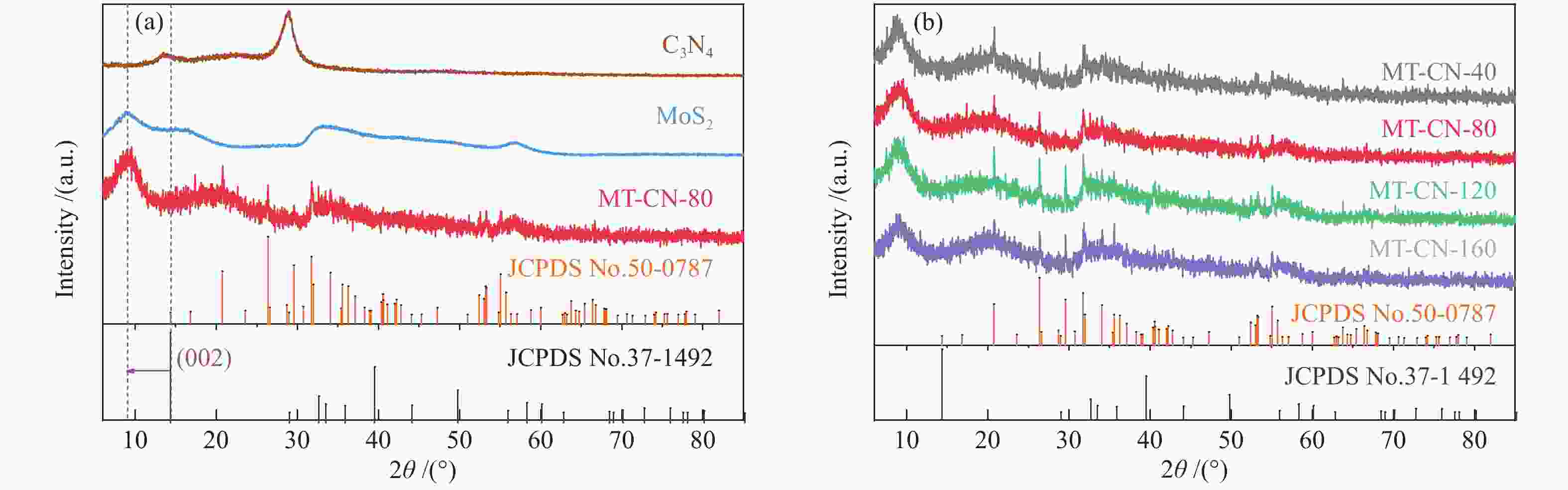
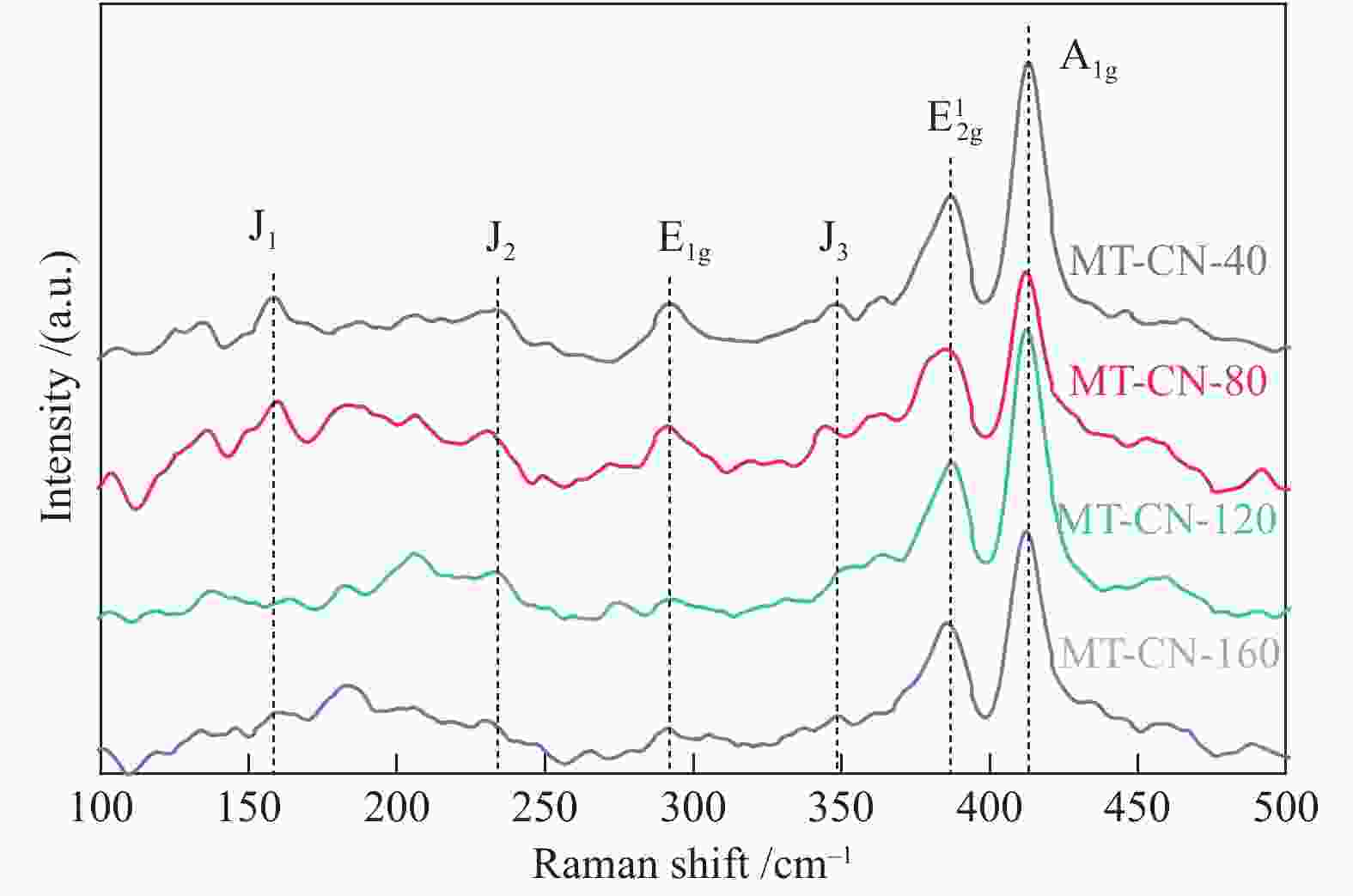
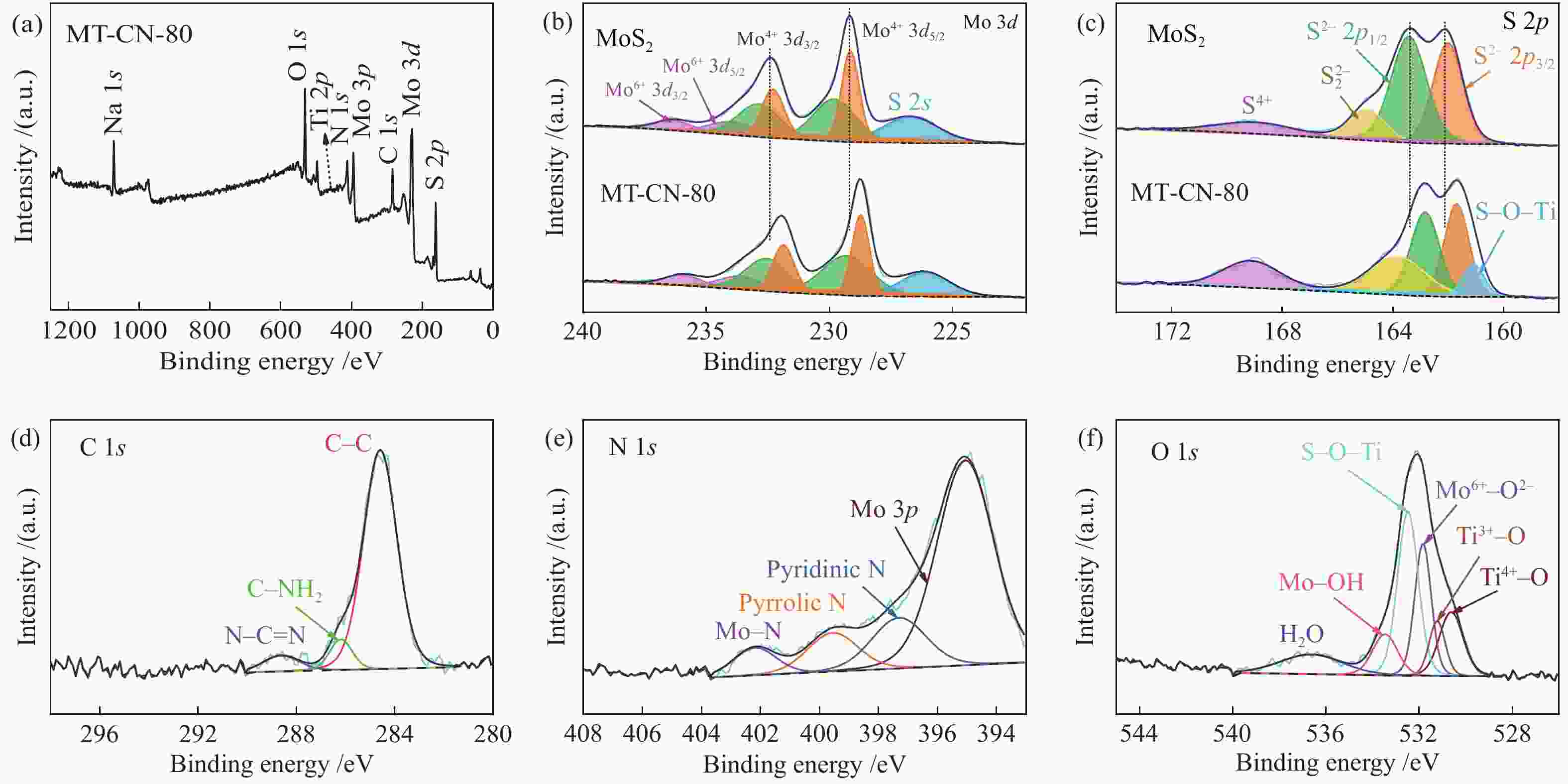
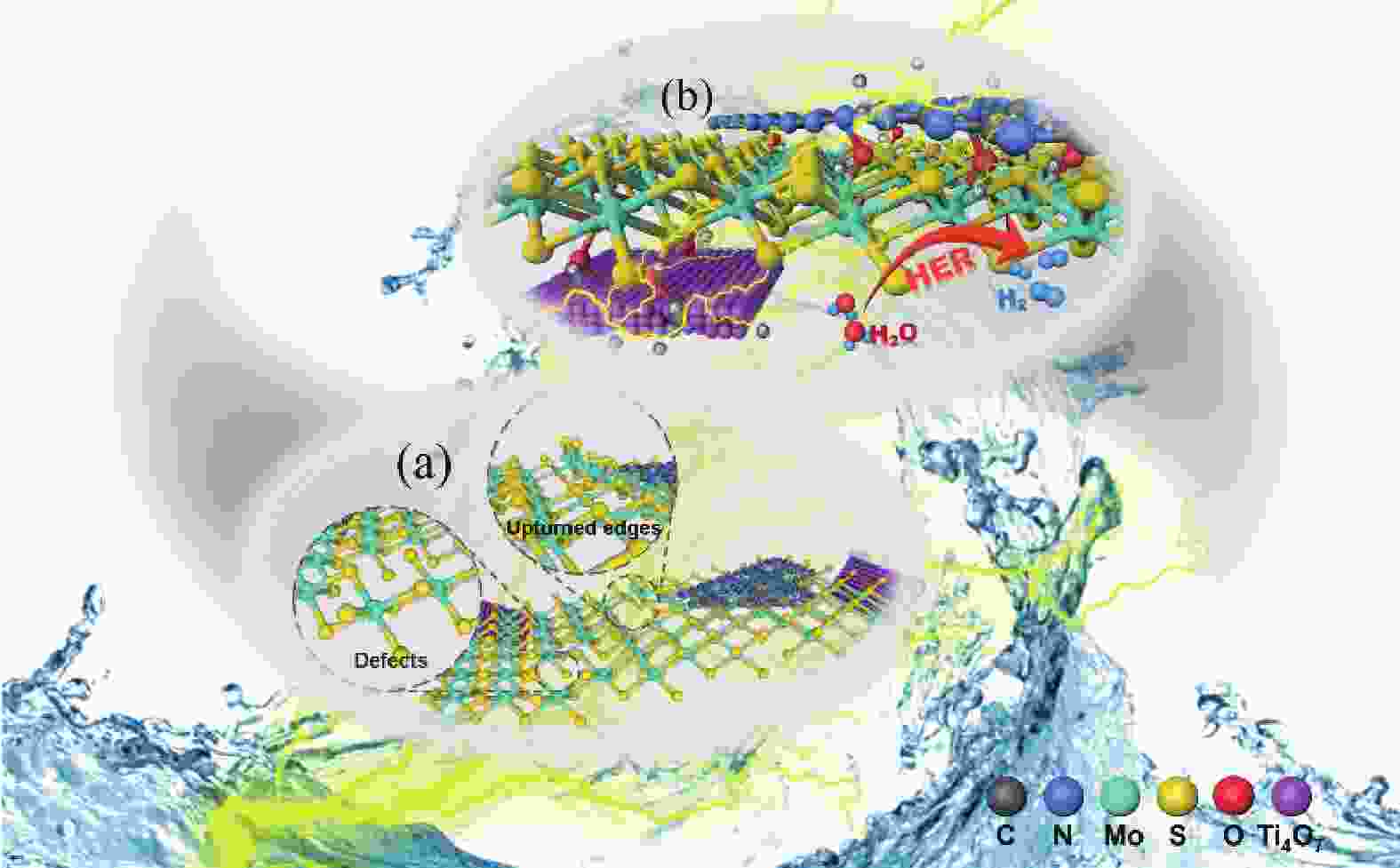
摘要: 电催化水裂解是目前最有前景的制氢技术之一。二硫化钼(MoS2)作为最有前途的非贵金属电解水制氢催化剂之一,受有限的催化位点和弱电导率的困扰,迫切地需要被进一步优化。本文采用简单的水热方法构建了C3N4-Ti4O7-MoS2异质催化剂,利用活性组分间的界面相互作用,实现了催化剂活性位点的高度暴露、表面电荷的再分布、氢吸附动力学和稳定性的优化,改进了MoS2的电催化析氢性能。结果表明,界面效应赋予C3N4-Ti4O7-MoS2催化剂优异的电催化活性,即300 mV的过电位下获得50 mA/cm2的电流密度以及较低的Tafel斜率(54 mV/dec),长达33 h的析氢反应后仍保持高的催化活性,其电催化析氢性能优于纯MoS2。结果表明,界面效应作为一种合理改进MoS2基电催化剂的策略,对开发新型高效制氢电催化剂的发展至关重要。Abstract: Electrocatalytic water splitting is one of the most prospective technology for hydrogen production. Molybdenum disulfide (MoS2), as one of the most promising non-noble metal electrocatalysts, suffers from the disadvantages of limited catalytic sites and weak conductivity which urgently needs to be further optimized. Herein, the C3N4-Ti4O7-MoS2 heterostructure is constructed through a simple hydrothermal strategy. The interfacial interaction between the active components leads to more exposed active sites, the redistribution of the surface charge, the optimization of the hydrogen adsorption kinetics and stability, which makes up the typical shortcomings of MoS2. The results indicate that the interface effect endows C3N4-Ti4O7-MoS2 catalyst with excellent electrocatalytic activity for hydrogen evolution reaction (HER). The current density of 50 mA/cm2 for HER is obtained at the overpotential of 300 mV, with the lower Tafel slope (54 mV/dec) and stable catalytic activity over 33 h, which is much better than that of the pure MoS2. This work indicates that the interface effect, as an effective strategy for rational design of MoS2-based electrocatalysts, is crucial to the future development of catalytic hydrogen production.
-
Table 1 Comparison of the double-layer capacitance (Cdl), electrochemical surface areas (ECSA) and roughness (RF) of the as-synthesized catalysts
Catalyst Cdl/(mF·cm−2) ECSA/cm2 RF MoS2 2.24 14.65 74.65 MT-CN-0 5.65 36.96 188.33 M-CN-80 5.94 38.86 198.00 MT-CN-80 16.24 106.24 541.33 -
[1] GREELEY J, JARAMILLO T F, BONDE J, CHORKENDORFF I B, NORSKOV J K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution[J]. Nat Mater,2006,5(11):909−913. doi: 10.1038/nmat1752 [2] HINNEMANN B, MOSES P G, BONDE J, JOERGENSEN K P, NIELSEN J H, HORCH S, CHORKENDORFF I, NOERSKOV J K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution[J]. J Am Chem Soc,2005,127(15):5308−5309. doi: 10.1021/ja0504690 [3] WYPYCH F, WEBER T, PRINS R. Scanning tunneling microscopic investigation of 1T-MoS2[J]. Chem Mater,1998,10:723−727. doi: 10.1021/cm970402e [4] ZHOU S, HAN J, SUN J, SROLOVITZ D J. MoS2 edges and heterophase interfaces: Energy, structure and phase engineering[J]. 2D Materials,2017,4(2):025080. doi: 10.1088/2053-1583/aa6d22 [5] DENG S, LUO M, AI C, ZHANG Y, LIU B, HUANG L, JIANG Z, ZHANG Q, GU L, LIN S, WANG X, YU L, WEN J, WANG J, PAN G, XIA X, TU J. Synergistic doping and intercalation: Realizing deep phase modulation on MoS2 arrays for high-efficiency hydrogen evolution reaction[J]. Angew Chem Int Ed,2019,58(45):16289−16296. doi: 10.1002/anie.201909698 [6] KIBSGAARD J, CHEN Z, REINECKE B N, JARAMILLO T F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis[J]. Nat Mater,2012,11(11):963−969. doi: 10.1038/nmat3439 [7] REN X, PANG L, ZHANG Y, REN X, FAN H, LIU S. One-step hydrothermal synthesis of monolayer MoS2 quantum dots for highly efficient electrocatalytic hydrogen evolution[J]. J Mater Chem A,2015,3(20):10693−10697. doi: 10.1039/C5TA02198G [8] KONG D, WANG H, CHA J J, PASTA M, KOSKI K J, YAO J, CUI Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers[J]. Nano Lett,2013,13(3):1341−1347. doi: 10.1021/nl400258t [9] LI Y, WANG H, XIE L, LIANG Y, HONG G, DAI H. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction[J]. J Am Chem Soc,2011,133(19):7296−7299. doi: 10.1021/ja201269b [10] CHEN L X, CHEN Z W, WANG Y, YANG C C, JIANG Q. Design of dual-modified MoS2 with nanoporous Ni and graphene as efficient catalysts for the hydrogen evolution reaction[J]. ACS Catal,2018,8(9):8107−8114. doi: 10.1021/acscatal.8b01164 [11] YU Y, NAM G-H, HE Q, WU X-J, ZHANG K, YANG Z, CHEN J, MA Q, ZHAO M, LIU Z, RAN F-R, WANG X, LI H, HUANG X, LI B, XIONG Q, ZHANG Q, LIU Z, GU L, DU Y, HUANG W, ZHANG H. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals[J]. Nat Chem,2018,10(6):638−643. doi: 10.1038/s41557-018-0035-6 [12] WANG D, ZHANG X, BAO S, ZHANG Z, FEI H, WU Z. Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution[J]. J Mater Chem A,2017,5(6):2681−2688. doi: 10.1039/C6TA09409K [13] LUKOWSKI M A, DANIEL A S, MENG F, FORTICAUX A, LI L, JIN S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets[J]. J Am Chem Soc,2013,135(28):10274−10277. doi: 10.1021/ja404523s [14] LI H, TSAI C, KOH A L, CAI L, CONTRYMAN A W, FRAGAPANE A H, ZHAO J, HAN H S, MANOHARAN H C, ABILD-PEDERSEN F, NøRSKOV J K, ZHENG X. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies[J]. Nat Mater,2016,15(3):364−364. [15] YIN Y, HAN J, ZHANG Y, ZHANG X, XU P, YUAN Q, SAMAD L, WANG X, WANG Y, ZHANG Z, ZHANG P, CAO X, SONG B, JIN S. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets[J]. J Am Chem Soc,2016,138(25):7965−7972. doi: 10.1021/jacs.6b03714 [16] JIAO S, YAO Z, XUE F, LU Y, LIU M, DENG H, MA X, LIU Z, MA C, HUANG H, RUAN S, ZENG Y-J. Defect-rich one-dimensional MoS2 hierarchical architecture for efficient hydrogen evolution: Coupling of multiple advantages into one catalyst[J]. Appl Catal B: Environ,2019,258:117964. doi: 10.1016/j.apcatb.2019.117964 [17] ZHANG H, YU L, CHEN T, ZHOU W, LOU X W D. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution[J]. Adv Funct Mater,2018,28(51):1807086. doi: 10.1002/adfm.201807086 [18] LIU P, ZHU J, ZHANG J, XI P, TAO K, GAO D, XUE D. P dopants triggered new basal plane active sites and enlarged interlayer spacing in MoS2 nanosheets toward electrocatalytic hydrogen evolution[J]. ACS Energy Lett,2017,2(4):745−752. doi: 10.1021/acsenergylett.7b00111 [19] ZANG Y, NIU S, WU Y, ZHENG X, CAI J, YE J, XIE Y, LIU Y, ZHOU J, ZHU J, LIU X, WANG G, QIAN Y. Tuning orbital orientation endows molybdenum disulfide with exceptional alkaline hydrogen evolution capability[J]. Nat Commun,2019,10(1):1217. doi: 10.1038/s41467-019-09210-0 [20] XIE J, ZHANG J, LI S, GROTE F, ZHANG X, ZHANG H, WANG R, LEI Y, PAN B, XIE Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution[J]. J Am Chem Soc,2013,135(47):17881−17888. doi: 10.1021/ja408329q [21] ZHAO Z, LUO S, MA P, LUO Y, WU W, LONG Y, MA J. In situ synthesis of MoS2 on C3N4 to form MoS2/C3N4 with interfacial Mo-N coordination for electrocatalytic reduction of N2 to NH3[J]. ACS Sustainable Chem Eng,2020,8(23):8814−8822. doi: 10.1021/acssuschemeng.0c02763 [22] LI M, ZHANG L, FAN X, WU M, DU Y, WANG M, KONG Q, ZHANG L, SHI J. Dual synergetic effects in MoS2/pyridine-modified g-C3N4 composite for highly active and stable photocatalytic hydrogen evolution under visible light[J]. Appl Catal B: Environ,2016,190:36−43. doi: 10.1016/j.apcatb.2016.02.060 [23] LIU Y, ZHANG H, KE J, ZHANG J, TIAN W, XU X, DUAN X, SUN H, O TADE M, WANG S. 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution[J]. Appl Catal B: Environ,2018,228:64−74. doi: 10.1016/j.apcatb.2018.01.067 [24] WU Q M, RUAN J M, ZHOU Z C, SANG S B. Magneli phase titanium sub-oxide conductive ceramic TinO2n-1 as support for electrocatalyst toward oxygen reduction reaction with high activity and stability[J]. J Cent South Univ,2015,22(4):1212−1219. doi: 10.1007/s11771-015-2635-2 [25] MA D, LI R, ZHENG Z, JIA Z, MENG K, WANG Y, ZHU G, ZHANG H, QI T. NiCoP/CoP nanoparticles supported on Ti4O7 as the electrocatalyst possessing an excellent catalytic performance toward the hydrogen evolution reaction[J]. ACS Sustainable Chem Eng,2018,6(11):14275−14282. doi: 10.1021/acssuschemeng.8b02935 [26] IBRAHIM K B, SU W N, TSAI M C, CHALA S A, KAHSAY A W, YEH M H, CHEN H M, DUMA A D, DAI H, HWANG B J. Robust and conductive Magnéli phase Ti4O7 decorated on 3D-nanoflower NiRu-LDH as high-performance oxygen reduction electrocatalyst[J]. Nano Energy,2018,47:309−315. doi: 10.1016/j.nanoen.2018.03.017 [27] ZHAO J, LI W, WU S, XU F, DU J, LI J, LI K, REN J, ZHAO Y. Strong interfacial interaction significantly improving hydrogen evolution reaction performances of MoS2/Ti4O7 composite catalysts[J]. Electrochim Acta,2020,337:135850. doi: 10.1016/j.electacta.2020.135850 [28] GUAN M, WANG C, LI S, DU H, YUAN Y. Understanding the enhanced electrocatalytic hydrogen evolutionvia integrating electrochemically inactive g-C3N4: the effect of interfacial engineering[J]. ACS Sustainable Chem Eng,2020,8(27):10313−10320. doi: 10.1021/acssuschemeng.0c03938 [29] TANG Y J, WANG Y, WANG X L, LI S L, HUANG W, DONG L Z, LIU C H, LI Y F, LAN Y Q. Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution[J]. Adv Energy Mater,2016,6(12):1600116. doi: 10.1002/aenm.201600116 [30] YAN J, WU H, CHEN H, PANG L, ZHANG Y, JIANG R, LI L, LIU S. One-pot hydrothermal fabrication of layered β-Ni(OH)2 /g-C3N4 nanohybrids for enhanced photocatalytic water splitting[J]. Appl Catal B: Environ,2016,194:74−83. doi: 10.1016/j.apcatb.2016.04.048 [31] LEI Z, ZHAN J, TANG L, ZHANG Y, WANG Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and storage[J]. Adv Energy Mater,2018,8(19):1703482. doi: 10.1002/aenm.201703482 [32] LIU Q, LI X, HE Q, KHALIL A, LIU D, XIANG T, WU X, SONG L. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: Applications for visible-light-driven photocatalytic hydrogen evolution[J]. Small,2015,11(41):5556−5564. doi: 10.1002/smll.201501822 [33] FAN X, XU P, ZHOU D, SUN Y, LI Y C, NGUYEN M A T, TERRONES M, MALLOUK T E. Fast and efficient preparation of exfoliated 2H MoS2 nanosheets by sonication-assisted lithium intercalation and infrared laser-induced 1T to 2H phase reversion[J]. Nano Lett,2015,15(9):5956−5960. doi: 10.1021/acs.nanolett.5b02091 [34] TAN S J R, SARKAR S, ZHAO X, LUO X, LUO Y Z, POH S M, ABDELWAHAB I, ZHOU W, VENKATESAN T, CHEN W, QUEK S Y, LOH K P. Temperature- and phase-dependent phonon renormalization in 1T′-MoS2[J]. ACS Nano,2018,12(5):5051−5058. doi: 10.1021/acsnano.8b02649 [35] LI M Y, SHI Y, CHENG C C, LU L S, LIN Y C, TANG H L, TSAI M L, CHU C W, WEI K H, HE J H. Epitaxial growth of a monolayer WSe2-MoS2 lateral p-n junction with an atomically sharp interface[J]. Science,2015,349(6247):524−528. doi: 10.1126/science.aab4097 [36] MOHAMED M A, M. ZAIN M F, JEFFERY MINGGU L, KASSIM M B, SAIDINA AMIN N A, W. SALLEH W N, SALEHMIN M N I, MD NASIR M F, MOHD HIR Z A. Constructing bio-templated 3D porous microtubular C-doped g-C3N4 with tunable band structure and enhanced charge carrier separation[J]. Appl Catal B: Environ,2018,236:265−279. doi: 10.1016/j.apcatb.2018.05.037 [37] MAITY N, MANDAL A, NANDI A K. High dielectric poly(vinylidene fluoride) nanocomposite films with MoS2 using polyaniline interlinker via interfacial interaction[J]. J Mater Chem C,2017,5(46):12121−12133. doi: 10.1039/C7TC03593D [38] QU D, ZHENG M, DU P, ZHOU Y, ZHANG L, LI D, TAN H, ZHAO Z, XIE Z, SUN Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts[J]. Nanoscale,2013,5(24):12272−12277. doi: 10.1039/c3nr04402e [39] SUN D, BAN R, ZHANG P-H, WU G-H, ZHANG J-R, ZHU J-J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties[J]. Carbon,2013,64:424−434. doi: 10.1016/j.carbon.2013.07.095 [40] CHANG Y-H, NIKAM R D, LIN C-T, HUANG J-K, TSENG C-C, HSU C-L, CHENG C-C, SU C-Y, LI L-J, CHUA D H C. Enhanced electrocatalytic activity of MoSx on TCNQ-treated electrode for hydrogen evolution reaction[J]. ACS Appl Mater Interfaces,2014,6(20):17679−17685. doi: 10.1021/am5039592 [41] QIN C, FAN A, ZHANG X, WANG S, YUAN X, DAI X. Interface engineering: Few-layer MoS2 coupled to a NiCo-sulfide nanosheet heterostructure as a bifunctional electrocatalyst for overall water splitting[J]. J Mater Chem A,2019,7(48):27594−27602. doi: 10.1039/C9TA10547F [42] ZHOU W, YIN Z, DU Y, HUANG X, ZENG Z, FAN Z, LIU H, WANG J, ZHANG H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities[J]. Small,2013,9(1):140−147. doi: 10.1002/smll.201201161 [43] YANG Y, WANG Y, HE H-L, YAN W, FANG L, ZHANG Y-B, QIN Y, LONG R, ZHANG X-M, FAN X. Covalently connected Nb4N5-xOx-MoS2 heterocatalysts with desired electron density to boost hydrogen evolution[J]. ACS Nano,2020,14(4):4925−4937. doi: 10.1021/acsnano.0c01072 [44] ZHAO S, LI C, WANG L, LIU N, QIAO S, LIU B, HUANG H, LIU Y, KANG Z. Carbon quantum dots modified MoS2 with visible-light-induced high hydrogen evolution catalytic ability[J]. Carbon,2016,99:599−606. doi: 10.1016/j.carbon.2015.12.088 [45] VRUBEL H, MERKI D, HU X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles[J]. Energy Environ Sci,2012,5(3):6136−6144. doi: 10.1039/c2ee02835b [46] ZHENG X, XU J, YAN K, WANG H, WANG Z, YANG S. Space-confined growth of MoS2 nanosheets within graphite: the layered hybrid of MoS2 and graphene as an active catalyst for hydrogen evolution reaction[J]. Chem Mater,2014,26(7):2344−2353. doi: 10.1021/cm500347r [47] YAN Y, GE X, LIU Z, WANG J Y, LEE J M, WANG X. Facile synthesis of low crystalline MoS2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction[J]. Nanoscale,2013,5(17):7768−7771. doi: 10.1039/c3nr02994h [48] KOROTEEV V O, BULUSHEVA L G, ASANOV I P, SHLYAKHOVA E V, OKOTRUB A V. Charge transfer in the MoS2/carbon nanotube composite[J]. J Phys Chem C,2011,115(43):21199−21204. doi: 10.1021/jp205939e [49] WU D, HU S, XUE H, HOU X, DU H, XU G, YUAN Y. Protonation and microwave-assisted heating induced excitation of lone-pair electrons in graphitic carbon nitride for increased photocatalytic hydrogen generation[J]. J Mater Chem A,2019,7(35):20223−20228. doi: 10.1039/C9TA05135J [50] DING Q, SONG B, XU P, JIN S. Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds[J]. Chem,2016,1(5):699−726. doi: 10.1016/j.chempr.2016.10.007 [51] WANG X, VASILEFF A, JIAO Y, ZHENG Y, QIAO S Z. Electronic and structural engineering of carbon‐based metal‐free electrocatalysts for water splitting[J]. Adv Mater,2018,31(13):1803625. -




 下载:
下载: