Study on regioselectivity in cobalt catalyzed hydroformylation of α-hexene
-
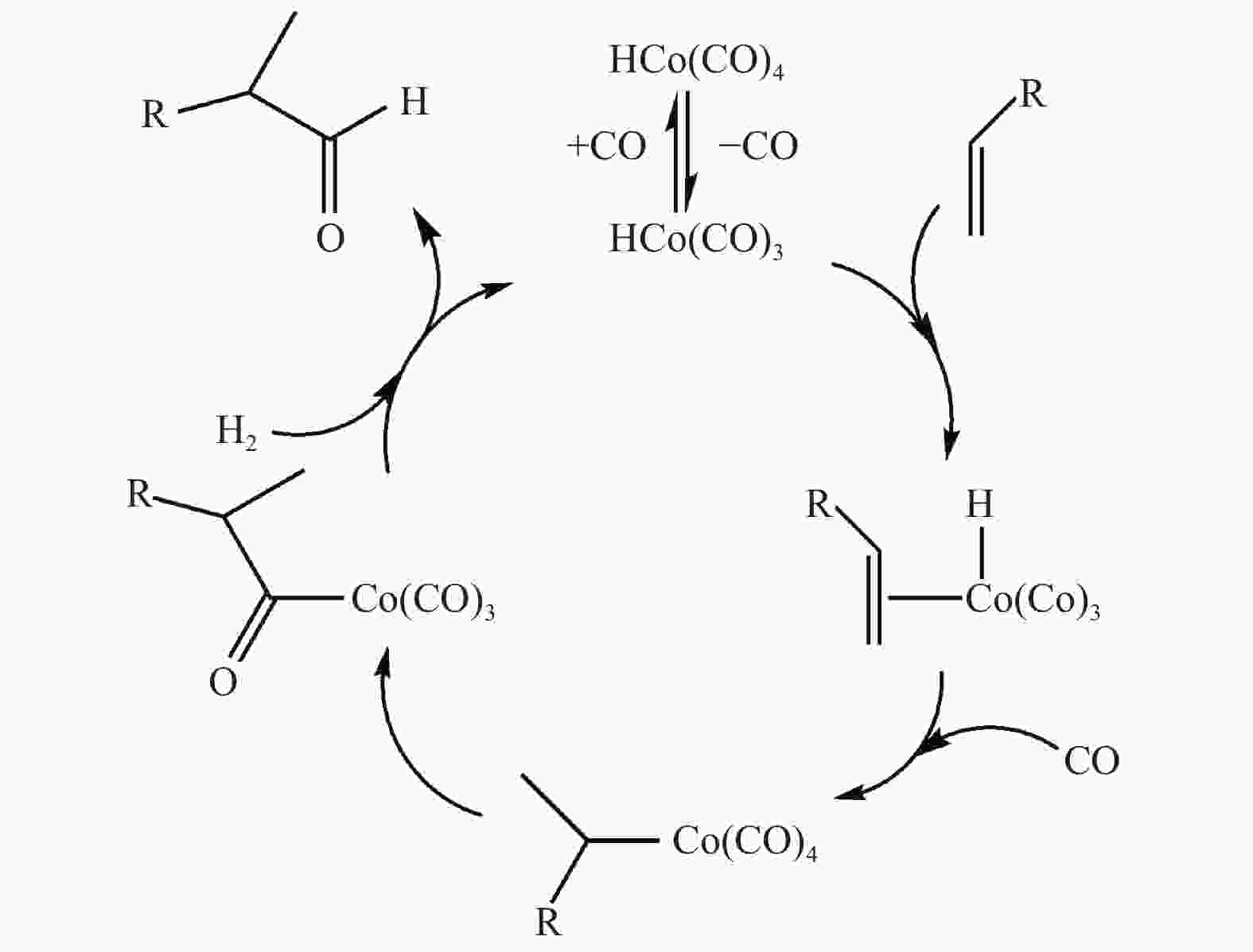
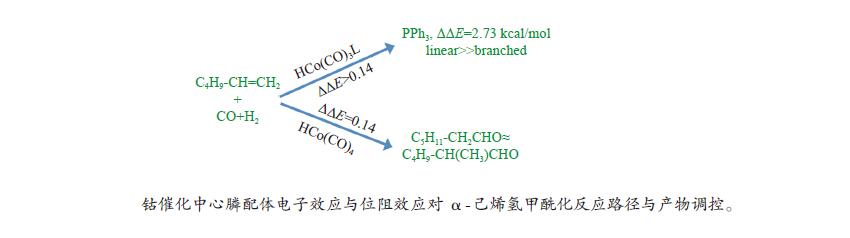
摘要: 采用密度泛函理论方法,研究了膦配体(L)配位催化活性中间体HCo(CO)2L的电子效应和位阻效应,对α-己烯氢甲酰化反应区域选择的影响。膦配体具有强吸电子能力,可提高HCo(CO)2L的稳定性;同时PPh3配体具有大的空间位阻,抑制了α-己烯吸附配位至HCo(CO)2L、以及C=C双键与Co–H键以支链反应路径加成。形成支链烷基Co中间体过渡态反应能垒(B-TS1)与形成直链烷基Co中间体过渡态(L-TS1)的反应能垒差(ΔΔE)为2.73 kcal/mol,表明前者发生相对困难,有利于按直链路经反应。膦配体的电子效应和位阻效应共同决定α-己烯C=C双键与Co–H键加成反应方式,且有利于直链反应路径加成,产物以直链醛为主。Abstract: Regioselective effects of electron and steric hindrance of catalytically active intermediate HCo(CO)2L coordinated by phosphine ligands on α-hexene hydroformylation were studied based on density functional theory. Phosphine ligands have strong electron-attracting capacity that raises the stability of HCo(CO)2L. PPh3 with large steric hindrance suppresses the coordination of α-hexene with HCo(CO)2L as well as the secondary reaction of the C=C with the Co–H via the branched chain pathway. The energy barrier for the transition state containing linear chain alkyl Co intermediate is lower about 2.73 kcal/mol than that for the transition state with branched chain alkyl Co intermediate, indicating that linear chain pathway is dominant in the addition reaction. Both the electron and steric hindrance effects of phosphine ligands determines the pathway of addition reaction between the C=C of α-hexene and the Co–H. The linear chain addition is preferable that mainly produces linear chain aldehydes.
-
Key words:
- cobalt /
- phosphine ligand /
- α-hexene /
- hydroformylation /
- regioselectivity /
- DFT
-
表 1 膦配体不同垂直配位构型及相对自由能
Table 1 Coordination configurations of phosphine ligands and free energies
Entry INT1 INT1a ΔG*/(kcal·mol−1) 1 

0.74 2 

0.87 3 

0.83 4 

0.90 5 

0.49 6 

0.50 *:ΔG = G(INT1a)−G(INT1) 表 2 α-己烯配位反应的自由能
Table 2 Free energy of coordination of α-hexene

Entry L ΔGr/(kcal·mol−1) 1 CO −3.82 2 PH3 −5.10 3 PF3 −9.90 4 PMe3 −3.30 5 PPh3 −3.26 6 PBu3 −2.77 表 3 HCo(CO)2L构象转化相对焓和自由能
Table 3 Enthalpy and free energy of conformational transformation of HCO(CO)2L
Entry Equatorial
(e)Axial
(a)ΔH*/
(kcal·mol−1)ΔG*/
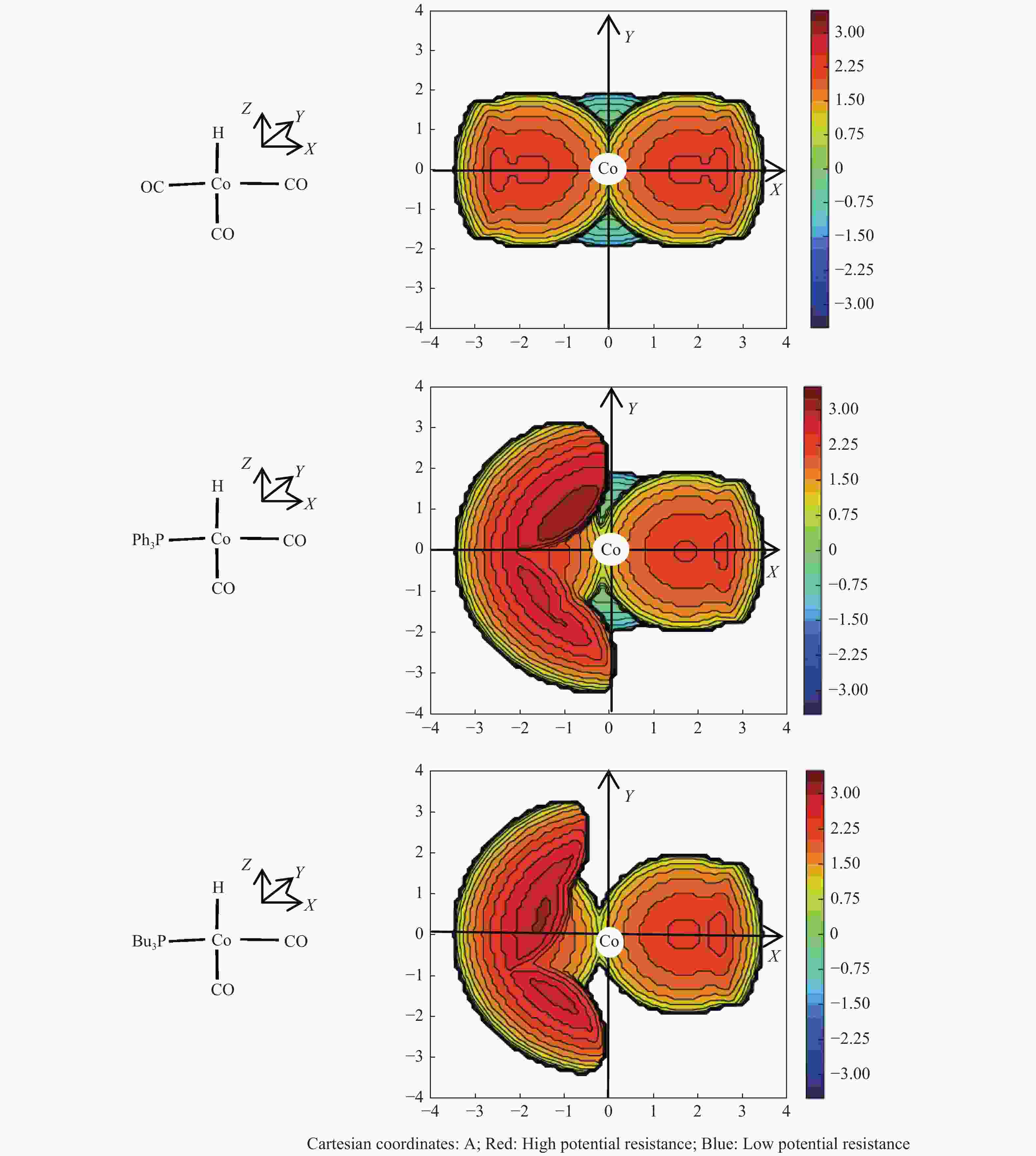
(kcal·mol−1)1 

0 0 2 

1.97 0.41 3 

1.70 1.05 4 

2.72 1.76 5 

1.46 1.14 6 

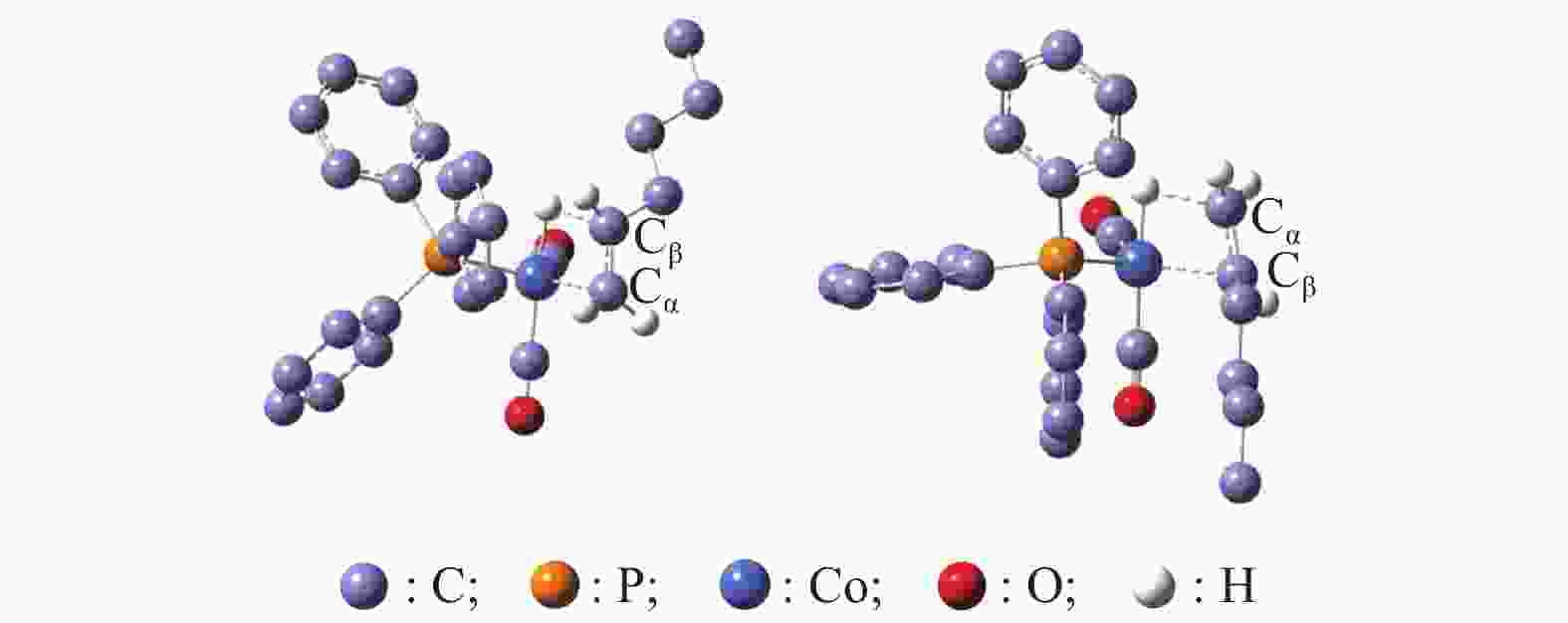
1.78 1.47 *:ΔG = G(a−G(e),ΔH = H(a)−H(e) 表 4 直链过渡态L-TS1和支链过渡态B-TS1结构参数
Table 4 Structural parameters of L-TS1 and B-TS1
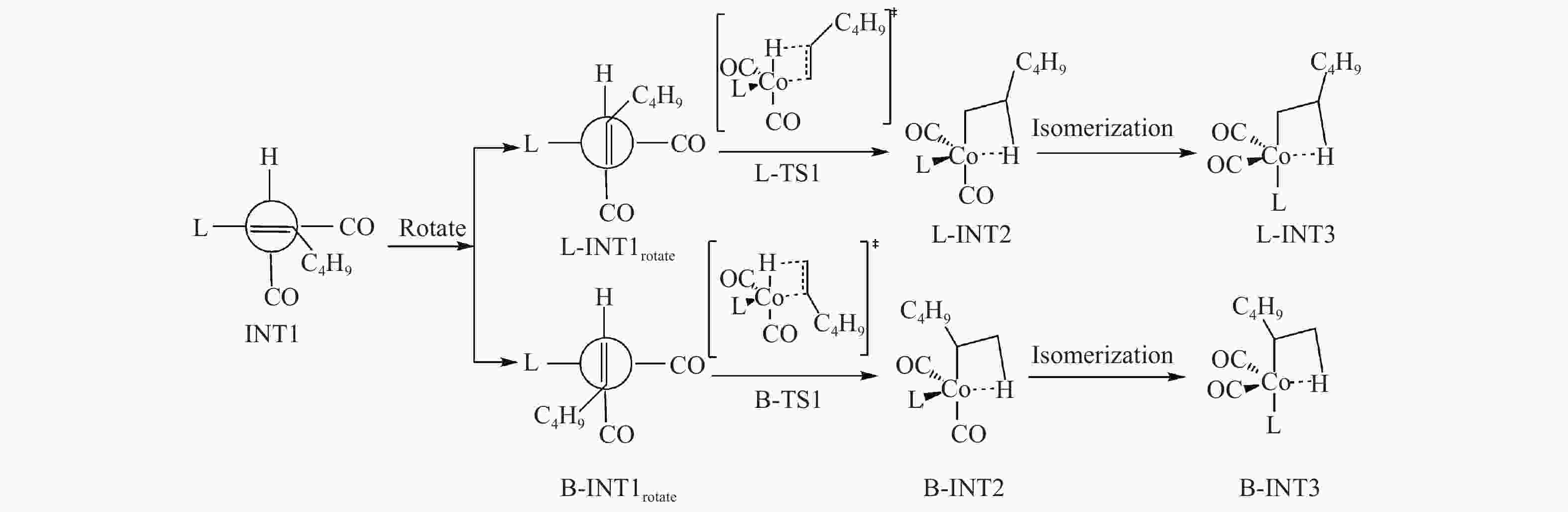
Parameter L-TS1(B-TS1) CO PH3 PF3 PMe3 PBu3 PPh3 R(Cα–Cβ) 1.405(1.400) 1.401(1.402) 1.403(1.402) 1.407(1.406) 1.402(1.406) 1.399(1.400) R(Co–Cβ) 2.291(2.162) 2.193(2.137) 2.214(2.135) 2.187(2.117) 2.199(2.219) 2.214(2.155) R(Co–Cα) 2.163(2.187) 2.106(2.154) 2.108(2.182) 2.095(2.159) 2.110(2.243) 2.117(2.164) R(Co–H) 1.544(1.516) 1.516(1.515) 1.514(1.518) 1.524(1.524) 1.512(1.555) 1.513(1.512) R(Co–P) − 2.234(2.554) 2.098(2.088) 2.220(2.222) 2.213(2.223) 2.210(2.243) R(Co–(CO)ax) 1.770(1.774) 1.765(1.765) 1.768(1.765) 1.751(1.749) 1.748(1.749) 1.754(1.754) ∠(Cα–Co–Cβ) 36.6(37.6) 38.0(38.1) 37.8(37.9) 38.3(38.4) 37.9(36.7) 37.6(37.8) ν* 662i(633i) 502i(557i) 553i(595i) 620i(657i) 545i(716i) 469i(572i) Bond Length: Å; Bond angle: ν*; Negative eigenvalue of Hessian matrix: cm−1 表 5 α-己烯嵌入Co−H键过程自由能
Table 5 Free energy of α-hexene intercalation into Co−H
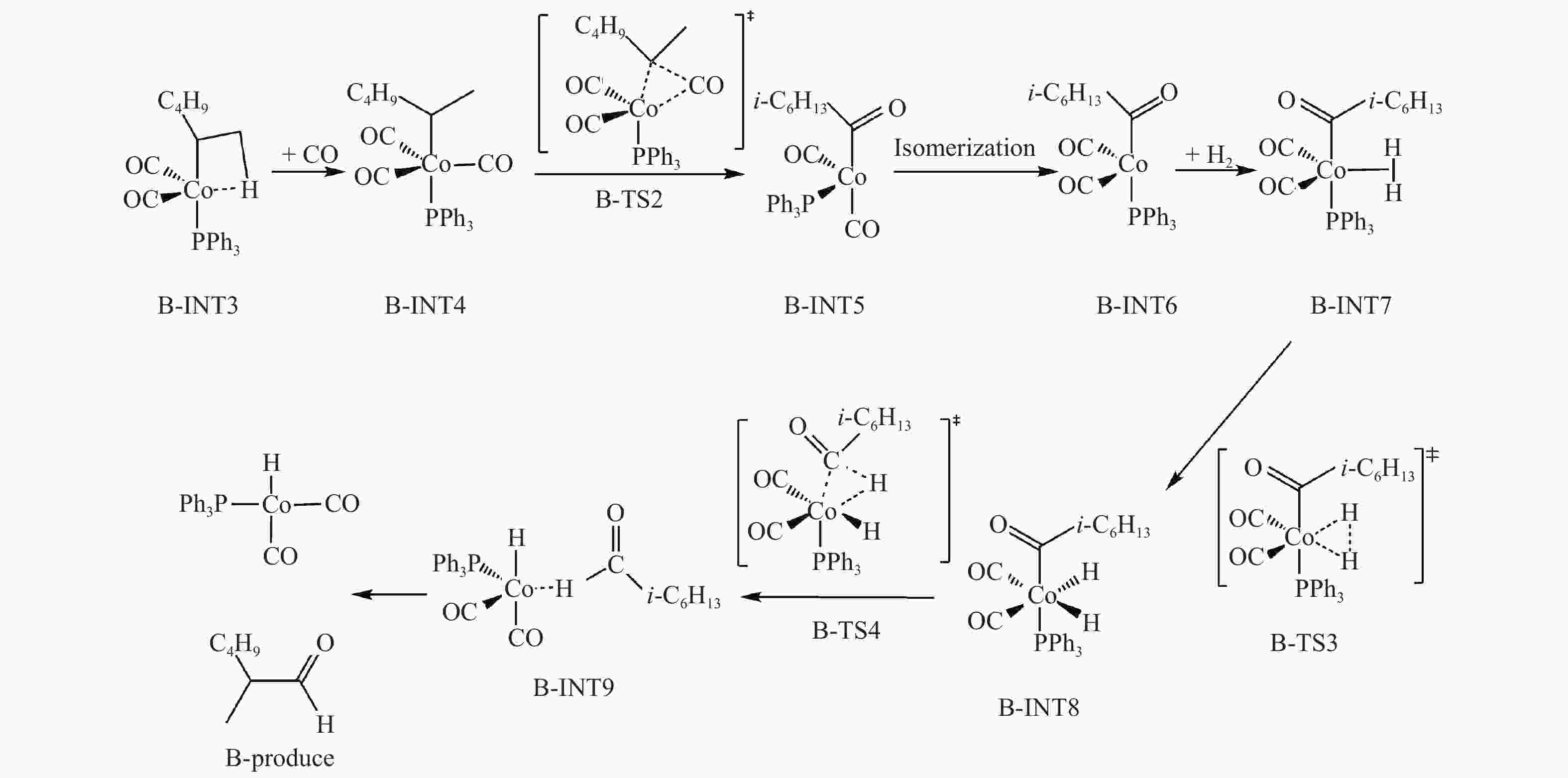
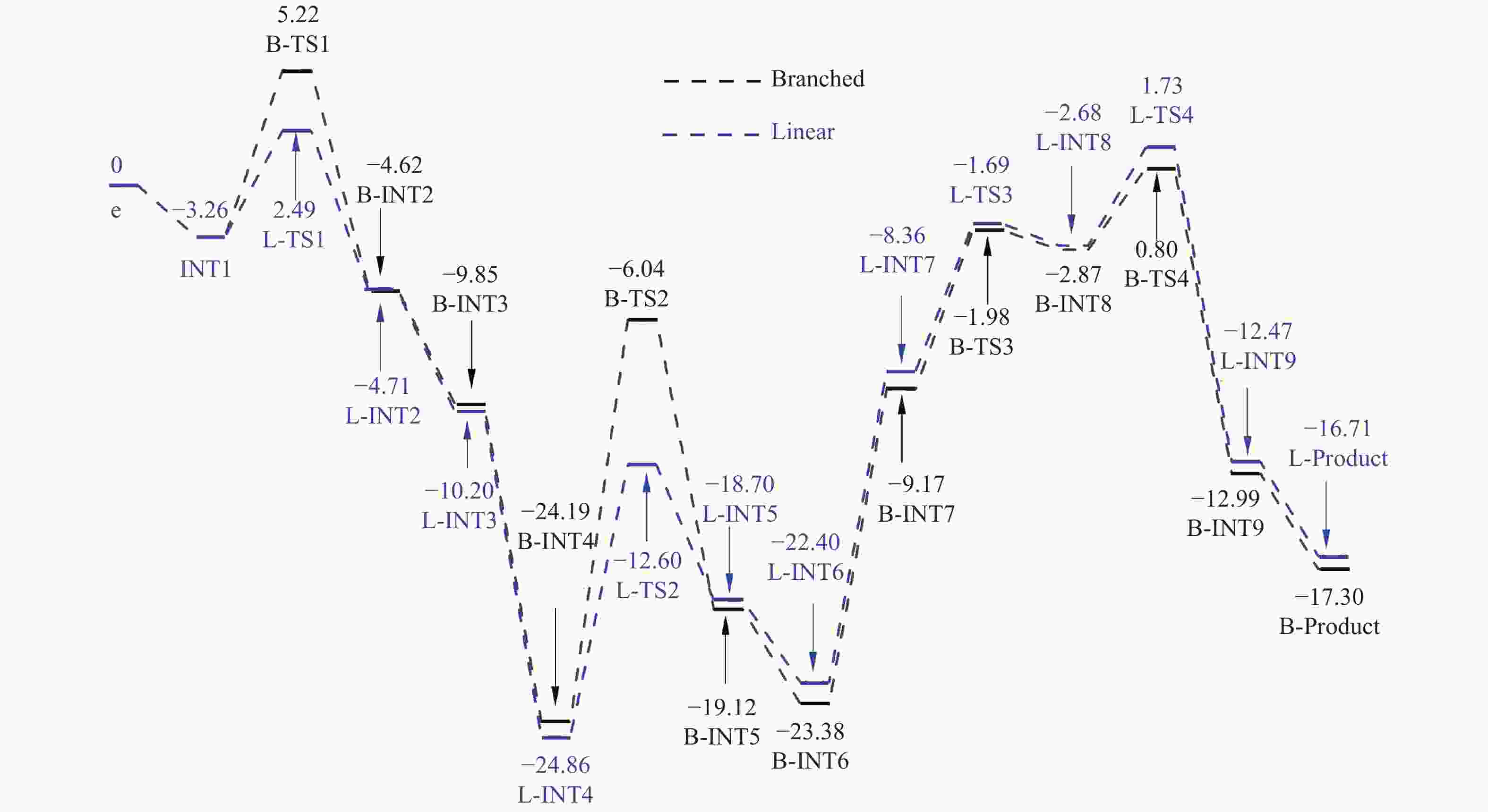
Entry L ΔG/(kcal·mol−1) L-TS1 B-TS1 ΔΔE* L-INT2 B-INT2 L-INT3 B-INT3 1 CO 6.54 6.68 0.14 −7.14 −7.19 − − 2 PH3 6.08 6.59 0.51 −7.15 −7.07 −7.93 −8.97 3 PF3 5.71 5.07 −0.64 −12.23 −13.25 −9.92 −11.25 4 PMe3 8.17 9.37 1.20 −3.21 −10.74 −4.68 −8.04 5 PPh3 5.75 8.48 2.73 −4.71 −4.62 −10.20 −9.85 6 PBu3 7.43 9.92 2.49 −4.20 −1.27 −8.43 −2.95 *:ΔΔE = ΔG (B-TS1)−ΔG(L-TS1) -
[1] FRANKE R, SELENT D, BÖRNER A. Applied hydroformylation[J]. Chem Rev,2012,112(11):5675−5732. doi: 10.1021/cr3001803 [2] ROELEN O. Production of oxygenated carbon compounds. US, 2327066[P]. 1943. [3] HEBRARD F, KALCK P. Cobalt-catalyzed hydroformylation of alkenes: Generation and recycling of the carbonyl species, and catalytic cycle[J]. Chem Rev,2009,109(9):4272−4282. doi: 10.1021/cr8002533 [4] SZLAPA E N, HARVEY J N. Computational modelling of selectivity in cobalt-catalyzed propene hydroformylation[J]. Chem Eur J,2018,24(64):17096−17104. doi: 10.1002/chem.201803490 [5] LIU Y, LI Z H, WANG B, ZHANG Y. A fine dispersed cobalt catalyst with macro-pore for hydroformylation of 1-hexene[J]. Catal Lett,2016,11(146):2252−2260. [6] HOOD D M, JOHNSON R A, CARPENTER A E, YOUNKER J M, VINYARD D J, STANLEY G G. Highly active cationic cobalt(II) hydroformylation catalysts[J]. Science,2020,367(6477):542−548. doi: 10.1126/science.aaw7742 [7] HECK R F, BRESLOE D S. The reaction of cobalt hydrotetracarbonyl with olefins[J]. J Am Chem Soc,1961,83:4023−4027. doi: 10.1021/ja01480a017 [8] LI P, SHEN C R, MIN J, MEI J Y, ZHENG H, HE L TIAN X X. Computational investigation of the ligand effect on the chemo/regioselectivity and reactivity of cobalt-catalysed hydroformylation[J]. Catal Sci Technol,2020,10(9):2994−3007. doi: 10.1039/C9CY02562F [9] VARELA J A, VÁZQUEZ S A, MARTÍNEZ-NÚÑEZ E. An automated method to find reaction mechanisms and solve the kinetics in organometallic catalysis[J]. Chem Sci,2017,8(5):3843−3851. [10] RUSH L E, PRINGLE P G, HARVEY J N. Computational kinetics of cobalt-catalyzed alkene hydroformylation[J]. Angew Chem Int Ed,2014,53(33):8672−8676. doi: 10.1002/anie.201402115 [11] FENG J H, GARLAND M. Unmodified homogeneous rhodium-catalyzed hydroformylation of styrene. The detailed kinetics of the regioselective synthesis[J]. Organometallics,1999,18(3):417−427. doi: 10.1021/om980514v [12] 雷鸣, 冯文林, 徐振峰. 羰基钴催化氢甲酰化反应的理论研究[J]. 物理化学学报,2000,16(6):522−526. doi: 10.3866/PKU.WHXB20000609LEI Ming, FENG Wen-lin, XU Zhen-feng. Theoretical study on the mechanisms of some elementary reactions catalyzed by modified carbonyl cobalt[J]. Acta Phys-Chim Sin,2000,16(6):522−526. doi: 10.3866/PKU.WHXB20000609 [13] HUO C F, LI Y W, BELLER M, JIAO H J. HCo(CO)3-catalyzed propene hydroformylation. Insight into detailed mechanism[J]. Organometallics,2003,22(23):4665−4677. doi: 10.1021/om0304863 [14] BERNALES V, FROESE R D. Rhodium catalyzed hydroformylation of olefins[J]. J Comput Chem,2019,40(2):342−348. doi: 10.1002/jcc.25605 [15] LEI M, FENG W L, XU Z F. Ab initio MO study of reactions mechanism for carbonyl migration of Co complex[J]. Chin Sci Bull,2000,45(13):1176−1178. doi: 10.1007/BF02886073 [16] KUMAR M, CHAUDHARI R V, SUBRAMANIAM B, JACKSON T A. Ligand effects on the regioselectivity of rhodium-catalyzed hydroformylation: Density functional calculations illuminate the role of long-range noncovalent interactions[J]. Organometallics,2014,33(16):4183−4191. doi: 10.1021/om500196g [17] PATEL P, WILSON A K. Computational chemistry considerations in catalysis: Regioselectivity and metal-ligand dissociation[J]. Catal Today,2020,358:422−429. doi: 10.1016/j.cattod.2020.07.057 [18] DIAS R P, PRATES M S L, DE ALMEIDA W B, ROCHA W R. DFT study of the ligand effects on the regioselectivity of the insertion reaction of olefins in the complexes [HRh(CO)2(PR3)(L)] (R = H, F, Et, Ph, OEt, OPh, and L = propene, styrene)[J]. Int J Quantum Chem,2011,111(7/8):1280−1292. doi: 10.1002/qua.22590 [19] BECKE A D. Density ‐ functional thermochemistry. III. The role of exact exchange[J]. J Chern Phys,1993,98(7):5648−5652. doi: 10.1063/1.464913 [20] GRIMME S, EHRLICH S, GOERIGK L. Effect of the damping function in dispersion corrected density functional theory[J]. J Comput Chem,2011,32(7):1456−1465. doi: 10.1002/jcc.21759 [21] FRISCH M J, TRUCKS G W, SCHLEGEL H B, SCUSERIA G E. Gaussian16 Rev. B. 01[M] Wallingford, CT, 2016. [22] AULLóN G, ALVAREZ S. The [M2(CO)8] complexes of the cobalt group[J]. Eur J Inorg Chem,2001,12:3031−3038. [23] GUO J D, PHAM H D, WU Y B, ZHANG D J, WANG X T. Mechanism of cobalt-catalyzed direct aminocarbonylation of unactivated alkyl electrophiles: Outer-sphere amine substitution to form amide bond[J]. ACS Catal,2020,10(2):1520−1527. doi: 10.1021/acscatal.9b04736 [24] 姜淼, 杜虹, 王国庆, 严丽, 丁云杰. Co-PPh3@POPs多相催化剂氢甲酰化反应研究[J]. 煤炭学报,2020,45(4):1250−1258.JIANG Miao, DU Hong, WANG Guo-qing, YAN Li, DING Yun-jie. Co-PPh3@POPs heterogeneous catalysts for hydroformylation of olefins[J]. J China Coal Soc,2020,45(4):1250−1258. [25] GRIMA J P, CHOPLIN F, KAUFMANN G. Theoretical study of the hydroformyltaion reaction mechanism[J]. J Organomet Chem,1977,129:221−237. doi: 10.1016/S0022-328X(00)92495-1 [26] BIRBECK J M, HAYNES A, ADAMS H, DAMOERNSE L, OTTO S. Ligand effects on reactivity of cobalt acyl complexes[J]. ACS Catal,2012,2(12):2512−2523. doi: 10.1021/cs300589n [27] HUO C F, ZENG T, LI Y W, BELLER M, JIAO H J. Switching end-on into side-on C≡N coordination: A computational approach[J]. Organometallics,2005,24(24):6037−6042. doi: 10.1021/om0505054 [28] FALIVENE L, CREDENDINO R, POATER A, PETTA A, SERRA L, OLIVA R, SCARANO V, CAVALLO L. SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps[J]. Organometallics,2016,35(13):2286−2293. doi: 10.1021/acs.organomet.6b00371 -




 下载:
下载: