ReaxFF MD study on the early stage co-pyrolysis of mixed PE/PP/PS plastic waste
-
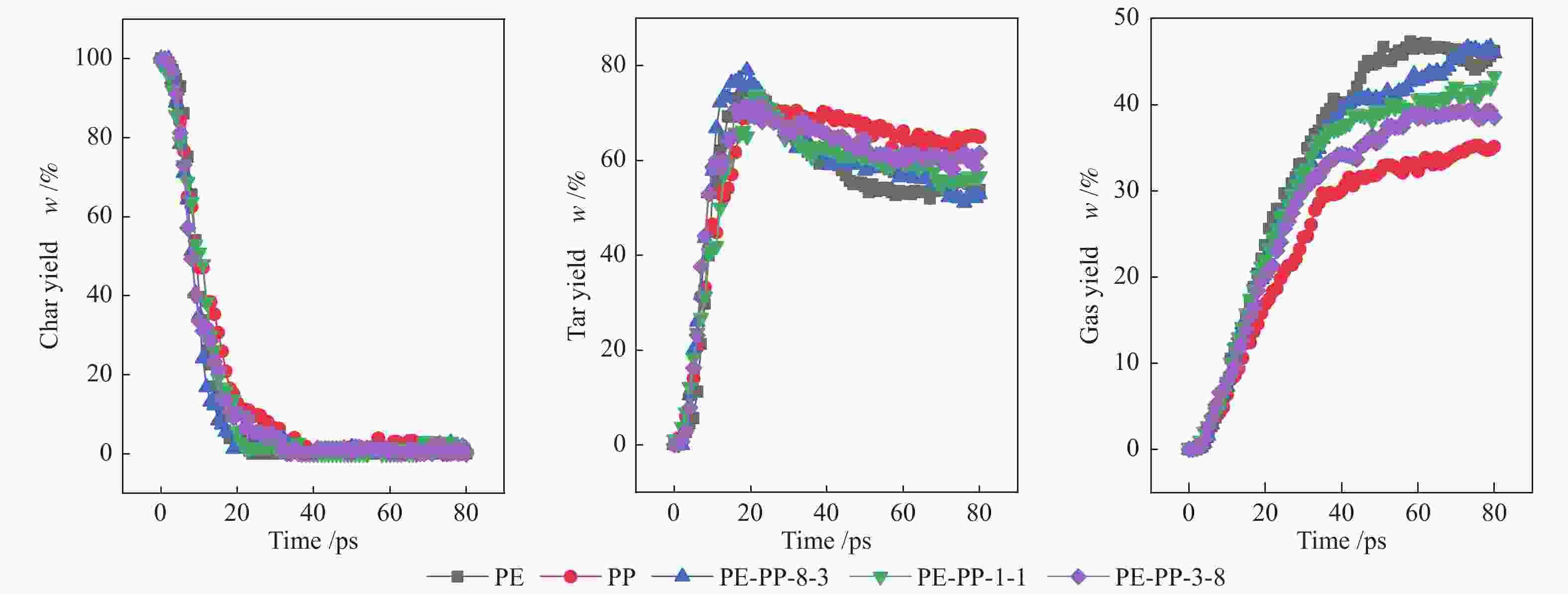
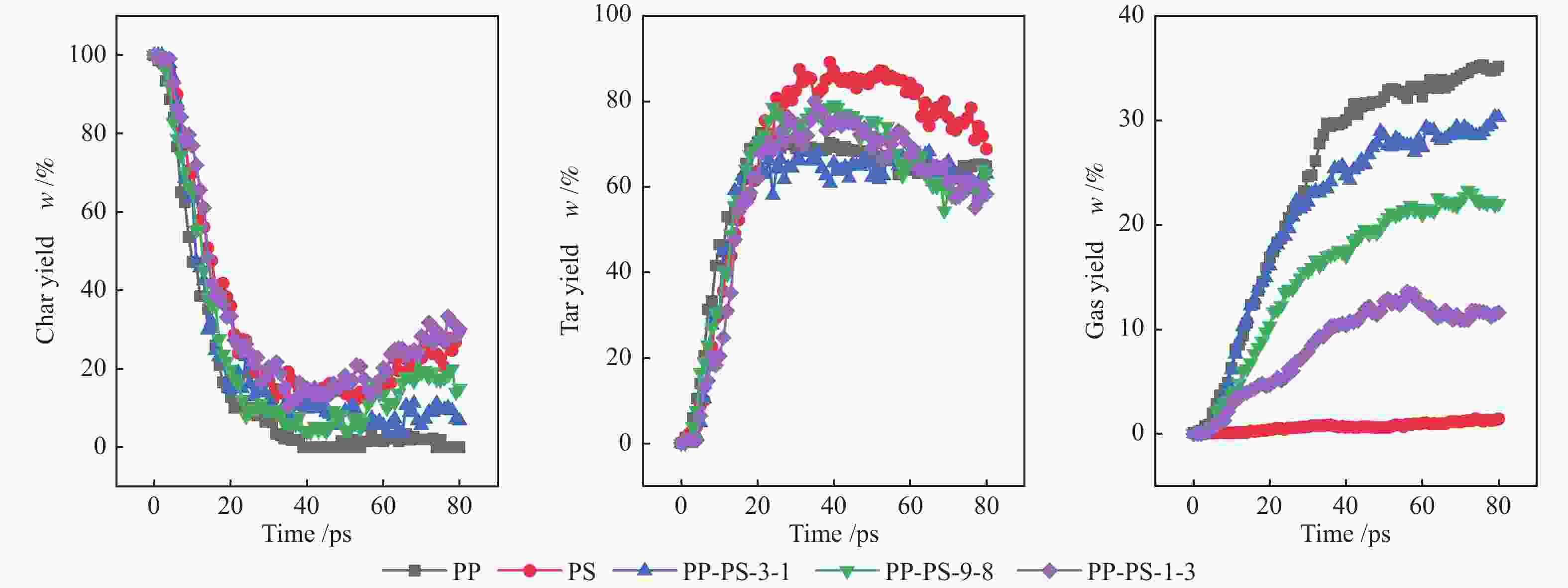
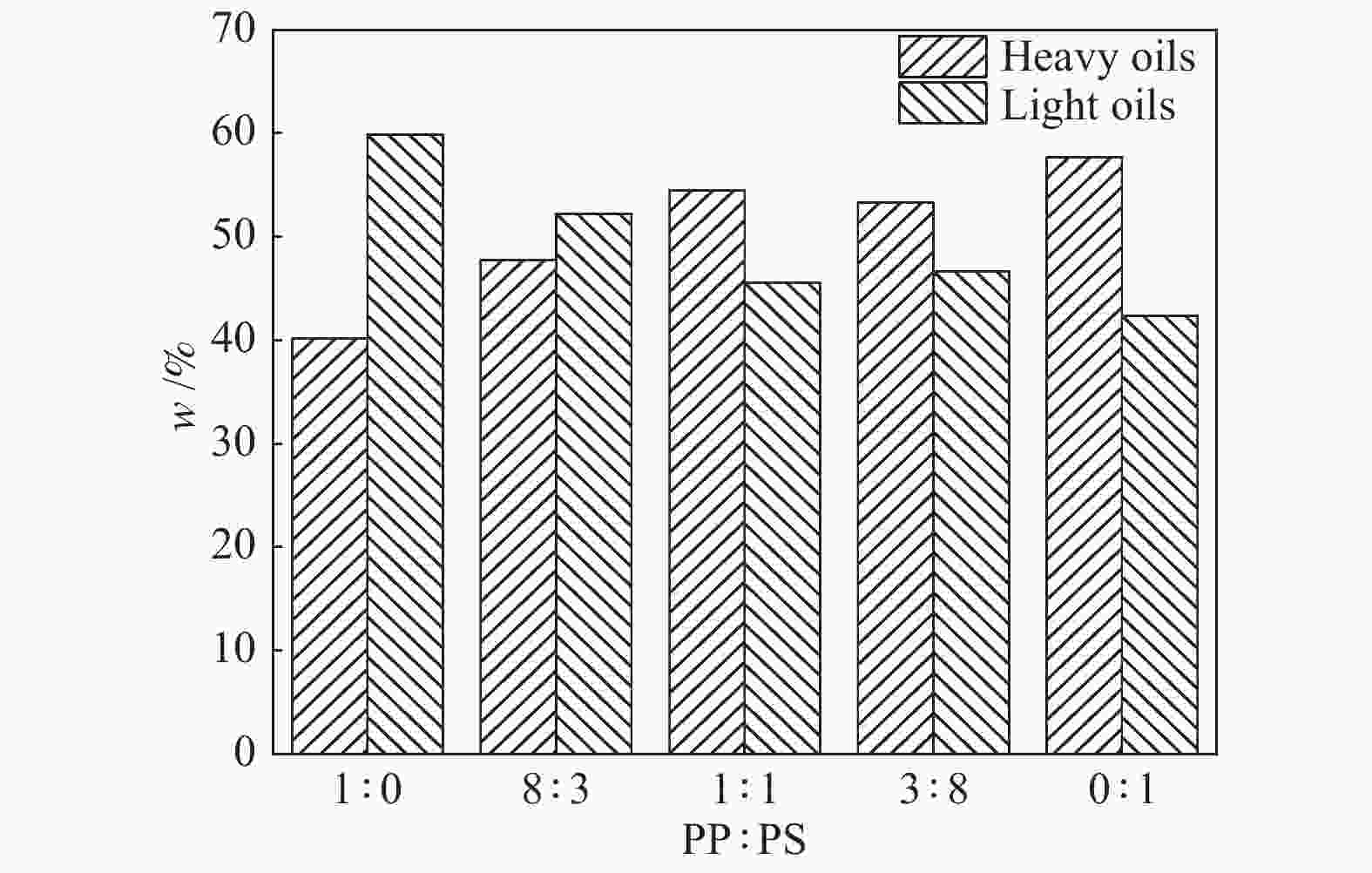
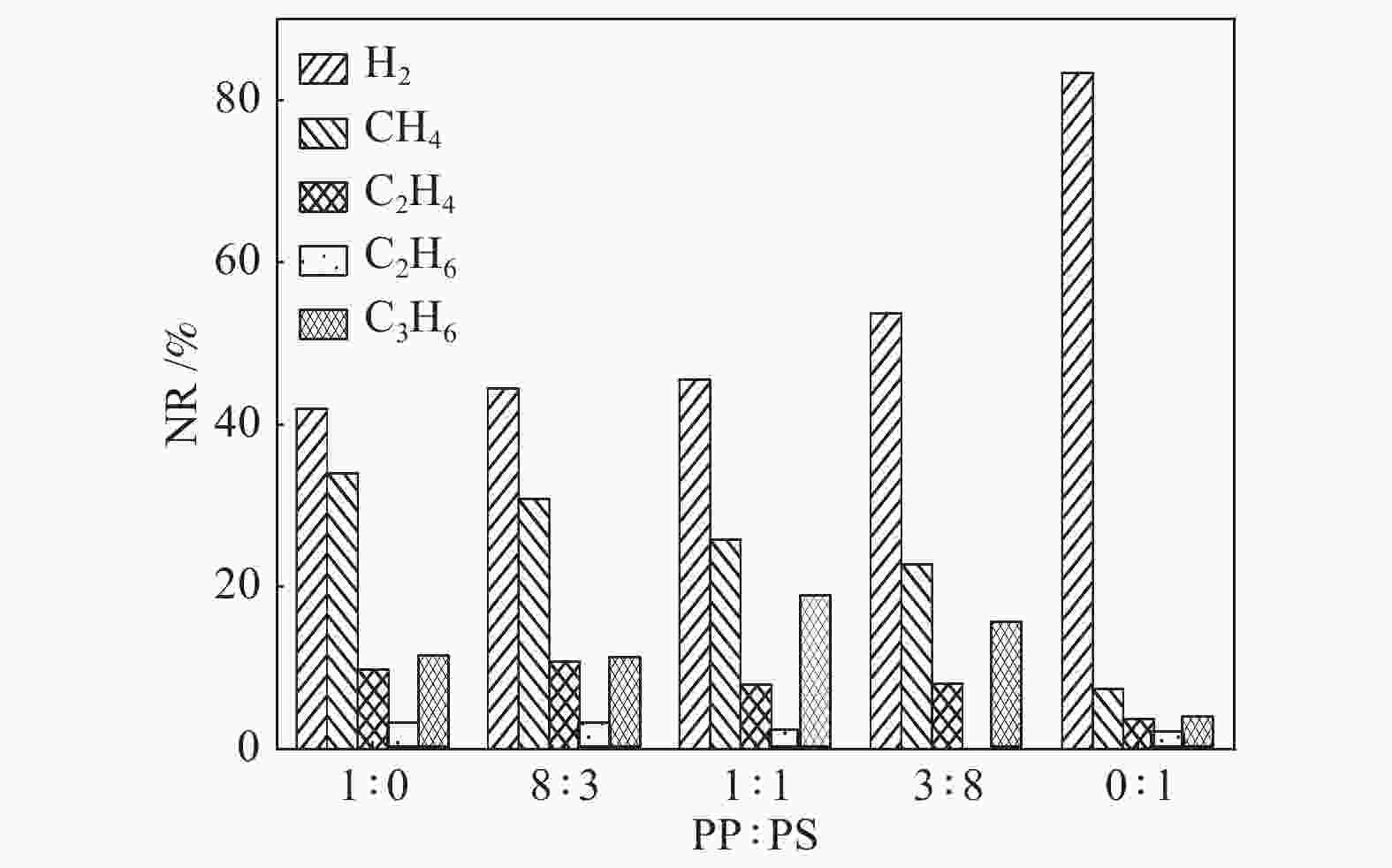
摘要: 利用反应力场分子模拟(ReaxFF MD)结合反应机理自分析(AutoRMA)工具,从动力学、热解产物及热解反应过程三方面在原子层面上,探究了典型聚乙烯(PE)、聚丙烯(PP)、聚苯乙烯(PS)废塑料共热解的反应机理。结果表明,PE/PP/PS共热解的动力学参数可通过C–C键和C–H键断裂的活化能加权求和(即Char Bonds方法) 获得,其活化能估计值与实验值的误差仅为±3.86%;因此可以由C–C键和C–H键的断裂来表征热解反应进程。对于PP-PE混合塑料热解体系,增加其中PP的含量可以提高油和可燃气的产率,而对于PP-PS体系,增加其中PS的含量可以提高炭和油产率。在PE-PP-PS混合塑料热解体系中,高温有利于重油裂解为轻油,轻油相对含量从2400 K的44.77%升高到3000 K的56.18%;同时,高温也会促使烃类小分子进一步裂解生成更小分子产物,随热解温度升高,H2和CH4的产率明显上升,但C2H4和C3H6的产率先上升后降低。相比单独热解,混合热解体系开始反应时间有所延迟,但达到第一次平衡的总反应时间缩短,并且更倾向于生成较小分子的产物。PE和PP单独热解时,首先生成其单体,继而生成烷烃和小分子气体,但在共热解过程中,首先生成烷烃和小分子气体,而后生成其单体。PS在共热解体系中更倾向于提供·H自由基从而与PE和PP生成的自由基结合,形成小分子烷烃或H2。
-
关键词:
- 共热解 /
- 废塑料 /
- 反应力场分子模拟(ReaxFF MD) /
- 动力学 /
- 聚乙烯(PE)、聚丙烯(PP)、聚苯乙烯(PS)
Abstract: The early stage co-pyrolysis of typical plastic waste including polyethylene (PE), polypropylene (PP) and polystyrene (PS) were investigated by using the reactive force field molecular dynamics (ReaxFF MD) simulation with an automatic reaction mechanism analysis software (AutoRMA); the kinetic model, product yields and reaction process of co-pyrolysis were analyzed at atomic level. The results show that the kinetic parameters of PE/PP/PS co-pyrolysis can be obtained through the weighted sum of the parameters for the fracture of C–C and C–H bonds; the estimated activation energy is very close to the experimental one with a small error of ±3.86%, indicating that the fracture of C–C and C–H bonds can accurately characterize the co-pyrolysis process. For the co-pyrolysis of PE-PP mixture, an increase of PP content can improve the yields of oil and combustible gas, whereas for the co-pyrolysis of PP-PS mixture, the increase of PS content can improve the yields of tar and oil. In contrast, for the co-pyrolysis of PE-PP-PS mixture, a higher temperature is beneficial for the conversion of heavy oil into light oil; the light oil content increases from 44.77% at 2400 K to 56.18% at 3000 K. In addition, as a higher temperature can promote the further cracking of light hydrocarbons into gas products of smaller molecules, the yields of H2 and CH4 increase significantly with the increase of pyrolysis temperature, whereas the yields of C2H4 and C3H6 increase first and then decrease with the temperature. In comparison with the separated pyrolysis, the co-pyrolysis commences later, but displays shorter time to reach the first equilibrium state and generates products with smaller molecules. For the separate pyrolysis of PE and PP, their monomers emerge first, hereafter the alkanes and small molecule gases are produced; for the co-pyrolysis process, in contrast, the alkanes and small molecule gases are generated prior to the monomers. Moreover, PS tends to provide ·H radicals in the co-pyrolysis process, which can combine with the free radicals generated from PE and PP pyrolysis, forming small molecule alkanes and H2. -
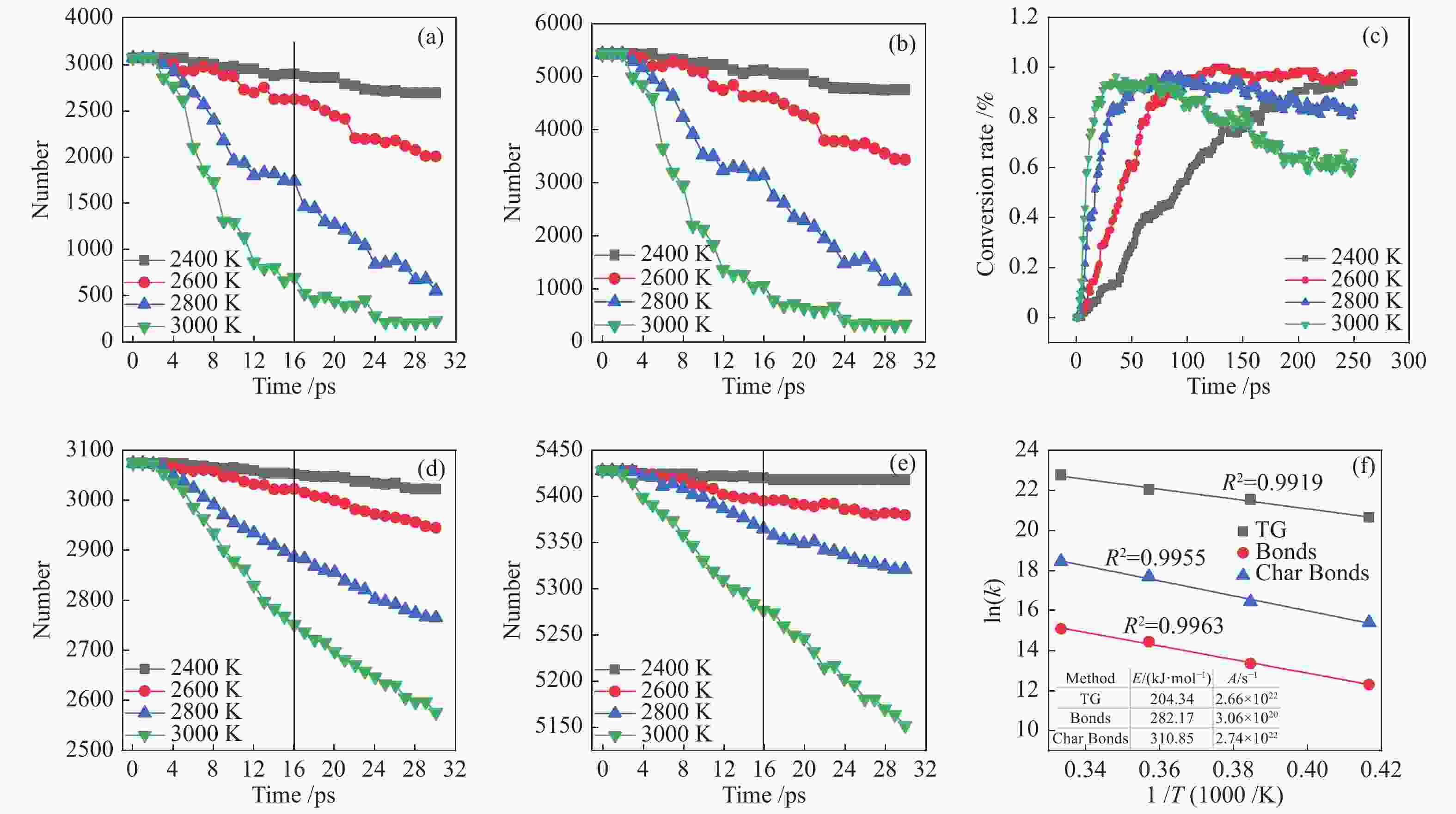
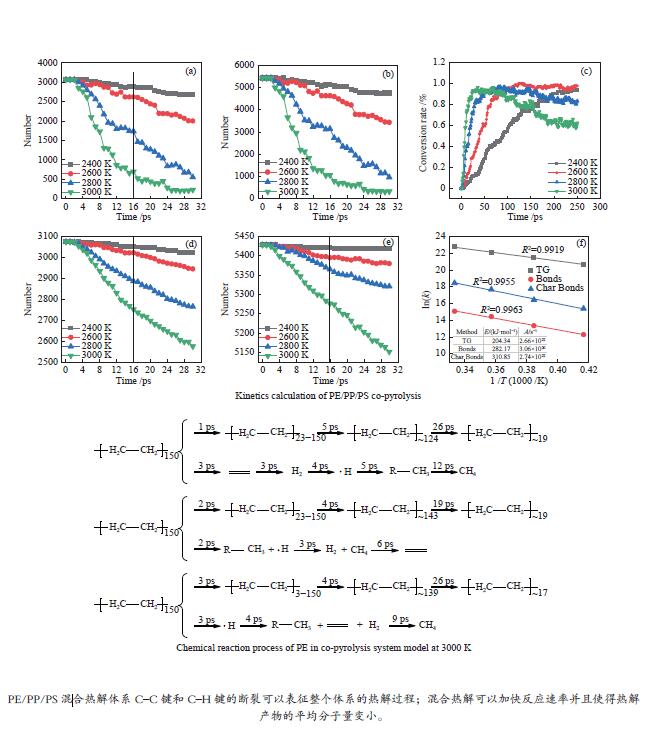
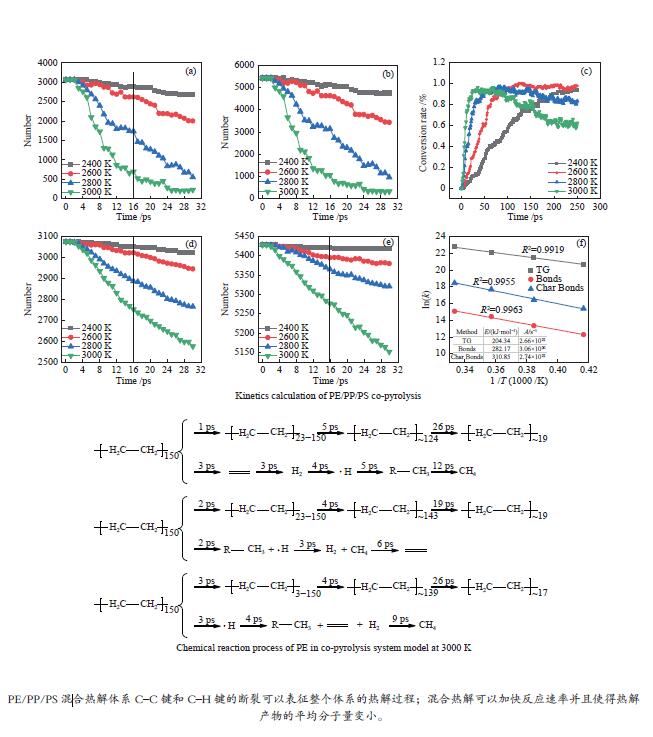
图 2 不同温度下PE-PP-PS体系共热解过程C–C键和C–H键数量变化、固体转换率及动力学计算
Figure 2 Number change of C–C and C–H bonds in the pyrolysis system at different temperatures as well as the solid conversion rate and related kinetic profiles: (a) C–C bond number change; (b) C–H bond number change; (c) solid conversion rate; (d) C–H bond number in solid products; (e) C–C bond number in solid products; (f) kinetics calculation
表 1 废塑料典型组分主要来源及其热解产品分布
Table 1 Main sources of typical plastics and their pyrolysis products at low and high temperatures
Component Source Low temperature pyrolysis products High temperature pyrolysis products PE household, industrial packaging, agricultural film wax, oil[5, 6] gas, light oil[6, 7] PP household, industrial packaging, automotive industry wax, oil[5, 6] gas, light oil[6, 7] PS household, industrial packaging, construction, WEEE toluene, styrene and
its oligomers [8]styrene and its oligomers, PAH[8] 表 2 模拟体系分子构成
Table 2 Molecular composition of simulation system
System model PE(C300H602) chain number PP(C300H602) chain number PS(C304H306) chain number PE 9 − − PP − 9 − PS − − 14 PE-PP-8-3 8 3 − PE-PP-1-1 5 5 − PE-PP-3-8 3 8 − PP-PS-9-8 − 6 6 PP-PS-3-1 − 6 2 PP-PS-1-3 − 2 6 PE-PP-PS 6 2 2 表 3 PE/PP/PS废塑料单独热解及共热解过程动力学参数
Table 3 Pyrolysis kinetic parameters of PE, PP, PS and mixtures of PE-PP, PP-PS and PE-PP-PS
System Simulation time
t/psActivation energy Ea/(kJ·mol−1) Preexponential factor A/s−1 Correlation coefficient experimental simulated experimental simulated experimental simulated PE 20 362.9 367.89a 4.09 × 1023 1.17 × 1022 0.978 0.973 PP 7 332.3 332.28b 1.38 × 1022 2.55 × 1022 0.996 0.897 PS 12 257.4 252.27b 2.61 × 1016 9.87 × 1020 0.997 0.983 PE-PP-8-3 9 338.4 337.75b 6.71 × 1022 7.37 × 1022 0.979 0.990 PP-PS-9-8 17 300.4 302.60b 1.65 × 1019 1.44 × 1022 0.999 0.997 PE-PP-PS 16 314.7 310.85b 9.24 × 1019 2.74 × 1022 0.998 0.995 a: calculated by bonds method; b: calculated by char bonds method 表 4 3000 K下PE/PP/PS在等温混合热解化学反应进程
Table 4 Chemical reaction process during isothermal co-pyrolysis of PE/PP/PS at 3000 K
Plastic System model Chemical reaction process PE PE 
PE-PP-8-3 
PE-PP-PS 
PP PP 
PP PE-PP-8-3 
PP-PS-9-8 
PE-PP-PS 
PS PS 
PP-PS-9-8 
PE-PP-PS 
-
[1] WILLIAMS P T, WILLIAMS E A. Interaction of plastics in mixed-plastics pyrolysis[J]. Energy Fuels,1999,13(1):188−196. doi: 10.1021/ef980163x [2] 赵娟. 废塑料回收利用的研究进展[J]. 现代塑料加工应用,2020,32(4):60−63.ZHAO Juan. Research progress on plastic easte recycling[J]. Mod Plast Process Appl,2020,32(4):60−63. [3] 张振华, 汪华林, 陈于勤, 胥培军. 聚乙烯类废弃塑料延迟焦化方法制取燃料油的研究[J]. 燃料化学学报,2008,36(2):223−226. doi: 10.3969/j.issn.0253-2409.2008.02.019ZHANG Zhen-hua, WANG Hua-lin, CHEN Yu-qin, XU Pei-jun. Preparation of fuel oil from waste polyethylene by delayed coking[J]. J Fuel Chem Technol,2008,36(2):223−226. doi: 10.3969/j.issn.0253-2409.2008.02.019 [4] KAMINSKY W, PREDEL M, SADIKI A. Feedstock recycling of polymers by pyrolysis in a fluidised bed[J]. Polym Degrad Stab,2004,85(3):1045−1050. doi: 10.1016/j.polymdegradstab.2003.05.002 [5] DAS P, TIWARI P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel[J]. Waste Manage,2018,79:615−624. doi: 10.1016/j.wasman.2018.08.021 [6] DONAJ P J, KAMINSKY W, BUZETO F, YANG W. Pyrolysis of polyolefins for increasing the yield of monomers’ recovery[J]. Waste Manage,2012,32(5):840−846. doi: 10.1016/j.wasman.2011.10.009 [7] HONUS S, KUMAGAI S, FEDORKO G, MOLNÁR V, YOSHIOKA T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—Part I: Production and physical properties[J]. Fuel,2018,221:346−360. doi: 10.1016/j.fuel.2018.02.074 [8] ONWUDILI J A, INSURA N, WILLIAMS P T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time[J]. J Anal Appl Pyrolysis,2009,86(2):293−303. doi: 10.1016/j.jaap.2009.07.008 [9] PREDEL M, KAMINSKY W. Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes[J]. Polym Degrad Stab,2000,70(3):373−385. doi: 10.1016/S0141-3910(00)00131-2 [10] JIN Z C, YIN L J, CHEN D Z, JIA Y J, YUAN J, HU Y Y. Co-pyrolysis characteristics of typical components of waste plastics in a falling film pyrolysis reactor[J]. Chin J Chem Eng,2018,26(10):2176−2184. doi: 10.1016/j.cjche.2018.07.005 [11] VAN DUIN A C T, DASGUPTA S, LORANT F, GODDARD W A. ReaxFF: A reactive force field for hydrocarbons[J]. J Phys Chem A,2001,105(41):9396−9409. doi: 10.1021/jp004368u [12] LIU X L, LI X X, LIU J, WANG Z, KONG B, GONG X M, YANG X Z, LIN W G, GUO L. Study of high density polyethylene (HDPE) pyrolysis with reactive molecular dynamics[J]. Polym Degrad Stab,2014,104:62−70. doi: 10.1016/j.polymdegradstab.2014.03.022 [13] KNYAZEV V D. Effects of chain length on the rates of C−C bond dissociation in linear alkanes and polyethylene[J]. J Phys Chem A,2007,111(19):3875−3883. doi: 10.1021/jp066419e [14] 贺兴处, 陈德珍, 梅振飞, 阿迪力·巴吐尔, 安青. CaO催化PE热解及H2O对催化过程影响的ReaxFF MD研究与机理分析[J]. 化工学报,2021,:1−15. doi: 10.11949/0438-1157.20201566HE Xing-chu, CHEN De-zhen, MEI Zhen-fei, ADILI Batuer, AN Qing. ReaxFF MD study on the pyrolysis of PE catalyzed by Cao and the effect of H2O on the catalytic process and mechanism analysis[J]. J Chem Ind Eng,2021,1−15. doi: 10.11949/0438-1157.20201566 [15] 同济大学. 全自动 ReaxFF 反应机理分析软件[简称: AutoRMA] V1.0: 2021SR0108488[P]. 2021-01-20Tongji university. Automatic ReaxFF reaction mechanism analyzer [abbreviation: AutoRMA] V1.0: 2021SR0108488[P]. 2021-01-20. [16] Sandia National Laboratories. LAMMPS[EB/OL]. http://lammps.sandia.gov. [17] ZHANG J L, GU J T, HAN Y, LI W, GAN Z X, GU J J. Supercritical water oxidation vs supercritical water gasification: Which process is better for explosive wastewater treatment?[J]. Ind Eng Chem Res,2015,54(4):1251−1260. doi: 10.1021/ie5043903 [18] PITMAN M C, VAN DUIN A C T. Dynamics of confined reactive water in smectite clay-zeolite composites[J]. J Am Chem Soc,2012,134(6):3042−3053. doi: 10.1021/ja208894m [19] PONOMAREV I, VAN DUIN A C T, KROLL P. Reactive force field for simulations of the pyrolysis of polysiloxanes into silicon oxycarbide ceramics[J]. J Phys Chem C,2019,123(27):16804−16812. doi: 10.1021/acs.jpcc.9b03810 [20] PAAJANEN A, VAARI J. High-temperature decomposition of the cellulose molecule: a stochastic molecular dynamics study[J]. Cellul,2017,24(7):2713−2725. doi: 10.1007/s10570-017-1325-7 [21] BHOI S, BANERJEE T, MOHANTY K. Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF[J]. Fuel,2014,136:326−333. doi: 10.1016/j.fuel.2014.07.058 [22] 张秀霞, 吕晓雪, 肖美华, 林日亿, 周志军. 典型烟煤热解机理的反应动力学模拟[J]. 燃料化学学报,2020,48(9):1035−1046. doi: 10.3969/j.issn.0253-2409.2020.09.002ZHANG Xiu-xia, LU Xiao-xue, XIAO Mei-hua, LIN Ri-yi, ZHOU-Zhi-jun. Molecular re action dynamics simulation of pyrolysis mechanism of typical bituminous coal via ReaxFF[J]. J Fuel Chem Technol,2020,48(9):1035−1046. doi: 10.3969/j.issn.0253-2409.2020.09.002 [23] ZHONG Q F, MAO Q Y, XIAO J, VAN DUIN A C T, MATHEWS J P. ReaxFF simulations of petroleum coke sulfur removal mechanisms during pyrolysis and combustion[J]. Combust Flame,2018,198:146−157. doi: 10.1016/j.combustflame.2018.09.005 [24] ZHANG Z J, GUO L, ZHANG H Y, ZHAN J H. Comparing product distribution and desulfurization during direct pyrolysis and hydropyrolysis of Longkou oil shale kerogen using reactive MD simulations[J]. Int J Hydrogen Energy,2019,44(47):25335−25346. doi: 10.1016/j.ijhydene.2019.08.036 [25] CHEN C, ZHAO L L, WU X, LIN S C. Theoretical understanding of coal char oxidation and gasification using reactive molecular dynamics simulation[J]. Fuel,2020,260:116300. doi: 10.1016/j.fuel.2019.116300 [26] CHENOWETH K, CHEUNG S, VAN DUIN A C T, GODDARD III W A, KOBER E M. Simulations on the thermal decomposition of a poly(dimethylsiloxane) polymer using the ReaxFF reactive force field[J]. J Am Chem Soc,2005,127:7192−7202. doi: 10.1021/ja050980t [27] BATUER A, CHEN D Z, HE X C, HUANG Z. Simulation methods of cotton pyrolysis based on ReaxFF and the influence of volatile removal ratio on volatile evolution and char formation[J]. Chem Eng J,2021,405:126633. doi: 10.1016/j.cej.2020.126633 [28] LI D, LEI S J, WANG P, ZHONG L, MA W C, CHEN G Y. Study on the pyrolysis behaviors of mixed waste plastics[J]. Renewable Energy,2021,173:662−674. doi: 10.1016/j.renene.2021.04.035 -




 下载:
下载: