Catalytic depolymerization of kraft lignin for liquid fuels and phenolic monomers over molybdenum-based catalysts: The effect of supports
-
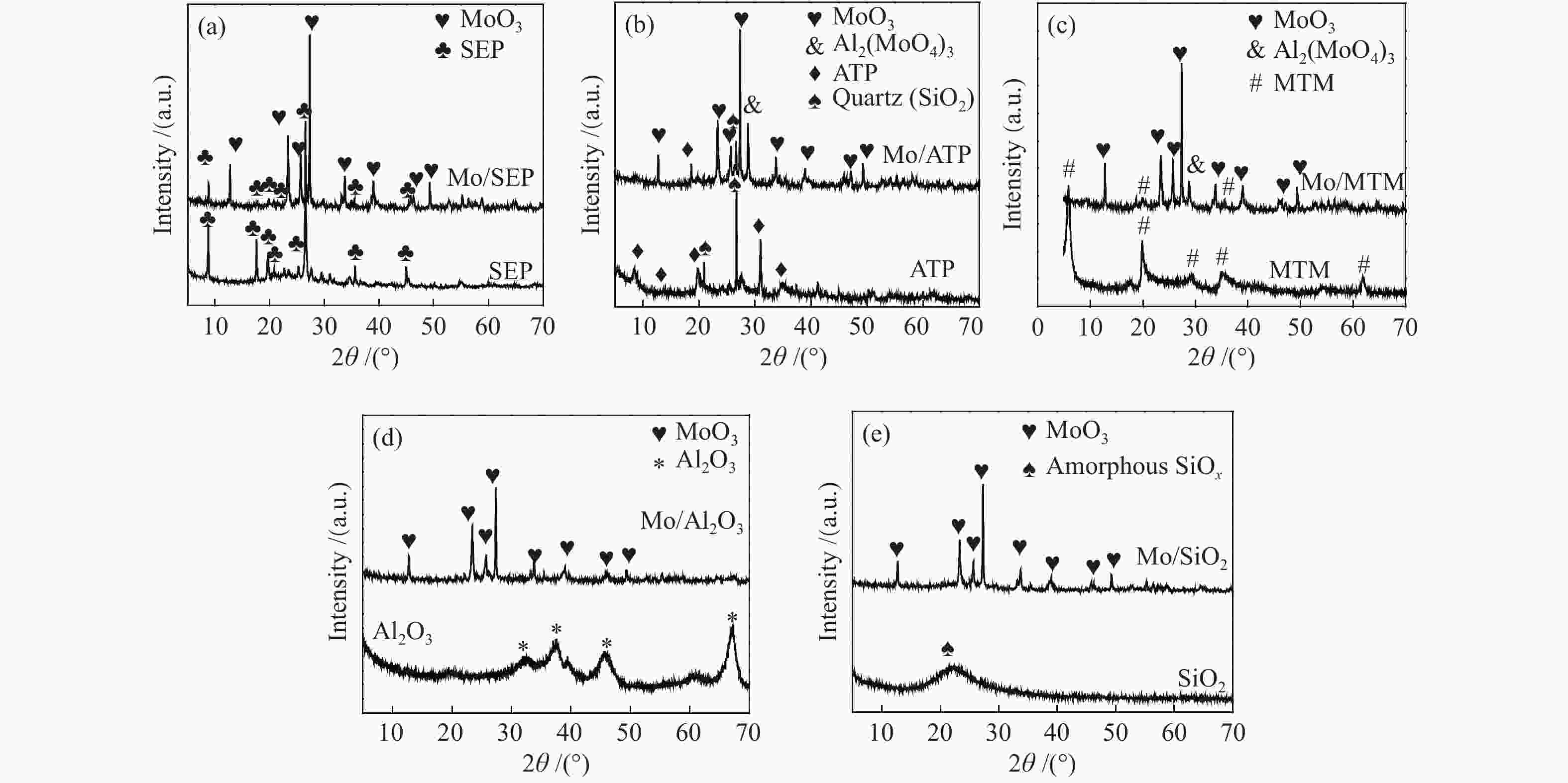
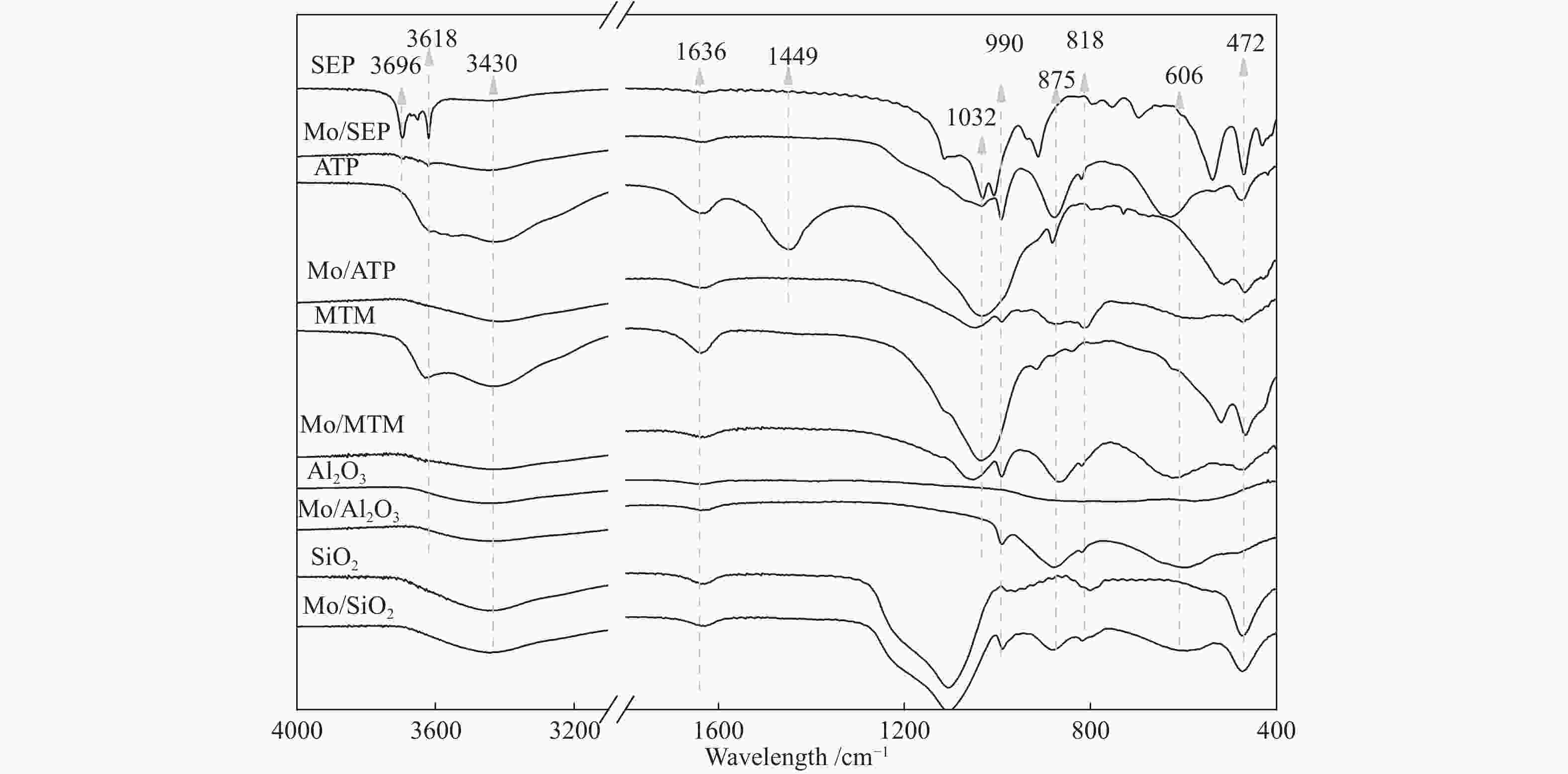
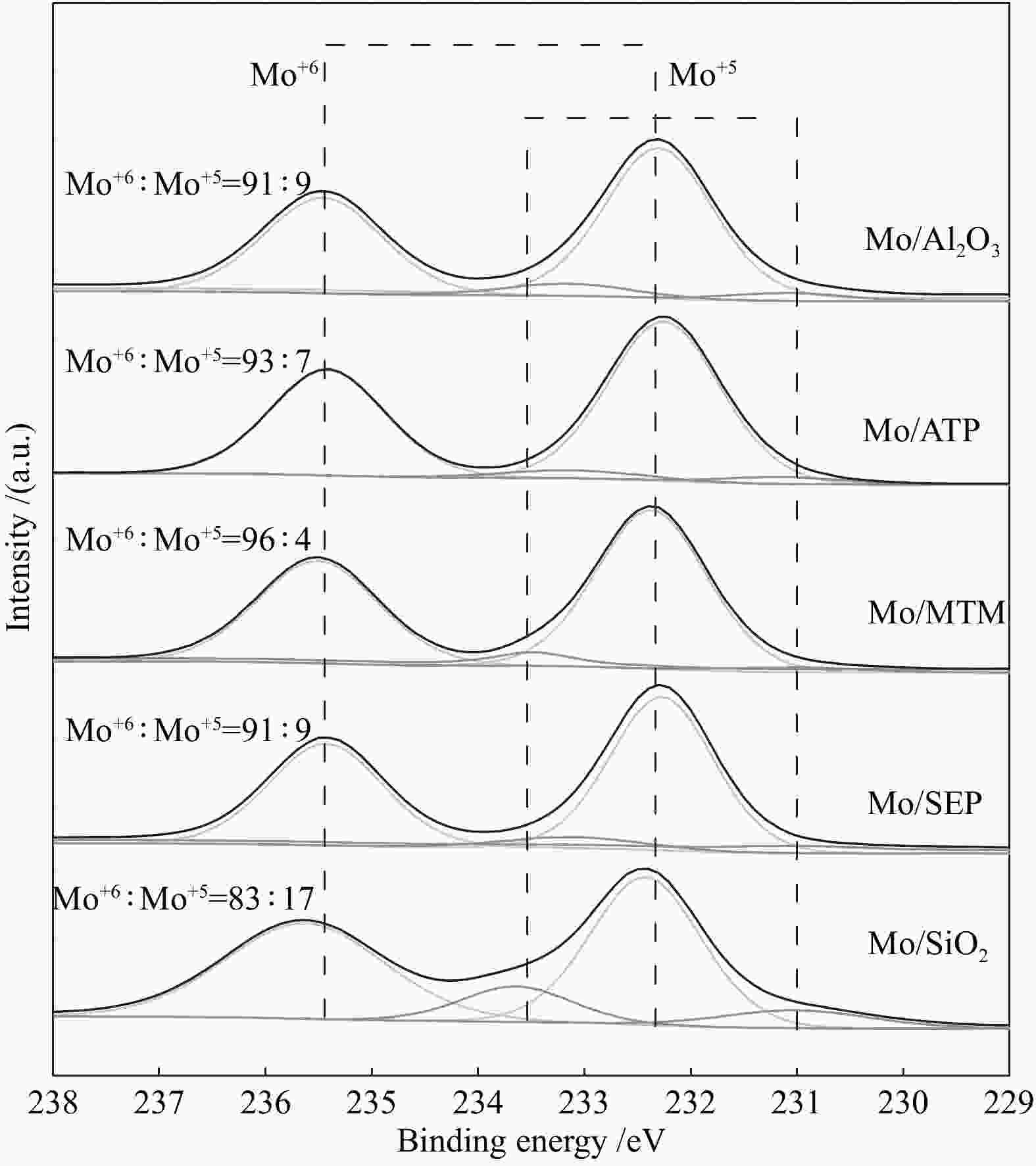
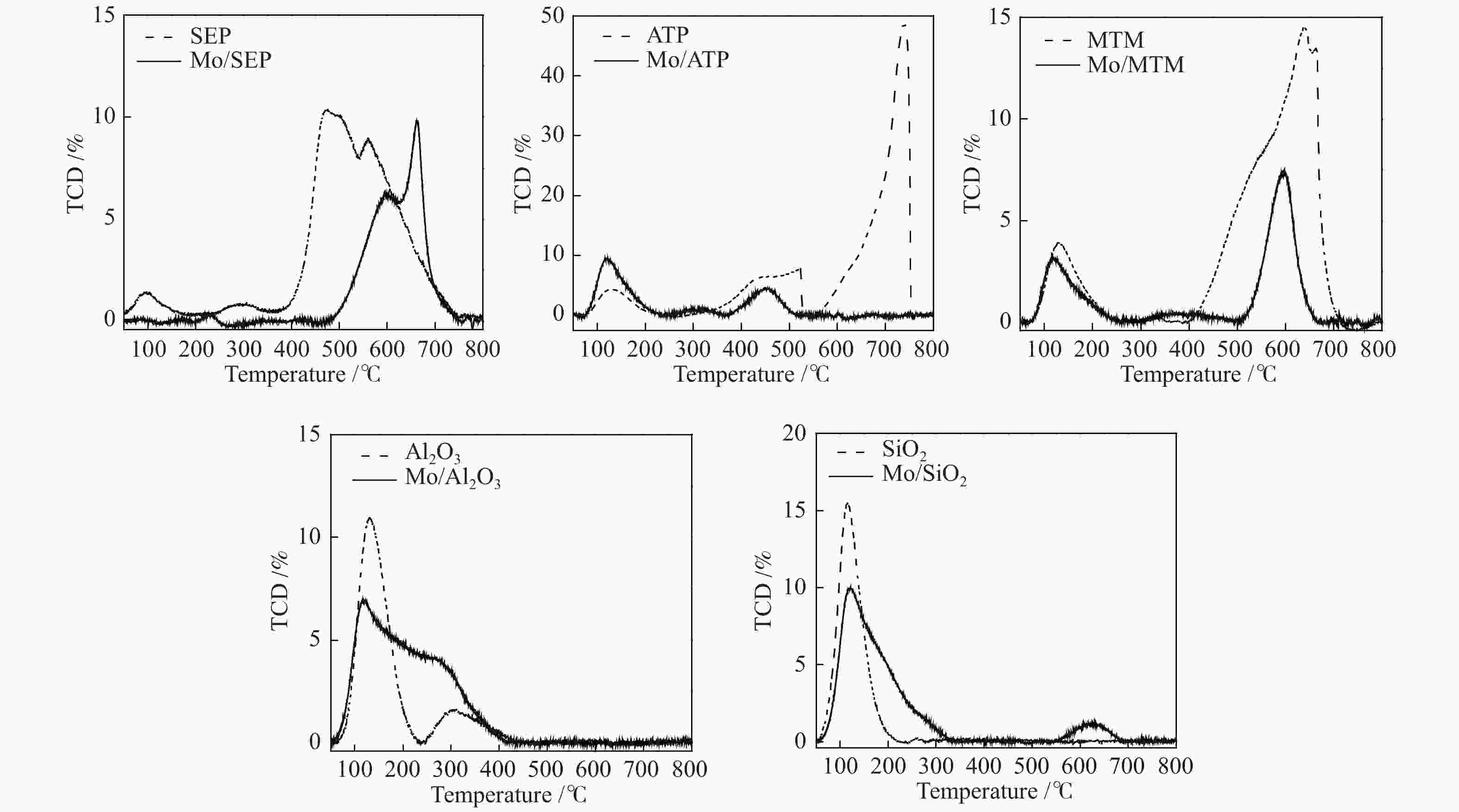
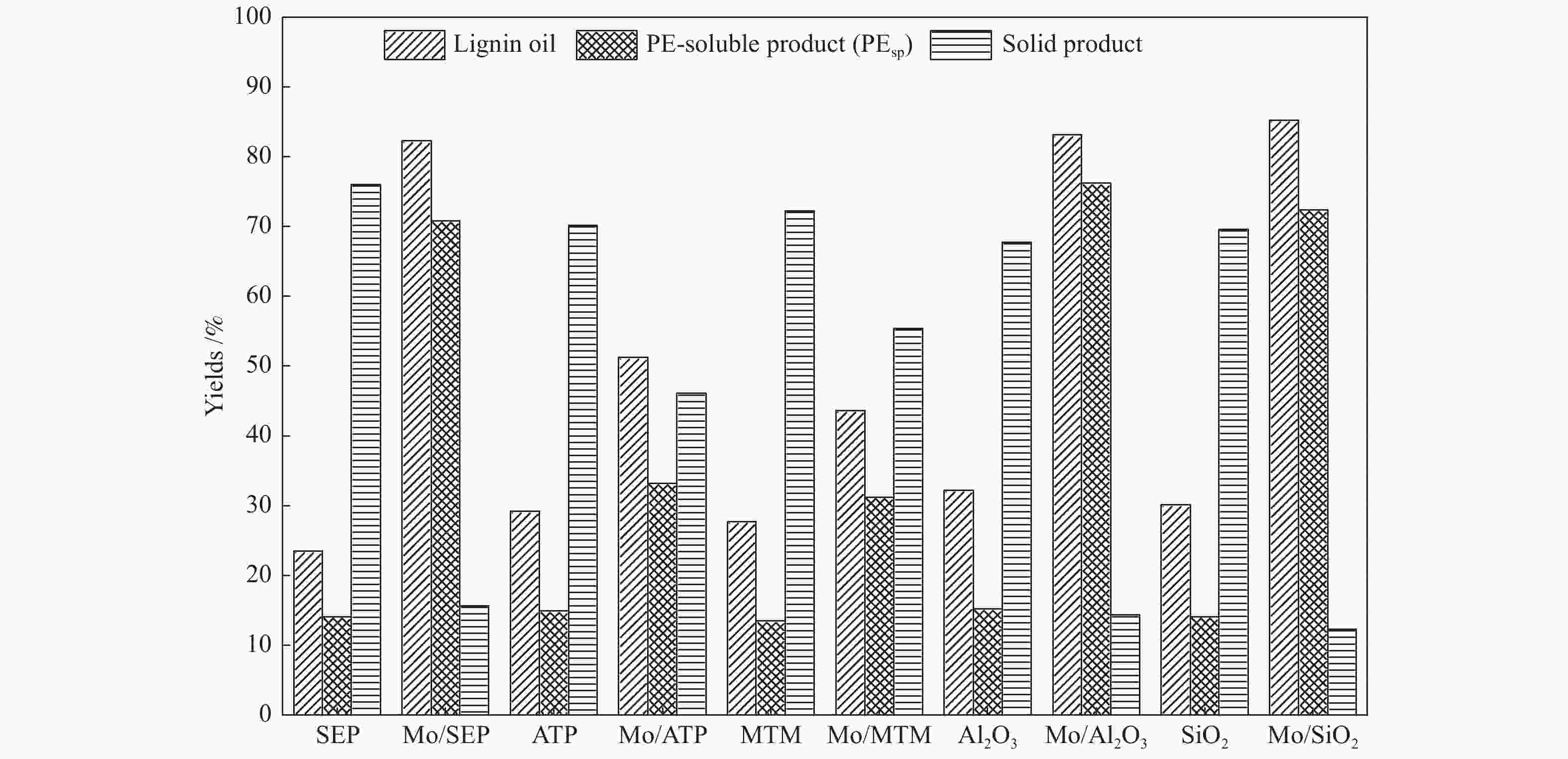
摘要: 本文研究了不同载体(黏土(海泡石(SEP)、凹凸棒石(ATP)、蒙脱土(MTM))和氧化物(Al2O3和SiO2)及其负载的Mo基催化剂对超临界乙醇体系中催化解聚木质素制备液体燃料和酚类单体的影响。催化剂表征结果证明,不同结构性质的载体会影响Mo基催化剂结构、表面Mo5+含量和酸性位分布。与Al2O3和SiO2相比,黏土基载体具有更多的强酸位,不利于木质素油(LO)的生成,形成更多的固体产物。仅使用载体获得的LO中得到的石油醚溶性产物(PEsp)主要为烷基/烷氧基取代苯酚,而Mo基催化剂中Mo物种(尤其是Mo5+)显著提高了LO和PEsp的产量。Mo/SiO2表面Mo5+物种最多,LO产率最高,为85.2%,其中生成的烷基/烷氧基取代苯酚达450.3 mg/glignin。在黏土负载的Mo催化剂中,Mo/SEP的LO产率(82.3%)和PEsp产率(70.8%)较高,所获得取代酚达到398.8 mg/glignin。本研究系统地报道了绿色环保型黏土基材料在木质素转化中的应用,为黏土基材料在生物质转化中的应用提供了关键信息。Abstract: Catalytic lignin depolymerization (CCLD) for liquid fuels and phenolic monomers was investigated over various supports including clays (e.g., sepiolite (SEP), attapulgite (ATP), and montmorillonite (MTM)), and oxides (e.g., Al2O3 and SiO2) as well as their supported Mo-based catalysts under supercritical ethanol. The characterization results demonstrated that different supports with diverse structural properties could affect the textural structures, surface Mo5+ content, and acid sites distribution. Clay-based supports had more strong acid sites as compared with Al2O3 and SiO2, which went against the production of lignin oil (LO) and led to form more solid products during CLD experiments. Meanwhile, the obtained petroleum ether-soluble product (PEsp) in LO catalyzed by sole supports was mainly alkyl/alkoxy substituted phenols. Additionally, Mo species (especially Mo5+) significantly increased the yields of LO and PEsp. Mo/SiO2 had the highest surface Mo5+ species, showing the highest LO yield of 85.2%, in which the produced alkyl/alkoxy substituted phenols reached 450.3 mg/glignin. Among the clay-supported Mo catalysts, Mo/SEP presented superior LO (82.3%) and PEsp (70.8%) yields and the generated substituted phenols reached 398.8 mg/glignin. This paper systematically reported the application of green and environmentally friendly clay-based materials in lignin conversion, which provides some key information for the development of clay catalysts for biomass conversion.
-
Key words:
- kraft lignin /
- catalytic depolymerization /
- lignin oil /
- Mo-based catalysts /
- clay materials /
- phenolic monomers
-
Table 1 Ultimate and proximate analysis of lignin
Ultimate analysis w/% Proximate analysis w/% C H O N S M A V FC 57.84 4.63 22.98 0.89 2.28 5.78 4.05 55.79 34.38 Table 2 Textural properties of supports and Mo-based catalysts
Sample SBET /(m2·g−1)* v /(cm3·g−1)** d /nm** NH3 monolayer uptake /(mol NH3·g−1 sample)*** SEP 9.346 0.060 18.7 1443.2 Mo/SEP 5.632 0.037 25.6 1268.7 ATP 127.571 0.449 15.8 5593.4 Mo/ATP 6.904 0.056 22.4 1195.3 MTM 64.227 0.134 5.7 3044.0 Mo/MTM 8.961 0.052 10.1 1462.2 Al2O3 92.224 0.226 6.1 777.5 Mo/Al2O3 34.835 0.099 7.6 679.9 SiO2 234.081 1.647 30.3 1176.1 Mo/SiO2 65.317 0.530 27.9 1040.4 * SBET was calculated using the BET method;
** v and d were calculated using the BJH method based on the desorption isotherms;
*** data was obtained from NH3-TPD analysisTable 3 Yields of identified monomer products in PEsp obtained over various supports
SN Monomer name ${\rm{Yields} } /({\rm{mg} }\cdot{\rm{g} }_{ {\rm{lignin} } }^{ - 1})$ PEsp-a PEsp-b PEsp-c PEsp-d PEsp-e 2 1,2-dimethoxy-4-(1-methoxyethenyl) benzene 10.2 13.3 3.8 − 14.3 4 phenol,3-(1,1-dimethylethyl)-4-methoxy- 11.4 12.8 − − 13.9 6 2-hexenoic acid, ethyl ester 4.4 4.2 5.8 6.6 − 8 1,6-heptadien-4-ol 3.9 4.4 6.5 5.7 4.3 9 phenol,2-methoxy- 24.4 30.1 27.6 14.3 32.5 11 octanoic acid, ethyl ester 3.0 − 3.5 − − 13 phenol,2-ethoxy- 8.7 5.5 9.7 30.0 5.1 14 phenol,4-methoxy-3-methyl- 27.0 21.1 29.0 13.3 21.2 15 ethyl P-hydroxybenzoate 3.1 3.4 3.9 3.2 3.5 16 benzene,1-ethoxy-4-methoxy- 3.1 3.2 4.1 7.9 6.2 17 2-pentenoic acid,4-methyl-, ethyl ester, 3.4 3.6 3.0 3.1 − 18 2-ethoxy-4-methylphenol 5.1 4.5 6.7 9.7 − 20 phenol,4-ethyl-2-methoxy- 15.9 21.7 15.8 13.2 12.2 23 P-cymene-2,5-diol 3.1 4.0 3.8 5.4 3.3 24 phenol,2-methoxy-4-propyl- 9.8 11.4 7.9 5.99 12.54 25 durohydroquinone − − − 6.1 − 26 1,4-benzenediol,2,3,5-trimethyl- − − − 2.6 − 27 propofol − − − 3.0 − 28 benzene,2-(1,1-dimethylethyl)-1,4-methoxyl- − − − 3.1 − 29 phenol,3,5-bis(1,1-dimethylethyl)- − − − 4.9 − 31 benzene,1,4-dimethoxy-2-methyl-5-isopropyl- − − − 2.8 − Aliphatic oxygenates 14.7 12.2 18.8 15.4 4.3 Alkyl and oxyalkylated benzenes 13.3 16.5 7.9 13.8 20.5 Alkyl and alkoxy substituted phenols 108.5 114.5 104.2 111.6 104.2 Total 136.5 143.2 130.9 140.8 129.0 Table 4 Yields of identified monomer products in PEsp obtained over various Mo-based catalysts
SN Monomer name Yields/(mg·${\rm{g} }_{ {\rm{lignin} } }^{ - 1}$) PEsp-A PEsp-B PEsp-C PEsp-D PEsp-E 1 pentanoic acid,3-methyl-, ethyl ester 20.4 4.8 11.4 22.4 22.2 3 2-hexenoic acid, ethyl ester 76.2 6.9 45.6 37.8 78.0 5 3-hexenoic acid, ethyl ester 71.6 10.1 35.2 33.9 73.2 7 6-hepten-1-ol,5-methyl- 8.0 4.5 3.3 9.3 10.3 9 phenol,2-methoxy- 34.8 17.3 14.8 23.1 32.5 10 octanoic acid,2-ethyl-, ethyl ester 36.0 16.1 9.3 34.1 18.7 11 octenoic acid, ethyl ester 50.4 12.7 11.5 53.3 30.6 12 phenol,2-ethoxyl- 35.9 18.4 16.4 23.7 34.5 13 phenol,3-ethoxyl- 9.3 8.4 2.7 13.9 6.9 16 benzene,1-ethoxy-4-methoxy- 16.5 18.8 5.0 35.9 17.2 17 2-pentenoic acid,4-methyl-, ethyl ester, 19.8 6.9 4.8 23.5 14.6 19 2,5-diethylphenol 6.1 − 3.4 9.9 7.0 21 1,4-benzenediol,2,6-dimethyl- 7.8 4.0 4.7 12.1 6.4 22 phenol,2-(1,1-dimethylethyl)-6-methyl- 15.1 11.1 6.2 19.3 16.2 27 propofol 41.9 26.7 30.7 83.8 64.9 29 phenol,3,5-bis(1,1-dimethylethyl)- 16.9 15.5 13.6 35.0 28.9 30 phenol,2,6-bis(1,1-dimethylethyl)- 182.5 96.8 46.7 200.7 189.9 32 phenol,2,4,6-tris(1-methylethyl)- 15.6 16.5 8.5 24.8 22.4 33 4-tert-butyl-2,6-diisopropylphenol 32.9 30.4 17.6 49.6 40.7 Aliphatic oxygenates 282.4 62.0 121.1 214.3 247.6 Alkyl and oxyalkylated benzenes 16.5 18.8 5.0 35.9 17.2 Alkyl and alkoxy substituted phenols 398.8 245.1 165.3 496.2 450.3 Total 697.7 325.9 291.4 746.4 715.1 -
[1] ZHAO B, WU K, ZHONG L P, WEI G, HU Z H, ZHENG, W G, RUAN H F, YAN X M, MA Y, WANG B, JIANG T L, ZHANG H Y. Experimental study on catalytic pyrolysis of lignin under char and ZSM-5 for preparation of aromatics[J]. J Fuel Chem Technol,2021,49(3):304−311. doi: 10.1016/S1872-5813(21)60015-4 [2] ZAKZESKI J, BRUIJNINCX P C A, JONGERIUS A L, WECKHUYSEN B M. The catalytic valorization of lignin for the production of renewable chemicals[J]. Chem Rev,2010,110(6):3552−3599. doi: 10.1021/cr900354u [3] LI H J, BUNRIT A, LI N, WANG F. Heteroatom-participated lignin cleavage to functionalized aromatics[J]. Chem Soc Rev,2020,49(12):3748−3763. doi: 10.1039/D0CS00078G [4] CHEN M Q, SHI J J, WANG Y S, TANG Z Y, YANG Z L, WANG J, ZHANG H. Conversion of Kraft lignin to phenol monomers and liquid fuel over trimetallic catalyst W-Ni-Mo/sepiolite under supercritical ethanol[J]. Fuel,2021,303:121332. doi: 10.1016/j.fuel.2021.121332 [5] NGUYEN L T, PHAN D P, SARWAR A, TRAN M H, LEE O K, LEE E Y. Valorization of industrial lignin to value-added chemicals by chemical depolymerization and biological conversion[J]. Ind Crop Prod,2021,161:113219. doi: 10.1016/j.indcrop.2020.113219 [6] OUYANG X P, TAN Y D, QIU X Q. Oxidative degradation of lignin for producing monophenolic compounds[J]. J Fuel Chem Technol,2014,42(6):677−682. doi: 10.1016/S1872-5813(14)60030-X [7] DOU X M, LI W Z, ZHU C F. Catalytic hydrotreatment of Kraft lignin into liquid fuels over porous ZnCoOx nanoplates[J]. Fuel,2021,283:118801. doi: 10.1016/j.fuel.2020.118801 [8] DOU X M, LI W Z, ZHU C F, JIANG X. Catalytic waste Kraft lignin hydrodeoxygenation to liquid fuels over a hollow Ni-Fe catalyst[J]. Appl Catal B: Environ,2021,287:119975. doi: 10.1016/j.apcatb.2021.119975 [9] WANG J D, LI W Z, WANG H Z, MA Q Z, LI S, CHANG H M, JAMEEL H. Liquefaction of kraft lignin by hydrocracking with simultaneous use of a novel dual acid-base catalyst and a hydrogenation catalyst[J]. Bioresour Technol,2017,243:100−106. doi: 10.1016/j.biortech.2017.06.024 [10] LI W Z, DOU X M, ZHU C F, WANG J D, CHANG H M, JAMEEL H, LI X S. Production of liquefied fuel from depolymerization of kraft lignin over a novel modified nickel/H-beta catalyst[J]. Bioresour Technol,2018,269:346−354. doi: 10.1016/j.biortech.2018.08.125 [11] YU Y X, XU Y, WANG T J, MA L L, ZHANG Q, ZHANG X H, ZHANG X. In-situ hydrogenation of lignin depolymerization model compounds to cyclohexanol[J]. J Fuel Chem Technol,2013,41(4):443−448. doi: 10.1016/S1872-5813(13)60023-7 [12] WU Y S, LIN Z X, HU X, GHOLIZADEH M, SUN H Q, HUANG Y, ZHANG S, ZHANG H. Hydrogenolysis of lignin to phenolic monomers over Ru based catalysts with different metal-support interactions: Effect of partial hydrogenation of C(sp2)-O/C[J]. Fuel,2021,302:121184. doi: 10.1016/j.fuel.2021.121184 [13] LONG J X, XU Y, WANG T J, ZHANG X H, ZHANG Q, MA L L, LI Y P. Catalytic depolymerization and hydrogenolysis of lignin[J]. Adv New Renewable Energy,2014,2(2):83−88. [14] XIAO L P, WANG S Z, LI H L, LI Z W, SHI Z J, XIAO L, SUN R C, FANG Y M, SONG G Y. Catalytic hydrogenolysis of lignins into phenolic compounds over carbon nanotube supported molybdenum oxide[J]. ACS Catal,2017,7(11):7535−7542. doi: 10.1021/acscatal.7b02563 [15] DU B Y, LIU C, WANG X, HAN Y, GUO Y Z, LI H M, ZHOU J H. Renewable lignin-based carbon nanofiber as Ni catalyst support for depolymerization of lignin to phenols in supercritical ethanol/water[J]. Renewable Energy,2020,147:1331−1339. doi: 10.1016/j.renene.2019.09.108 [16] CHEN M Q, LU H T, WANG Y S, TANG Z Y, ZHANG J H, WANG C S, YANG Z L, WANG J, ZHANG H. Effect of reduction treatments of Mo/sepiolite catalyst on lignin depolymerization under supercritical ethanol[J]. Energy Fuels,2020,34(3):3394−3405. doi: 10.1021/acs.energyfuels.9b04533 [17] HUANG X M, KORÁNYI T I, BOOT M D, HENSEN E J M. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics[J]. Green Chem,2015,17:4941. doi: 10.1039/C5GC01120E [18] HUANG X M, ATAY C, KORÁNYI T I, BOOT M D, HENSEN E J M. Role of Cu-Mg-Al mixed oxide catalysts in lignin depolymerization in supercritical ethanol[J]. ACS Catal,2015,5(12):7359−7370. doi: 10.1021/acscatal.5b02230 [19] KORÁNYI T I, HUANG X M, COUMANS A E, HENSEN E J M. Synergy in lignin upgrading by a combination of Cu-based mixed oxide and Ni-phosphide catalysts in supercritical ethanol[J]. ACS Sustainable Chem Eng,2017,5(4):3535−3543. doi: 10.1021/acssuschemeng.7b00239 [20] WANG Y S, TANG Z Y, CHEN M Q, ZHANG J H, SHI J J, WANG C S, YANG Z L, WANG J. Effect of Mo content in Mo/Sepiolite catalyst on catalytic depolymerization of Kraft lignin under supercritical ethanol[J]. Energy Convers Manage,2020,222:113227. doi: 10.1016/j.enconman.2020.113227 [21] HUANG X M, KORÁNYI T I, BOOT M D, HENSEN E J M. Catalytic depolymerization of lignin in supercritical ethanol[J]. ChemSusChem,2014,7(8):2276−2288. doi: 10.1002/cssc.201402094 [22] JEONG S, JANG G H, KIM D H. Depolymerization of protobind lignin using MO-MgAlOy mixed oxide catalysts (M=Co, Ni and Cu) in supercritical ethanol[J]. Top Catal,2017,60:637−643. doi: 10.1007/s11244-017-0787-z [23] JEONG S, YANG S, KIM D H. Depolymerization of protobind lignin to produce monoaromatic compounds over Cu/ZSM-5 catalyst in supercritical ethanol[J]. Mol Catal,2017,442:140−146. doi: 10.1016/j.mcat.2017.09.010 [24] CHEN M Q, ZHANG J H, WANG Y S, TANG Z Y, SHI J J, WANG C S, YANG Z L, WANG J, ZHANG H. Lignin catalytic depolymerization for liquid fuel and phenols by using Mo/sepiolite catalysts calcined at different temperature[J]. J Environ Chem Eng,2021,9(4):105348. doi: 10.1016/j.jece.2021.105348 [25] TANG Z Y, WANG Y S, CHEN M Q, ZHANG J H, WANG C S, YANG Z L, ZHANG H, WANG J. Study of Mo-based sepiolite catalyst on depolymerization of lignin under supercritical ethanol[J]. Int J Energy Res,2020,44(1):257−268. doi: 10.1002/er.4901 [26] PRASOMSRI T, SHETTY M, MURUGAPPAN K, ROMÁN-LESHKOV Y. Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures[J]. Energy Environ Sci,2014,7(8):2660−2669. doi: 10.1039/C4EE00890A [27] ZHANG X H, CHEN Q, ZHANG Q, WANG C G, MA L L, XU Y. Conversion of pyrolytic lignin to aromatic hydrocarbons by hydrocracking over pristine MoO3 catalyst[J]. J Anal Appl Pyrolysis,2018,135:60−66. doi: 10.1016/j.jaap.2018.09.020 [28] PRASOMSRI T, NIMMANWUDIPONG T, ROMAN-LESHKOV Y. Effective hydrodeoxygenation of biomass-derived oxygenates into unsaturated hydrocarbons by MoO3 using low H2 pressures[J]. Energy Environ Sci,2013,6:1732−1738. doi: 10.1039/c3ee24360e [29] SHETTY M, ANDERSON E M, GREEN W H, ROMÁN-LESHKOV Y. Kinetic analysis and reaction mechanism for anisole conversion over zirconia-supported molybdenum oxide[J]. J Catal,2019,376:248−257. doi: 10.1016/j.jcat.2019.06.046 [30] HEWER T L R, SOUZA A G F, ROSENO K T C, MOREIRA P F, BONFIM R, ALVES R M B, SCHMAL M. Influence of acid sites on the hydrodeoxygenation of anisole with metal supported on SBA-15 and SAPO-11[J]. Renewable Energy,2018,119:615−624. doi: 10.1016/j.renene.2017.12.044 [31] RANGA C, ALEXIADIS V I, LAUWAERT J, LØDENG R, THYBAUT J W. Effect of Co incorporation and support selection on deoxygenation selectivity and stability of (Co)Mo catalysts in anisole HDO[J]. Appl Catal A: Gen,2019,571:61−70. doi: 10.1016/j.apcata.2018.12.004 [32] WU Z, ZHANG J, ZHAO X X, LI X, ZHANG Y, WANG F. Attapulgite-supported magnetic dual acid–base catalyst for the catalytic conversion of lignin to phenolic monomers[J]. J Chem Technol Biot,2019,94(4):1269−1281. doi: 10.1002/jctb.5881 [33] WU Z, ZHANG J, PAN Q Q, LI X, ZHANG Y, WANG F. Catalytic alcoholysis of alkaline extracted lignin for the production of aromatic esters over SO42-/ZrO2-ATP[J]. RSC Adv,2018,8:12344−12353. doi: 10.1039/C8RA00815A [34] BHATTI U H, SULTAN H, MIN G H, NAM S C, BAEK I H. Ion-exchanged montmorillonite as simple and effective catalysts for efficient CO2 capture[J]. Chem Eng J,2021,413:127476. doi: 10.1016/j.cej.2020.127476 [35] WANG Z H, JIAO M Y, CHEN Z P, HE H, LIU L C. Effects of montmorillonite and anatase TiO2 support on CeO2 catalysts during NH3-SCR reaction[J]. Microporous Mesoporous Mater,2021,320:111072. doi: 10.1016/j.micromeso.2021.111072 [36] WANG Y S, WANG C S, CHEN M Q, TANG Z Y, YANG Z L, HU J X, ZHANG H. Hydrogen production from steam reforming ethanol over Ni/attapulgite catalysts - Part I: Effect of nickel content[J]. Fuel Process Technol,2019,192:227−238. doi: 10.1016/j.fuproc.2019.04.031 [37] CHEN M Q, HU J X, WANG Y S, WANG C S, TANG Z Y, LI C, LIANG D F, CHENG W, YANG Z L, ZHANG H. Hydrogen production from acetic acid steam reforming over Ti-modified Ni/Attapulgite catalysts[J]. Int J Hydrog Energy,2021,46(5):3651−3668. doi: 10.1016/j.ijhydene.2020.10.196 [38] ZHANG X H, TANG J J, ZHANG Q, LIU Q Y, LI Y P, CHEN L G, WANG C G, MA L L. Hydrodeoxygenation of lignin-derived phenolic compounds into aromatic hydrocarbons under low hydrogen pressure using molybdenum oxide as catalyst[J]. Catal Today,2019,319:41−47. doi: 10.1016/j.cattod.2018.03.068 [39] CUI K, YANG L, MA Z W, YAN F, WU K, SANG Y S, CHEN H, LI Y D. Selective conversion of guaiacol to substituted alkylphenols in supercritical ethanol over MoO3[J]. Appl Catal A: Gen,2017,219:592−602. [40] MA X L, MA R, HAO W Y, CHEN M M, YAN F, CUI K, TIAN Y, LI Y D. Common pathways in ethanolysis of Kraft lignin to platform chemicals over molybdenum-based catalysts[J]. ACS Catal,2015,5(8):4803−4813. doi: 10.1021/acscatal.5b01159 [41] KIM S M, LEE Y J, BAE J W, POTDAR H S, JUN K W. Synthesis and characterization of a highly active alumina catalyst for methanol dehydration to dimethyl ether[J]. Appl Catal A: Gen,2008,348:113−120. doi: 10.1016/j.apcata.2008.06.032 [42] PAPAGERIDIS K N, CHARISIOU N D, DOUVARTZIDES S, SEBASTIAN V, HINDER S J, BAKER M A, ALKHOORI A A, ALKHOORI S I, POLYCHRONOPOULOU K, GOULA M A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3-Al2O3[J]. RSC Adv,2021,11:8569−8584. doi: 10.1039/D0RA08541C [43] CHEN M M, HAO W Y, MA R, MA X L, YANG L, YAN F, CUI K, CHEN H, LI Y D. Catalytic ethanolysis of Kraft lignin to small-molecular liquid products over an alumina supported molybdenum nitride catalyst[J]. Catal Today,2017,298:9−15. doi: 10.1016/j.cattod.2017.08.012 [44] CHEN M M, MA X L, MA R, WEN Z, YAN F, CUI K, CHEN H, LI Y D. Ethanolysis of Kraft lignin over a reduction modified MoO3 catalyst[J]. Ind Eng Chem Res,2017,56(47):14025−14033. doi: 10.1021/acs.iecr.7b03585 -





 下载:
下载: