Modification of the V2O5-WO3/TiO2 catalyst with Nb to reduce its activity for SO2 oxidation during the selective catalytic reduction of NOx
-
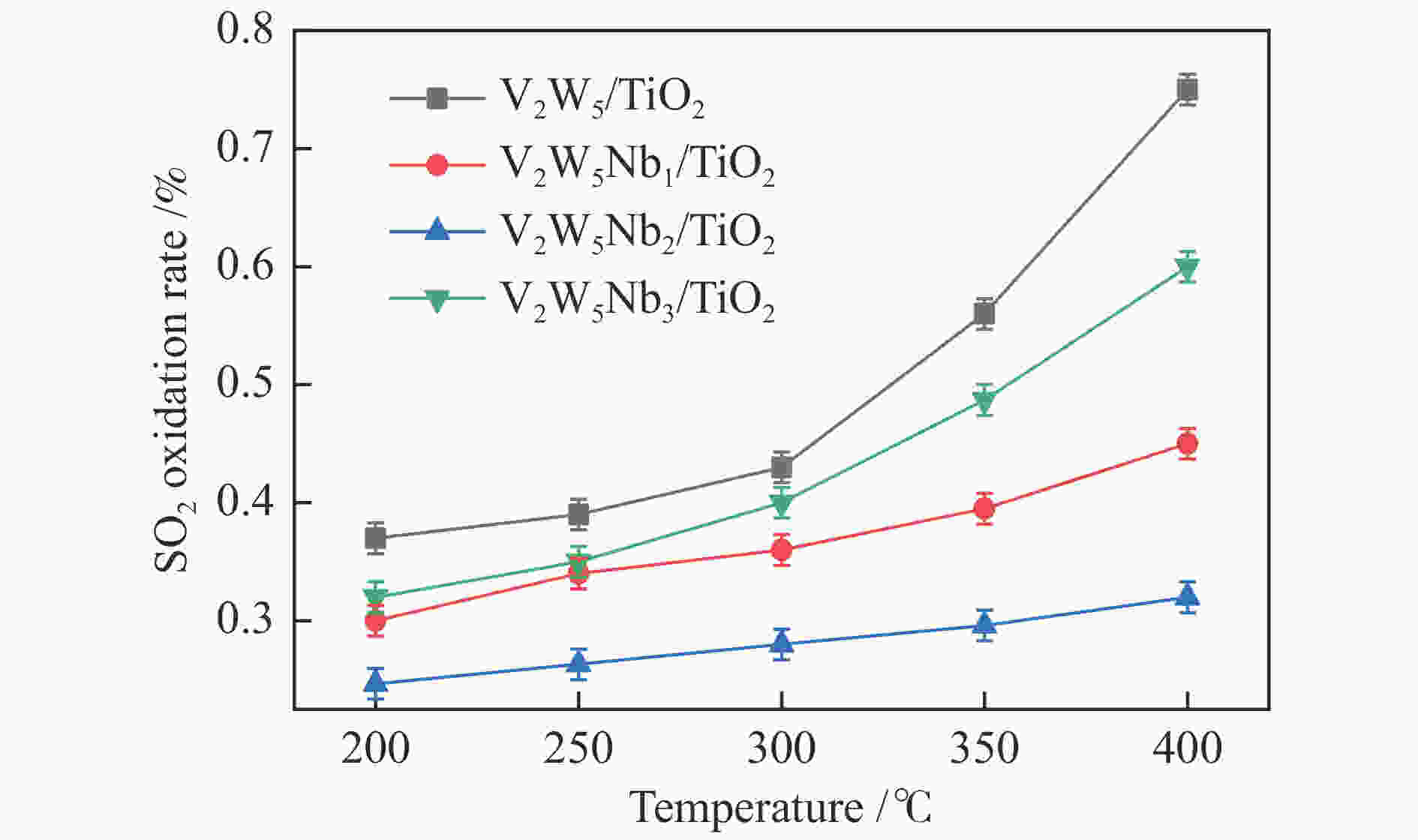
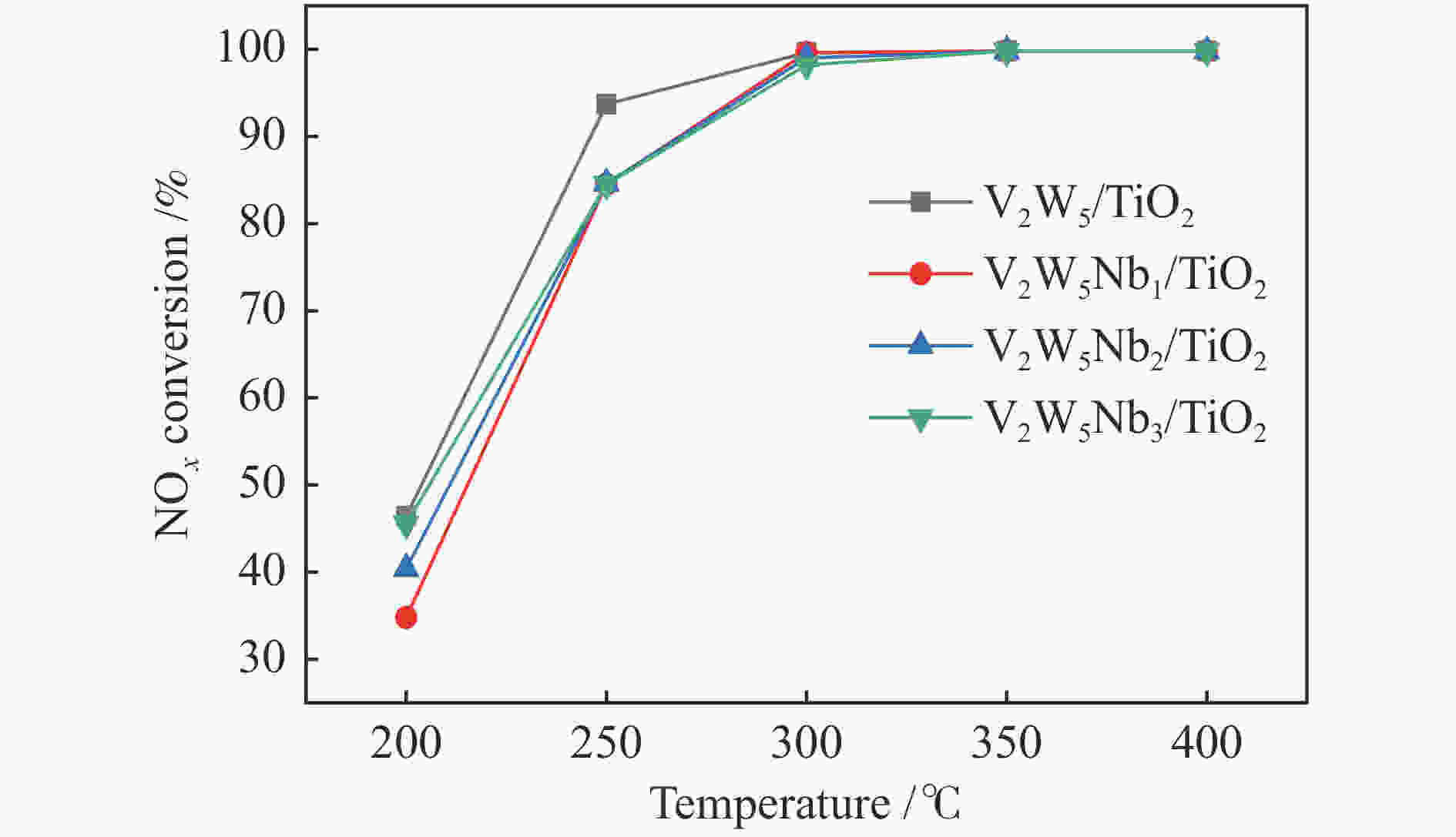
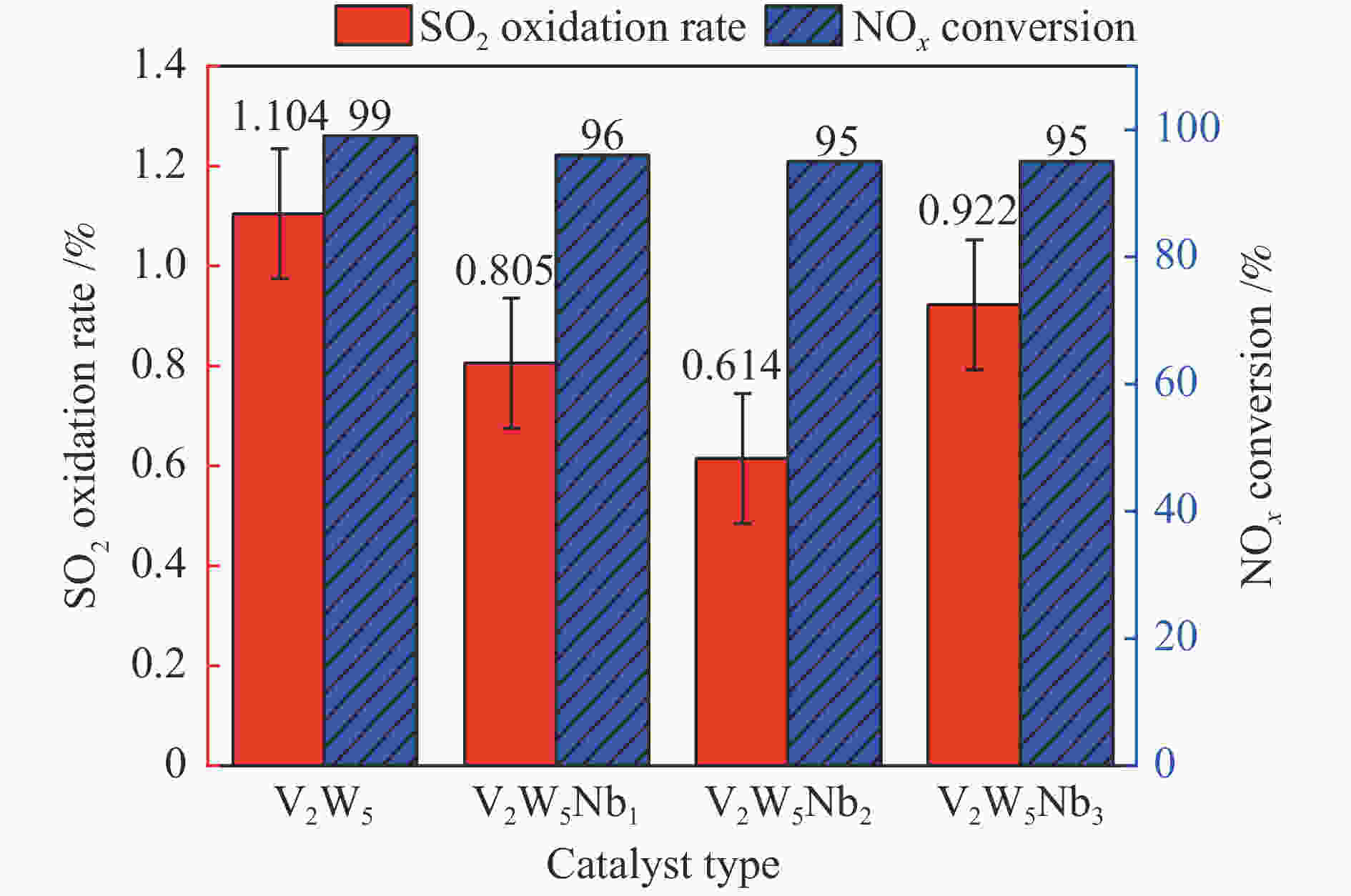
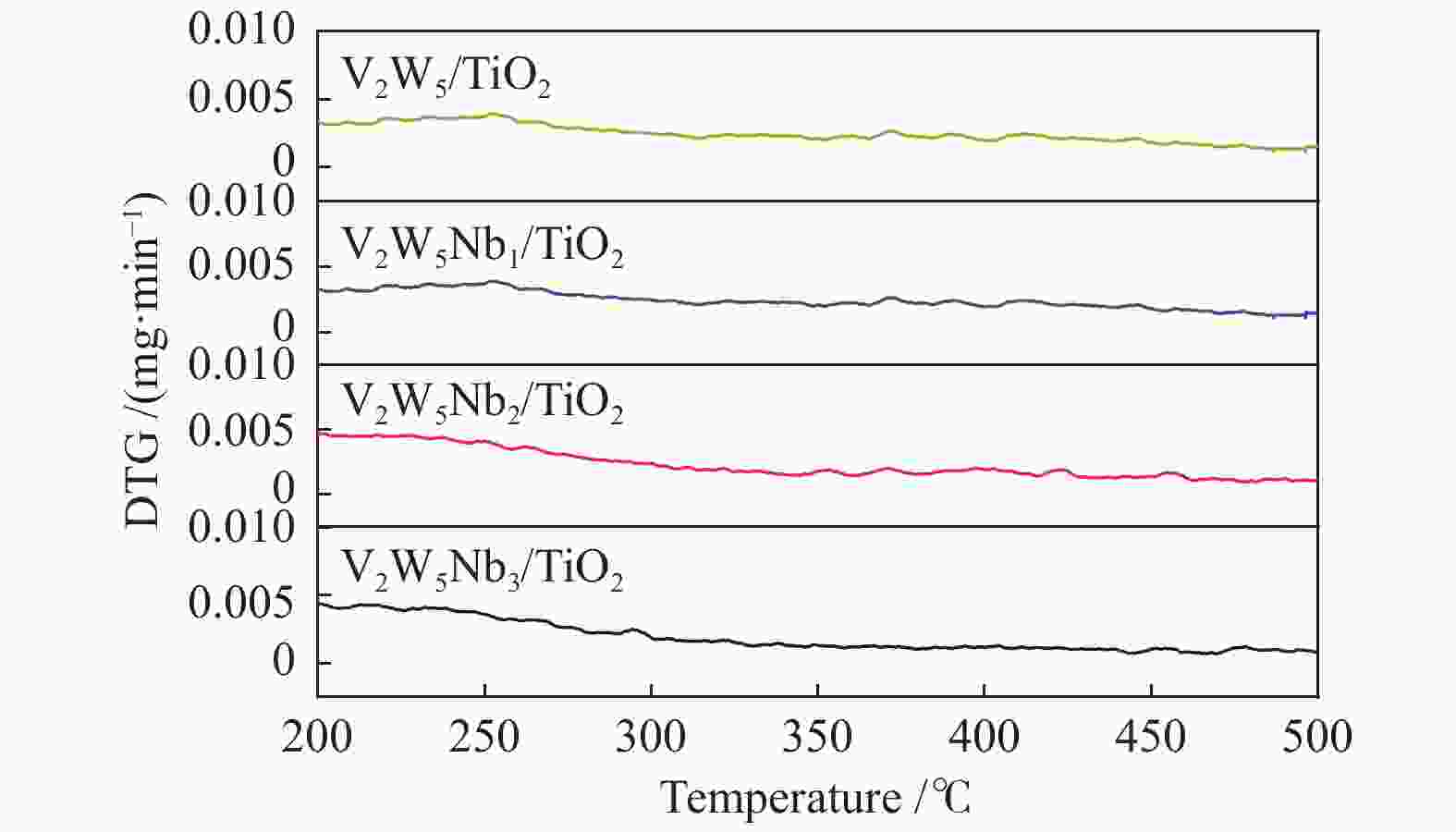
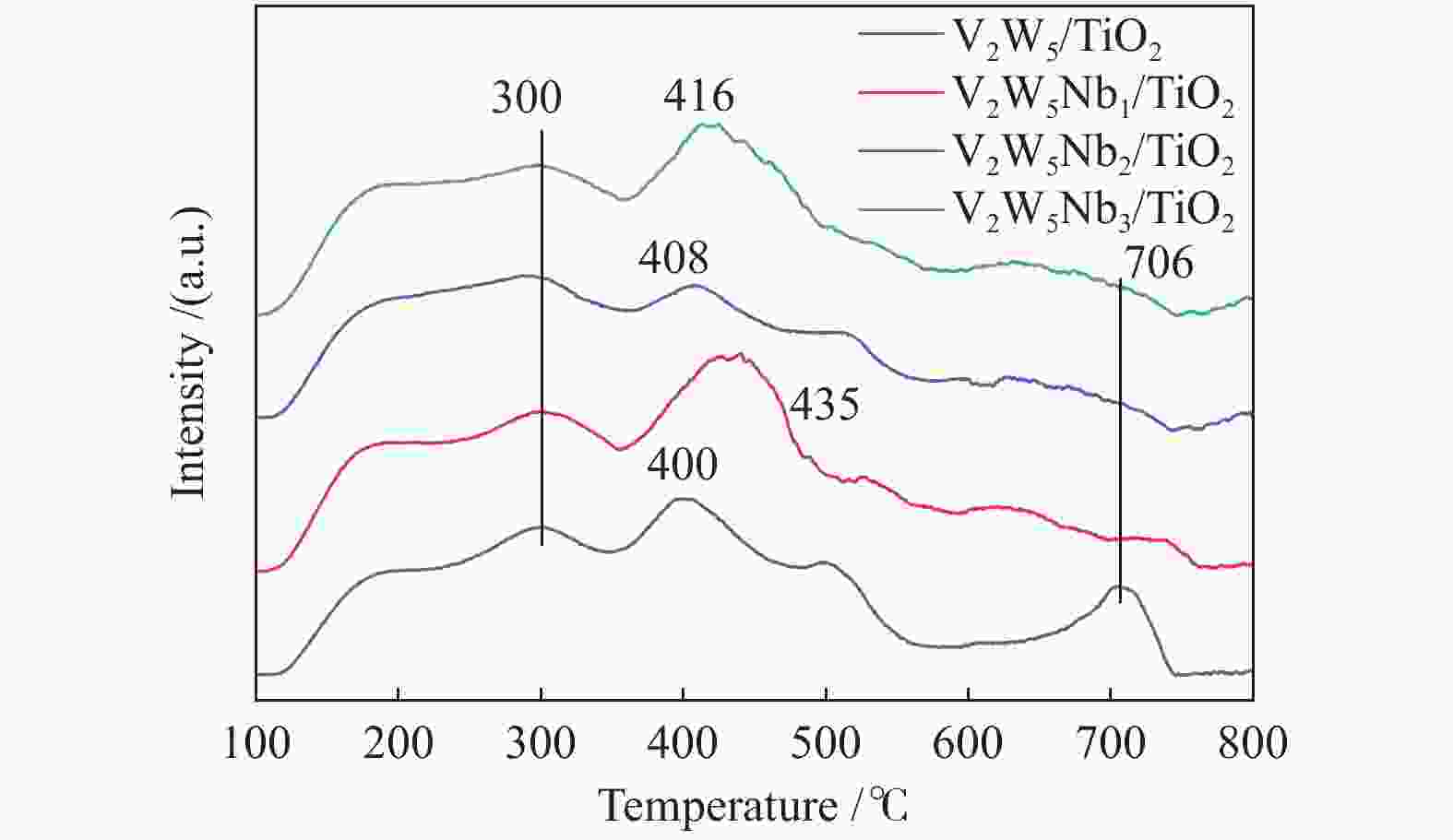
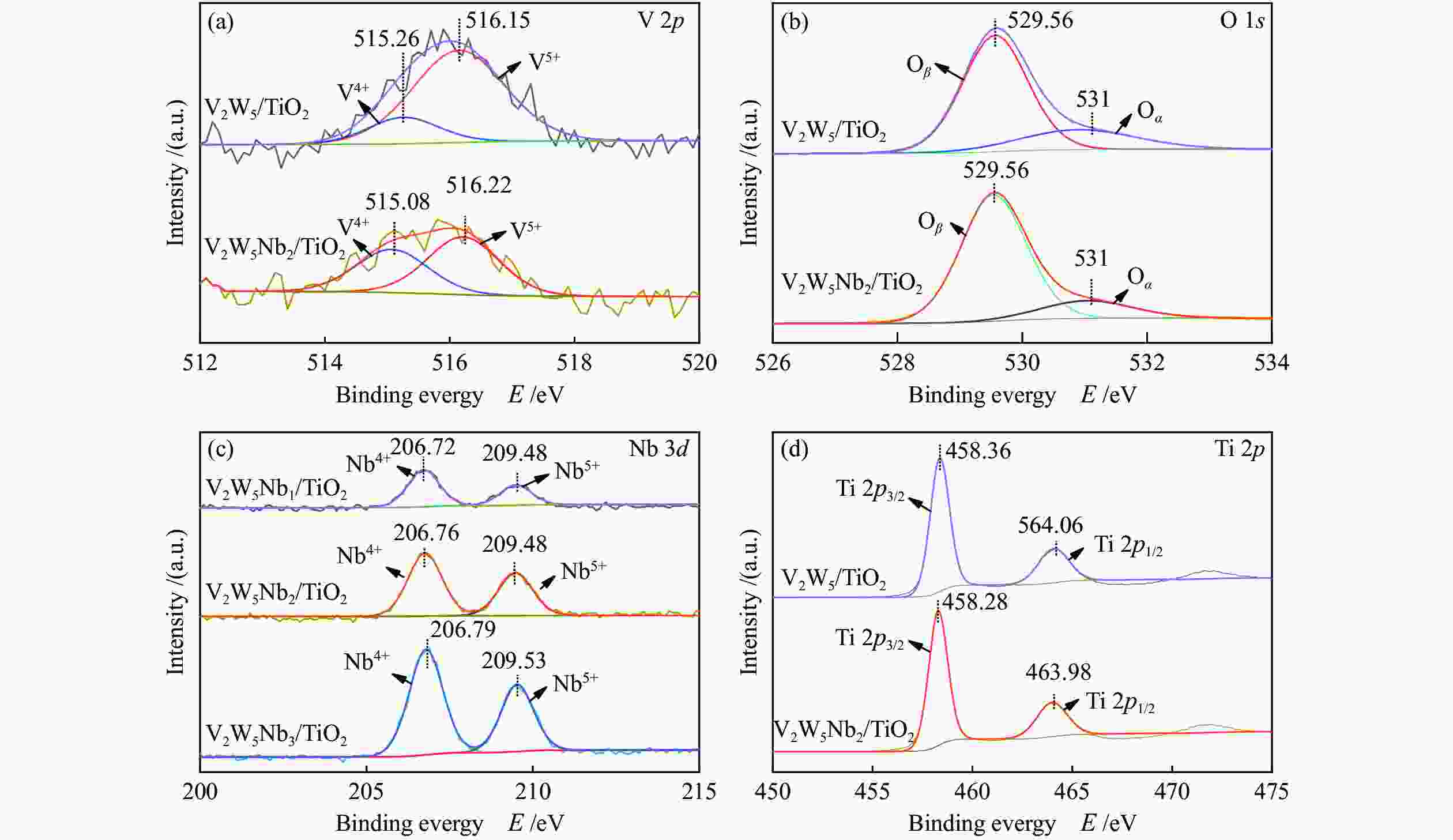
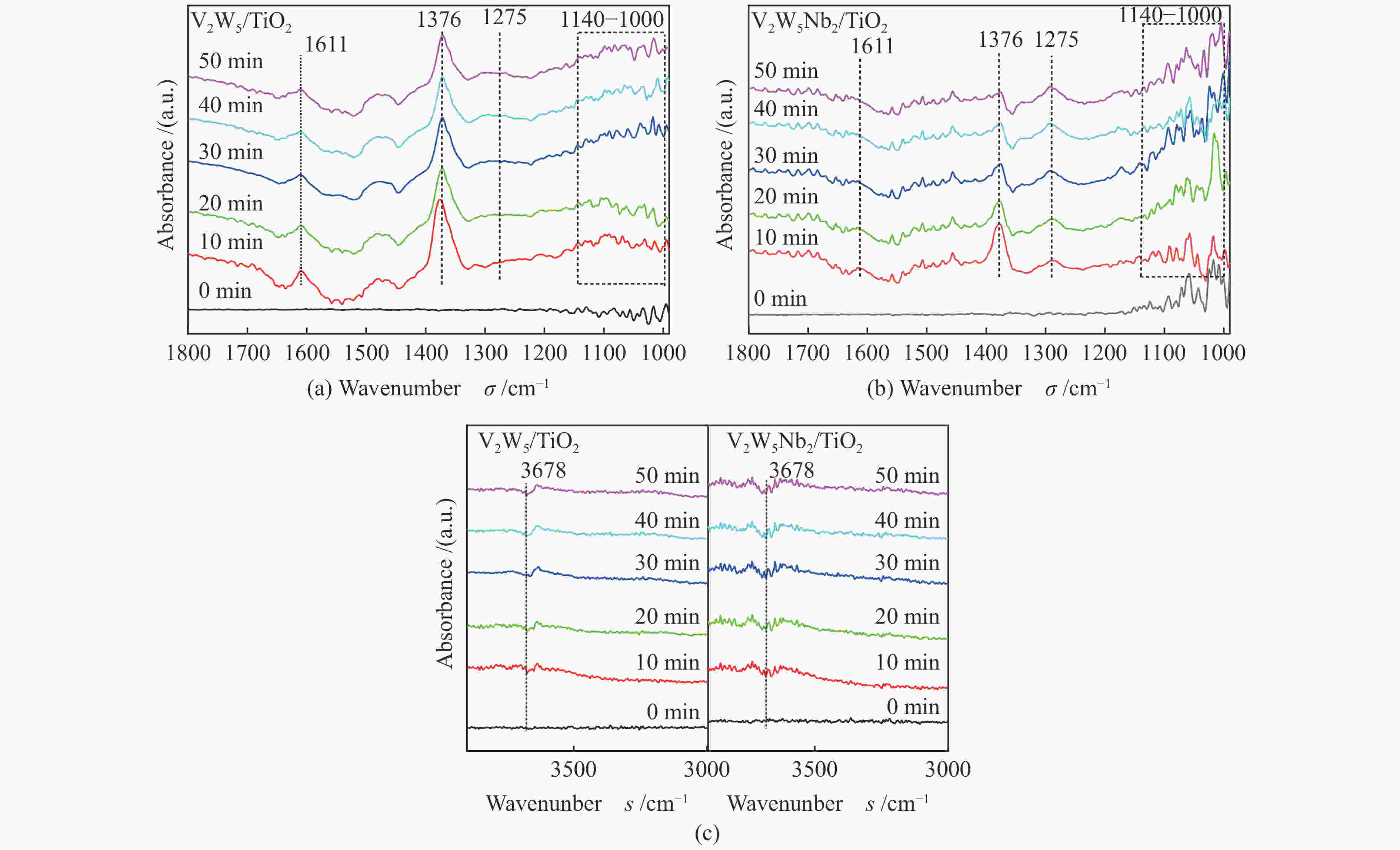
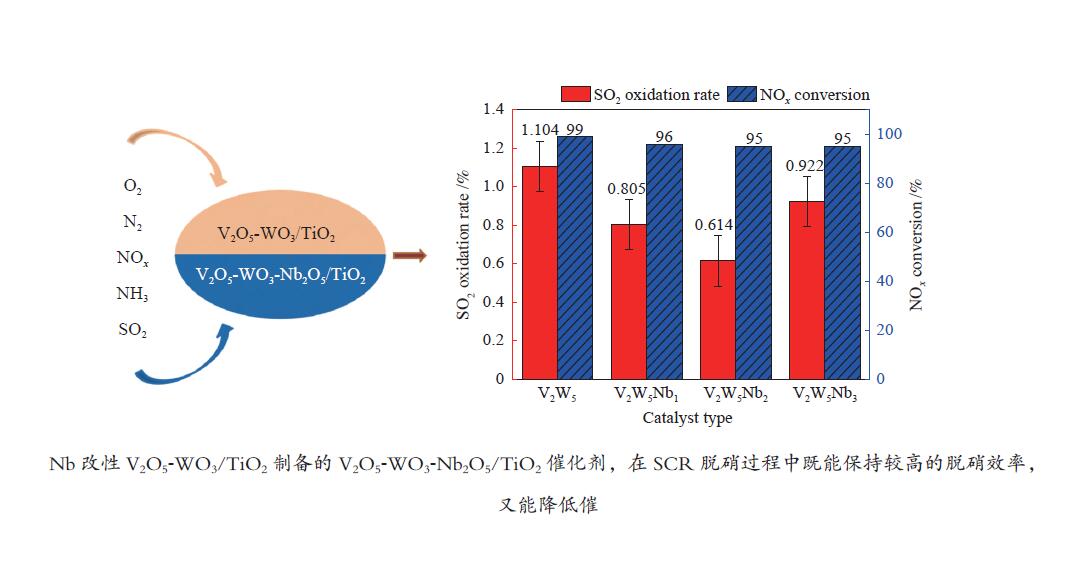
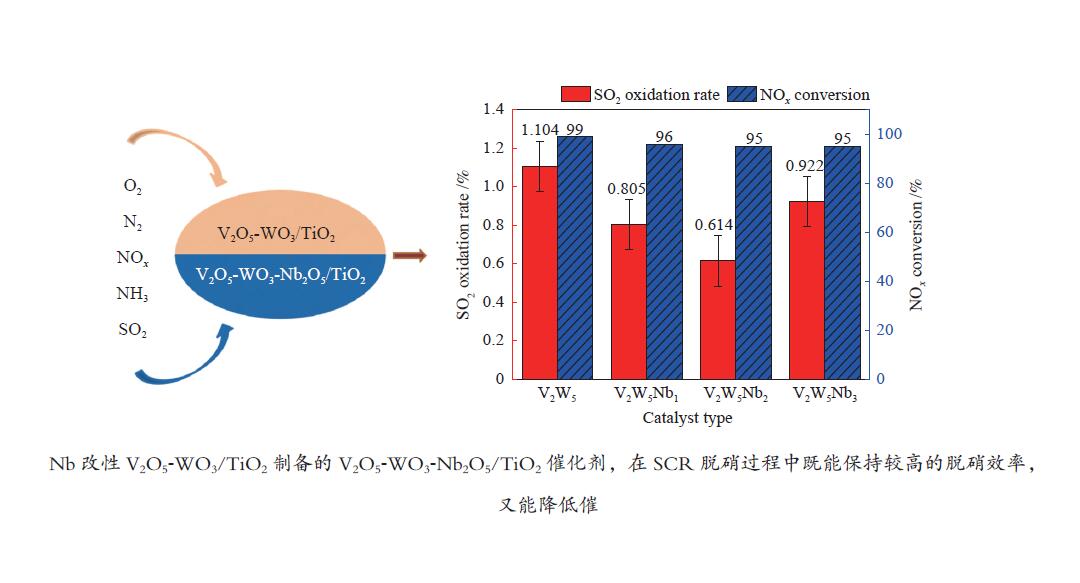
摘要: 本文采用浸渍法制备了Nb改性的V2O5-WO3/TiO2催化剂,研究了脱硝反应中Nb负载量对催化剂SO2氧化活性的影响。结果表明,在350 °C下,Nb2O5负载量为2%的Nb2O5-V2O5-WO3/TiO2催化剂上的SO2氧化率最低(0.6%),而同时NOx 的转化率仍能达到95%。采用TGA、氮吸附、XRD、H2-TPR、CO2-TPD、XPS和in- situ DRIFTS等对催化剂进行了表征分析,结果显示,Nb改性后V2O5-WO3/TiO2催化剂的晶体结构没有发生明显改变,但是其比表面积小幅度下降,有助于减少对SO2的吸附;同时,改性后催化剂表面的吸附氧含量下降,氧化还原性能也稍微减弱,这有利于降低其对SO2的氧化活性。in-situ DRIFTS结果表明,Nb改性后的Nb-V2O5-WO3/TiO2催化剂反应过程中表面中间产物VOSO4的含量明显下降,从而减少了SO3的生成量。
-
关键词:
- SO2氧化 /
- Nb改性 /
- V2O5-WO3/TiO2催化剂 /
- NH3-SCR脱硝
Abstract: A series of Nb-modified V2O5-WO3/TiO2 catalysts were prepared by the impregnation method and the effect of Nb loading on their SO2 oxidation activity during the selective catalytic reduction of NOx was investigated. The results indicate that the Nb2O5-V2O5-WO3/TiO2 catalyst with a Nb2O5 loading of 2% exhibits the lowest SO2 conversion of 0.6% for oxidation at 350 °C, whereas the conversion of NOx is still above 95%. The catalysts were characterized by TGA, BET, XRD, H2-TPR, CO2-TPD, XPS and in-situ DRIFTS. The results illustrate that the influence of Nb modification on the crystal structure of V2O5-WO3/TiO2 catalyst is rather insignificant; however, the surface area of the Nb2O5-V2O5-WO3/TiO2 catalyst decreases slightly after the modification with Nb, conducing to a decrease of SO2 adsorption on the catalyst. Meanwhile, the content of oxygen adsorbed on the catalyst surface decreases considerably upon the Nb modification, suggesting a weakened redox performance, which is beneficial to reducing the oxidation of SO2. The in-situ DRIFTS results illustrate that the content of the intermediate VOSO4 product on the catalyst surface decreases over the Nb-modified Nb2O5-V2O5-WO3/TiO2 catalyst, leading to a decrease of SO3 production.-
Key words:
- SO2 oxidation /
- Nb modification /
- V2O5-WO3/TiO2 /
- removal of NOx by NH3-SCR
-
表 1 实验所用标准气体
Table 1 The standard gas for the experiment
Gas Purity Producer N2 99.99% Sizhi Gas Co. LTD, Tianjin O2 99.99% Sizhi Gas Co. LTD, Tianjin NO 5% Sizhi Gas Co. LTD, Tianjin NH3 5% Sizhi Gas Co. LTD, Tianjin SO2 5% Haipu Gas Co. LTD, Beijing 表 2 样品的孔结构分析
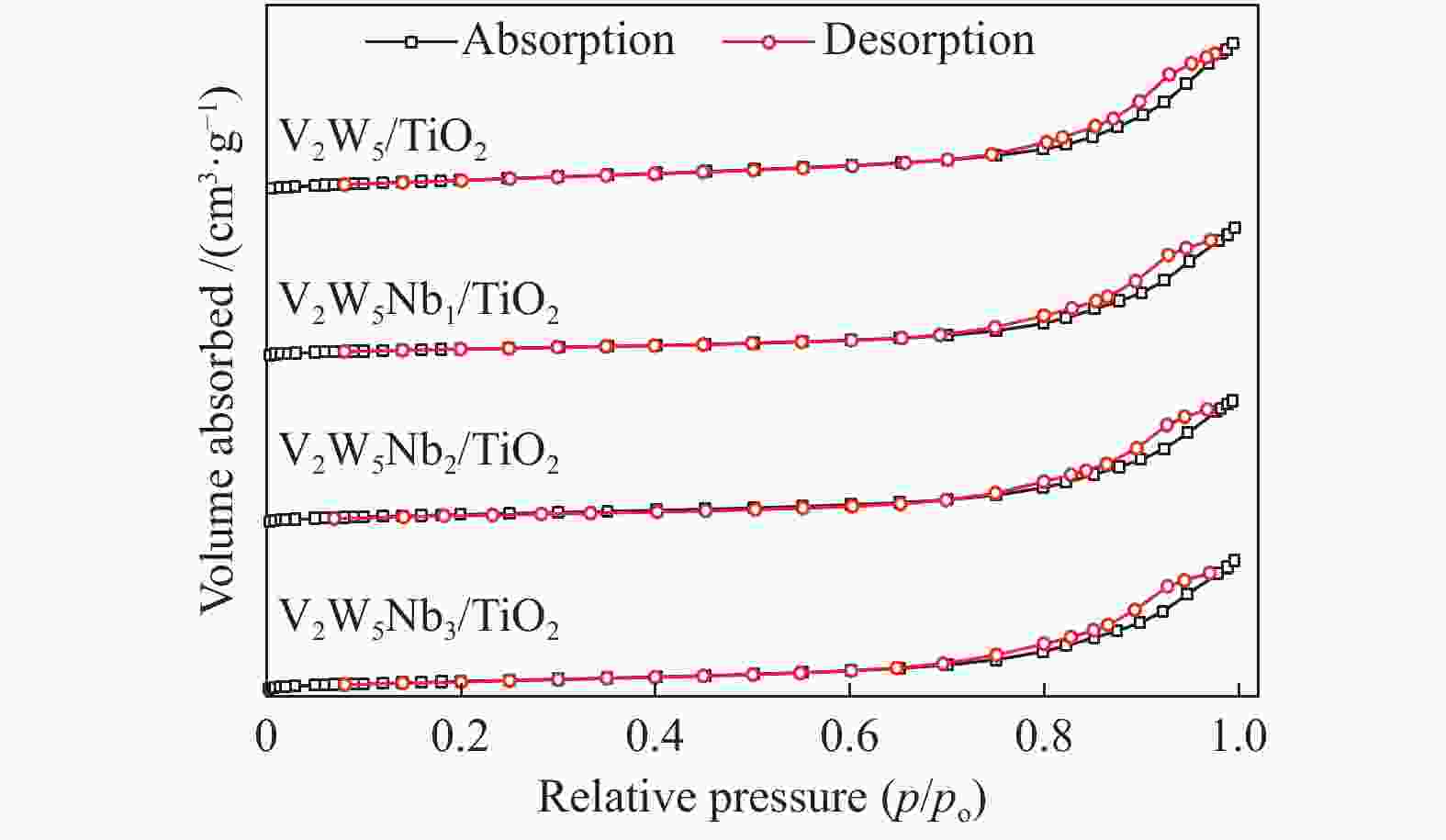
Table 2 Textural properties of various catalysts
Catalyst Surface area
A/(m2·g−1)Pore volume
v/(cm3·g−1)Pore size
d/nmV2W5/TiO2 129.2 0.494 15.31 V2W5Nb1/TiO2 100.68 0.38 17.39 V2W5Nb2/TiO2 111.4 0.417 14.97 V2W5Nb3/TiO2 111.9 0.441 15.72 表 3 催化剂表面组成分析
Table 3 Surface composition of various catalyst determined by XPS
Catalyst Surface atomic concentration / % Surface atomic ratio / % O V Ti W Nb V5+/(V5++V4+) O Oß Oα V2W5/TiO2 67.25 0.81 29.24 2.70 0 81.93 76.68 23.32 V2W5Nb2/TiO2 67.18 0.64 28.69 2.40 1.09 57.12 83.68 16.32 -
[1] 罗汉成, 潘卫国, 丁红蕾, 李付晓, 郭瑞堂, 金强, 丁承刚. 燃煤锅炉烟气中SO3的产生机理及其控制技术[J]. 锅炉技术,2015,46(6):69−72. doi: 10.3969/j.issn.1672-4763.2015.06.015LUO Hang-cheng, PAN Wei-guo, DING Hong-lei, LI Fu-xiao, GUO Rui-tang, JIN Qiang, DING Cheng-gang. Mechanism and control technology of SO3 in flue gas of coal-fired boiler[J]. Boiler Technol,2015,46(6):69−72. doi: 10.3969/j.issn.1672-4763.2015.06.015 [2] KAMATA H, OHARA H, TAKAHASHI K, YUKIMURA A, SEO Y. SO2 oxidation over the V2O5/TiO2 SCR catalyst[J]. Catal Lett,2001,73(1):79−83. doi: 10.1023/A:1009065030750 [3] 王飞. 燃煤电厂SO3抑制与脱除技术综述[J]. 科技资讯,2019,31:150−151.WANG Fei. Review of SO3 suppression and removal technologies in coal-fired power plants[J]. Sci Technol Inf,2019,31:150−151. [4] ZHANG Y, LAUMB J, LIGGETT R, HOLMES M, PAVLISH J. Impacts of acid gases on mercury oxidation cross SCR catalyst[J]. Fuel Process Technol,2007,88(10):929−934. doi: 10.1016/j.fuproc.2007.03.010 [5] 张道军, 马子然, 孙琦, 徐文强, 李永龙, 王宝东, 竹涛, 林德海, 季广辉, 马静. 硫酸氢氨在钒基选择性催化还原催化剂表面的生成、作用及防治[J]. 化工进展,2018,37(7):2635−2643.ZHANG Dao-jun, MA Zi-ran, SUN Qi, XU Wen-qiang, LI Yong-long, WANG Bao-dong, ZHU Tao, LIN De-hai, JI Guang-hui, MA Jing. Formation mechanism, effects and prevention of NH4HSO4 formed on the surface of V2O5 based catalysts[J]. Chem Ind Eng Prog,2018,37(7):2635−2643. [6] VEMOT E H, MACEWEN J D, HAUN C C, KINKEAD E R. Acute Toxicity and Skin Corrosion Data for Some Organic and Inorganic Compounds and Aqueous Solutions[J]. Toxicol Appl Pharmacol,1977,42(2):417−423. doi: 10.1016/0041-008X(77)90019-9 [7] CHEN M M, JIN Q J, TAO X J, PAN Y C, GU S S, SHEN Y S. Novel W-Zr-Ox/TiO2 catalyst for selective catalytic reduction of NO by NH3 at high temperature[J]. Catal Today,2020,358:254−262. doi: 10.1016/j.cattod.2019.06.045 [8] KOBAYASHI M, KUMA R, MASAKI S, SUGISHIMA N. TiO2-SiO2, and V2O5/TiO2-SiO2, catalyst: Physico-chemical characteristics and catalytic behavior in selective catalytic reduction of NO by NH3[J]. Appl Catal B: Environ, 2005, 60(3/4): 173–179. [9] CHOO S T, YIM S D, NAM I S, HAM S W, LEE J B. Effect of promoters including WO3 and BaO on the activity and durability of V2O5/sulfated TiO2 catalyst for NO reduction by NH3[J]. Appl Catal B: Environ,2003,44(3):237−252. doi: 10.1016/S0926-3373(03)00073-0 [10] HOU Y Q, WANG J C, LI Q Y, LIU Y J, BAI Y R, ZENG Z Q, HUANG Z G. Environmental-friendly production of FeNbTi catalyst with significant enhancement in SCR activity and SO2 resistance for NOx removal[J]. Fuel,2021,285:119133. doi: 10.1016/j.fuel.2020.119133 [11] CAO L, WU X D, CHEN Z, MA Y, MA Z R, RAN R, SI Z C, WENG D, WANG B D. A comprehensive study on sulfur tolerance of niobia modified CeO2/WO3-TiO2 catalyst for low-temperature NH3-SCR[J]. Appl Catal A: Gen,2019,580:121−130. doi: 10.1016/j.apcata.2019.05.007 [12] SAZONOVA N N, TSYKOZA L T, SIMAKOV A V, BARANNIK G B, ISMAGILOV Z R. Relationship between sulfur dioxide oxidation and selective catalytic NO reduction by ammonia on V2O5-TiO2 catalysts doped with WO3, and Nb2O5[J]. React Kinet Catal Lett,1994,52(1):101−106. doi: 10.1007/BF02129856 [13] AHN J, OKERLUND R, FRY A, EDDINGS E G. Sulfur trioxide formation during oxy-coal combustion[J]. Int J Greenhouse Gas Control,2011,5(12):127−135. [14] 张翰之. SCR催化法中SO2/SO3转化率的影响因素研究[D]. 保定: 华北电力大学, 2018.ZHANG Han-zhi. Influencing Factors of SO2/SO3 conversion rate in SCR catalytic process[D]. Baoding: North China Electric Power University, 2018. [15] REDDY B M, KHAN A, YAMADA Y, KOBAYASHI T, LORIDANT S, VOLTA J C. Raman and X-ray photoelectron spectroscopy study of CeO2-ZrO2 and V2O5/CeO2-ZrO2 catalysts[J]. Langmuir,2003,19:3025−3030. doi: 10.1021/la0208528 [16] 刘智. SO2在商用SCR催化剂表面氧化机理的研究[D]. 天津: 河北工业大学, 2019.LIU Zhi. Study on SO2 oxidation mechanism on commercial SCR catalysts surface[D]. Tianjin: Hebei University of Technology, 2019. [17] KANG T A, YOUN S, KIM D H. Improved catalytic performance and resistance to SO2 over V2O5-WO3/TiO2 catalyst physically mixed with Fe2O3 for low-temperature NH3-SCR[J]. Catal Today,2021,376:95−103. doi: 10.1016/j.cattod.2020.07.042 [18] 晁晶迪, 何洪, 宋丽云, 房玉娇, 梁全明, 张桂臻, 邱文革, 张然. Pr掺杂对V2W5-Mo3/TiO2催化剂NH3-SCR反应活性的影响[J]. 高等学校化学学报,2015,36(3):523−530.CHAO Jing-di, HE Hong, SONG Li-yun, FANG Yu-jiao, LIANG Quan-ming, ZHANG Gui-zhen, QIU Wen-ge, ZHANG Ran. Promotional effect of Pr-doping on the NH3-SCR activity over the V2O5-MoO3/TiO2 catalyst[J]. Chem J Chin Univ,2015,36(3):523−530. [19] ZHU L, ZHONG Z P, XUE J M, XU Y Y, WANG C H, WANG L X. NH3-SCR performance and the resistance to SO2 for Nb doped vanadium based catalyst at low temperatures[J]. J Environ Sci,2018,65:306−316. doi: 10.1016/j.jes.2017.06.033 [20] LIAN Z, LIU F, HE H, LIU K. Nb-doped VOx/CeO2 catalyst for NH3-SCR of NOx at low temperatures[J]. RSC Adv,2015,5(47):37675−37681. doi: 10.1039/C5RA02752G [21] WACHS I E, BRIAND L E, JEHNG J M, BURCHAM L, GAO X T. Molecular structure and reactivity of the group V metal oxides[J]. Catal Today,2000,57:323−330. doi: 10.1016/S0920-5861(99)00343-0 [22] MARSAL A, ROSSINYOL E, BIMBELA F, TELLEZ C, CORONAS J, CORENT A, MORANTE J R. Characterisation of LaOCl sensing materials using CO2-TPD, XRD, TEM and XPS[J]. Sens Actuators, B,2005,109(1):38−43. doi: 10.1016/j.snb.2005.03.022 [23] PI Z P, SHEN B X, ZHAO J G, LIU J C. CuO, CeO2 modified Mg-Al spinel for removal of SO2 from fluid catalytic cracking flue gas[J]. Ind Eng Chem Res,2015,54(43):10622−10628. doi: 10.1021/acs.iecr.5b02329 [24] LEE K M, LIM Y H, JO Y M. Evaluation of moisture effect on low-level CO2 adsorption by ion-exchanged zeolite[J]. Environ Technol,2012,33(1):77−84. doi: 10.1080/09593330.2011.551837 [25] YU Y K, MIAO J F, HE C, CHEN J S, LI C, DOUTHWAITE M. The remarkable promotional effect of SO2 on Pb-poisoned V2W5-WO3/TiO2 catalysts: An in-depth experimental and theoretical study[J]. Chem Eng J,2018,338:191−201. doi: 10.1016/j.cej.2018.01.031 [26] BOUDALI L K, GHORBEL A, GRANGE P. Characterization and reactivity of WO3-V2O5 supported on sulfated titanium pillared clay catalysts for the SCR-NO reaction[J]. Comptes Rendus Chim, 2009, 12(6/7): 779–786. [27] QING M X, SU S, WANG L L, LIU L J, XU K, HE L M, JUN X, HU S, WANG Y, XIANG J. Getting insight into the oxidation of SO2 to SO3 over V2O5-WO3/TiO2 catalysts: Reaction mechanism and effects of NO and NH3[J]. Chem Eng J,2019,361:1215−1224. doi: 10.1016/j.cej.2018.12.165 [28] ZHANG Y S, MEI D Q, WANG T, WANG J W, GU Y Z, ZHANG Z L, ROMERO C E, PAN W P. In-situ capture of mercury in coal-fired power plants using high surface energy fly ash[J]. Environ Sci Technol,2019,53(13):7913−7920. doi: 10.1021/acs.est.9b01725 [29] CAI W, ZHONG Q, ZHAO W, BU Y F. Focus on the modified CexZr1−xO2 with the rigid benzene-muti-carboxylate ligands and its catalysis in oxidation of NO[J]. Appl Catal B: Environ, 2014, 158–159: 258–268. [30] DU X S, DAO X, FU Y C, GAO F, LUO Z Y, CEN K F. The co-effect of Sb and Nb on the SCR performance of the V2O5/TiO2 catalyst[J]. J Colloid Interface Sci,2012,368:406−412. doi: 10.1016/j.jcis.2011.11.026 [31] GAO X, JIANG Y, ZHONG Y, LUO Z Y, CEN K F. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3[J]. J Hazard Mater, 2010, 174(1/3): 734–739. [32] YE D, QU R, SONG H, GAO X, LUO Z Y, NI M J, CEN K F. New insights into the various decomposition and reactivity behaviors of NH4HSO4 with NO on V2W5/TiO2 catalyst surfaces[J]. Chem Eng J,2016,283:846−854. doi: 10.1016/j.cej.2015.08.020 [33] LIU Y M, SHU H, XU Q S, ZHANG Y H, YANG L J. FT-IR study of the SO2 oxidation behavior in the selective catalytic reduction of NO with NH3 over commercial catalysts[J]. J Fuel Chem Technol,2015,43(8):1018−1024. doi: 10.1016/S1872-5813(15)30030-X [34] WEI L, CUI S P, GUO H X, MA X Y, ZHANG L J. DRIFT and DFT study of cerium addition on SO2 of manganese-based catalysts for low temperature SCR[J]. J Mol Catal A: Chem, 2016, 421: 102–108. [35] PAN S W, LUO H C, LI L, WEI Z L, HUANG B C. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3[J]. J Mol Catal A: Chem, 2013, 377: 154–161. [36] QING M X, SU S, WANG L L, LIU L J, XU K, HE L M, JUN X, HU S, WANG Y, XIANG J. Getting insight into the oxidation of SO2 to SO3 over V2O5-WO3/TiO2 catalysts: Reaction mechanism and effects of NO and NH3[J]. Chem Eng J, 2019, 361: 1215–1224. [37] JIN R B, LIU Y, WANG Y, CEN W L, WU Z B, WANG H Q, WENG X L. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature[J]. Appl Catal B: Environ, 2014, 148–149: 582-588. [38] 翁诗甫, 徐怡庄. 傅里叶变换红外光谱分析[M]. 3版. 北京: 化学工业出版社, 2016.WENG Shi-fu, XU Yi-zhuang. Fourier Transform Infrared Spectroscopy Analysis[M]. 3rd ed. Beijing: Chemical Industry Press, 2016. [39] LI H L, WU C Y, LI Y, LI L Q, ZHAO Y C, ZHANG J Y. Impact of SO2 on elemental mercury oxidation over CeO2-TiO2 catalyst[J]. Chem Eng J, 2013, 219: 319–326. [40] ULERSTAM M, JOHNSON M S, VOGT R, LJUNGSTROM E. DRIFTS and Kundsen cell study of the heterogeneous reactivity of SO2 and NO2 on mineral dust[J]. Atmos Chem Phys,2003,3:2043−2051. doi: 10.5194/acp-3-2043-2003 -




 下载:
下载: