Thermogravimetric analysis on the characteristics of oxy-fuel co-combustion of sub-bituminous coal and semi-coke
-
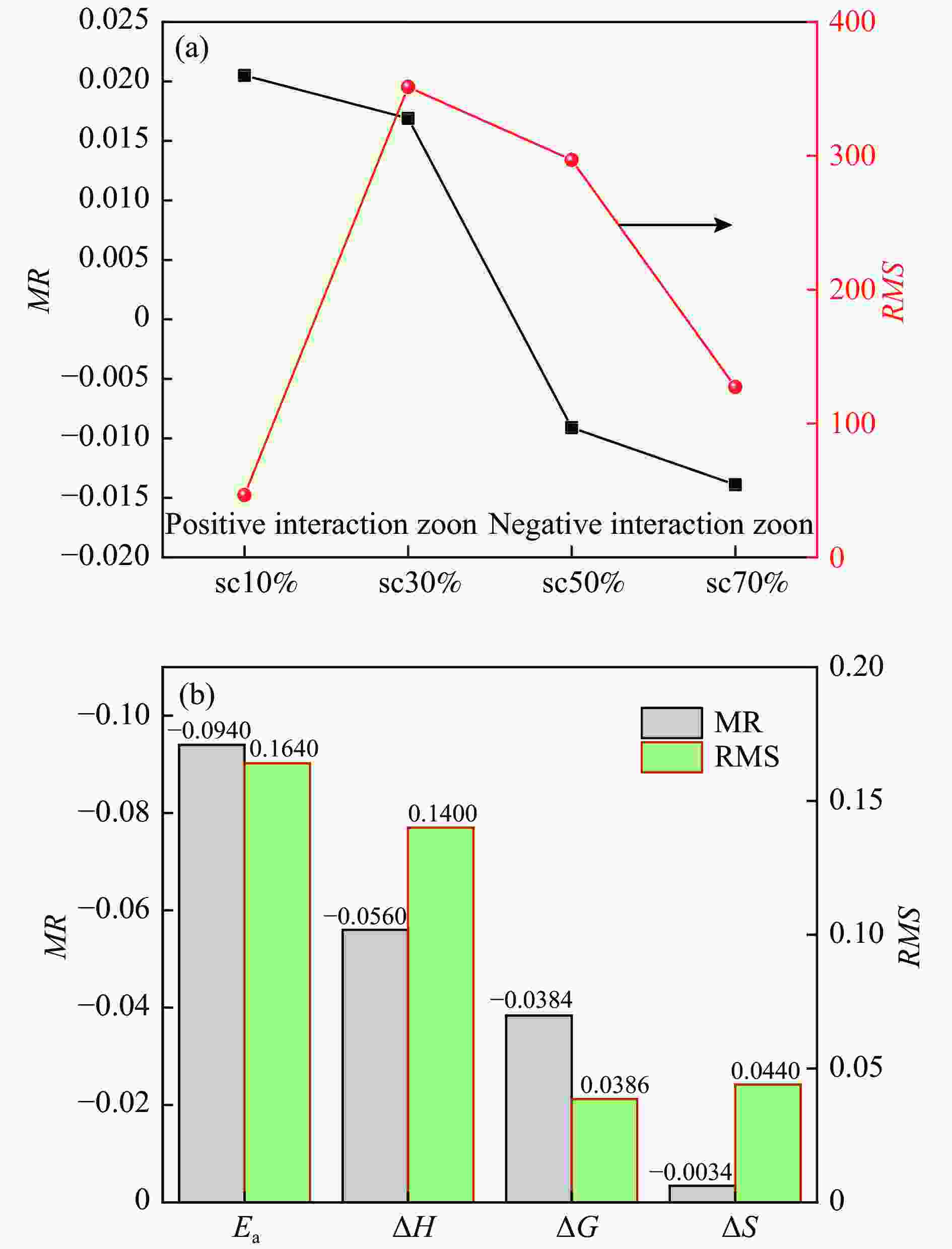
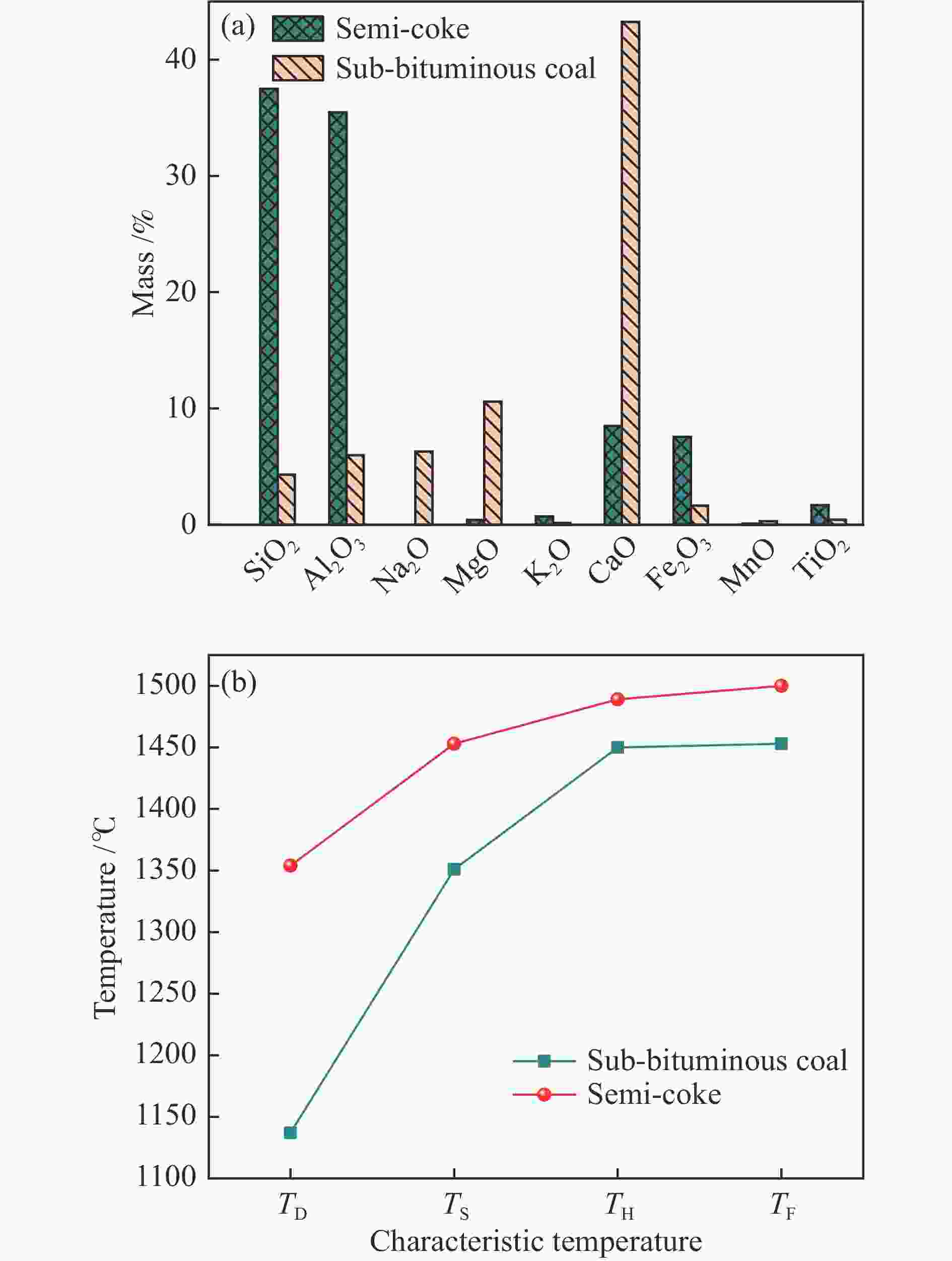
摘要: 低阶煤与煤制半焦混燃对解决中国半焦过剩的问题具有重要意义。通过热重分析研究了准东次烟煤与烟煤半焦的富氧混燃特性。与空气气氛相比,富氧燃烧将着火温度和燃尽温度分别提高了10和40 ℃。氧气浓度提高到30%可以大幅补偿富氧条件下燃烧参数的轻微降低并获得更好的共燃性能。利用Flynn-Wall-Ozawa (FWO)、Kissinger-Akahira-Sunose (KAS)和Starink法计算活化能,活化能随质量转化率的变化可分为两个不同阶段,次烟煤、混合燃料和半焦的平均活化能分别为49.31、50.82和59.00 kg/mol。计算了焓变、吉布斯自由能变和熵变等热力学参数,相互作用指数表明在共燃过程中两种燃料发生了明显的相互作用,动力学和热力学计算结果表明30%的半焦参比促进了共燃。同时,X射线荧光光谱(XRF)和灰熔融分析表明与半焦掺烧能降低次烟煤灰的结渣倾向。Abstract: The co-combustion of the low-rank coal with coal derived semi-coke is of great significance to solve the urgent problem of excessively produced semi-coke in China. In this research, the oxy-fuel co-combustion characteristics of Zhundong sub-bituminous coal with bituminous coal derived semi-coke are systematically investigated using thermogravimetric analysis. Compared with air combustion, oxy-fuel atmosphere increased the ignition and burnout temperature by 10 and 40 °C, respectively. Increasing the oxygen concentration to 30% strongly compensated for the slight reduction of the combustion parameters under oxy-fuel condition and much better co-combustion performance was obtained. Three iso-conversional methods, namely, Flynn-Wall-Ozawa (FWO), Kissinger-Akahira-Sunose (KAS) and Starink, were applied to estimate the activation energy, which can be divided into two stages during the co-combustion process. The average activation energy of sub-bituminous coal, the blend and semi-coke were 49.31, 50.82 and 59.00 kg/mol, respectively. Further, the pre-exponential factor and thermodynamic parameters of the enthalpy change, Gibbs free energy change and entropy change were calculated. Interaction indices were innovatively used for both kinetic-thermodynamic parameters and DTG values. An obvious interaction can be observed during the co-combustion process. The kinetic and thermodynamic results demonstrated that the 30% semi-coke ratio was beneficial to co-combustion. Meanwhile, X-ray fluorescence (XRF) and ash fusion analyses proved that the slagging tendency of sub-bituminous coal ash reduced by blending of semi-coke.
-
Key words:
- oxy-fuel co-combustion /
- coal semi-coke /
- thermogravimetric analysis /
- kinetics /
- thermodynamics /
- ash sintering
-
Table 1 Proximate and ultimate analyses of sub-bituminous coal and the bituminous coal derived semi-coke
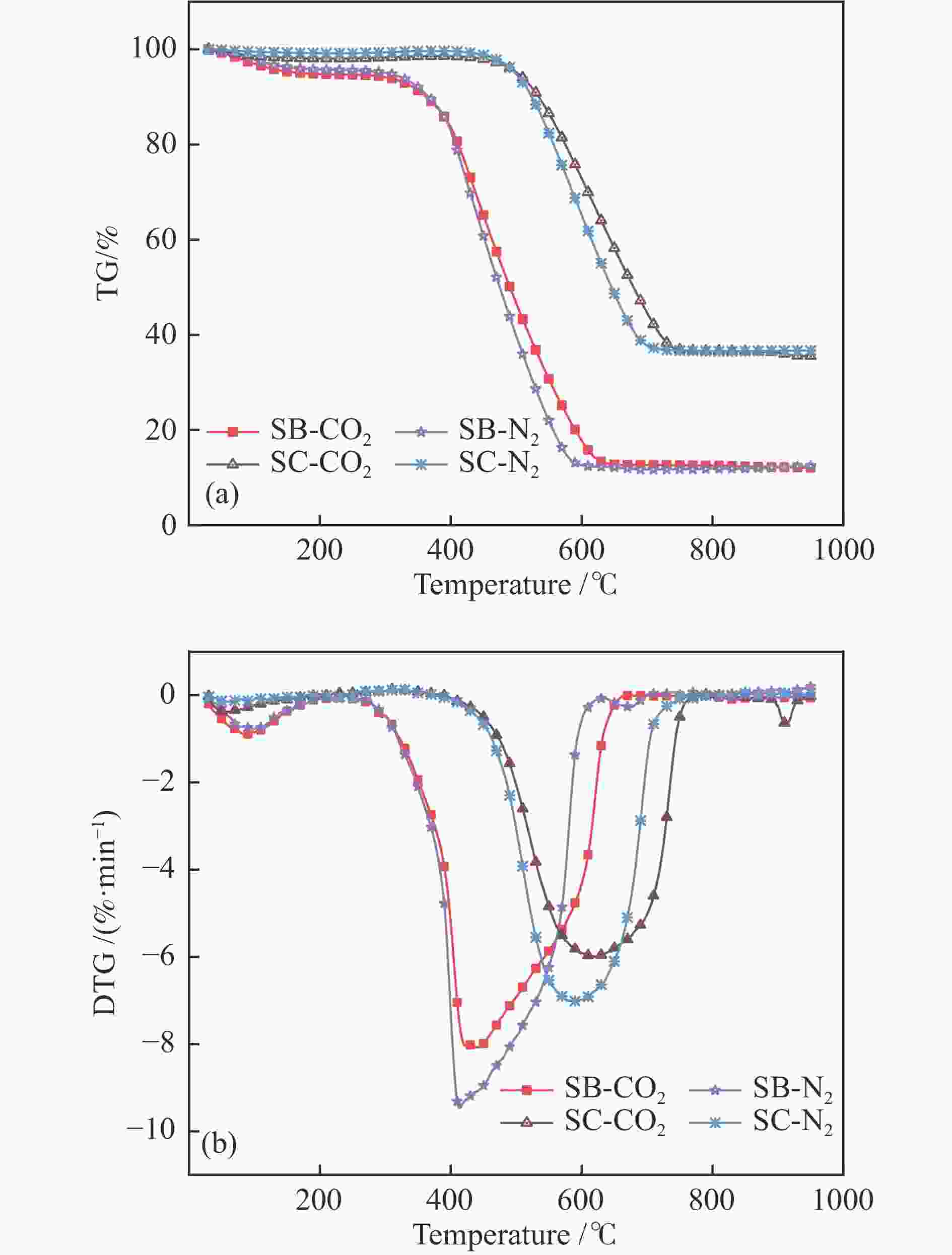
Fuel Proximate analysis wad/% Ultimate analysis wad/% M V FC A C H O N S SB 1.11 30.20 65.19 3.50 70.30 3.55 20.78 0.51 0.25 SC 0.50 8.78 58.45 32.27 60.10 1.92 2.31 1.06 1.84 Note: the subscript ad indicates the abbreviation of air-dried basis, M, V, FC and A indicate moisture, volatile, fixed carbon and ash, respectively Table 2 Combustion characteristic parameters of the blends
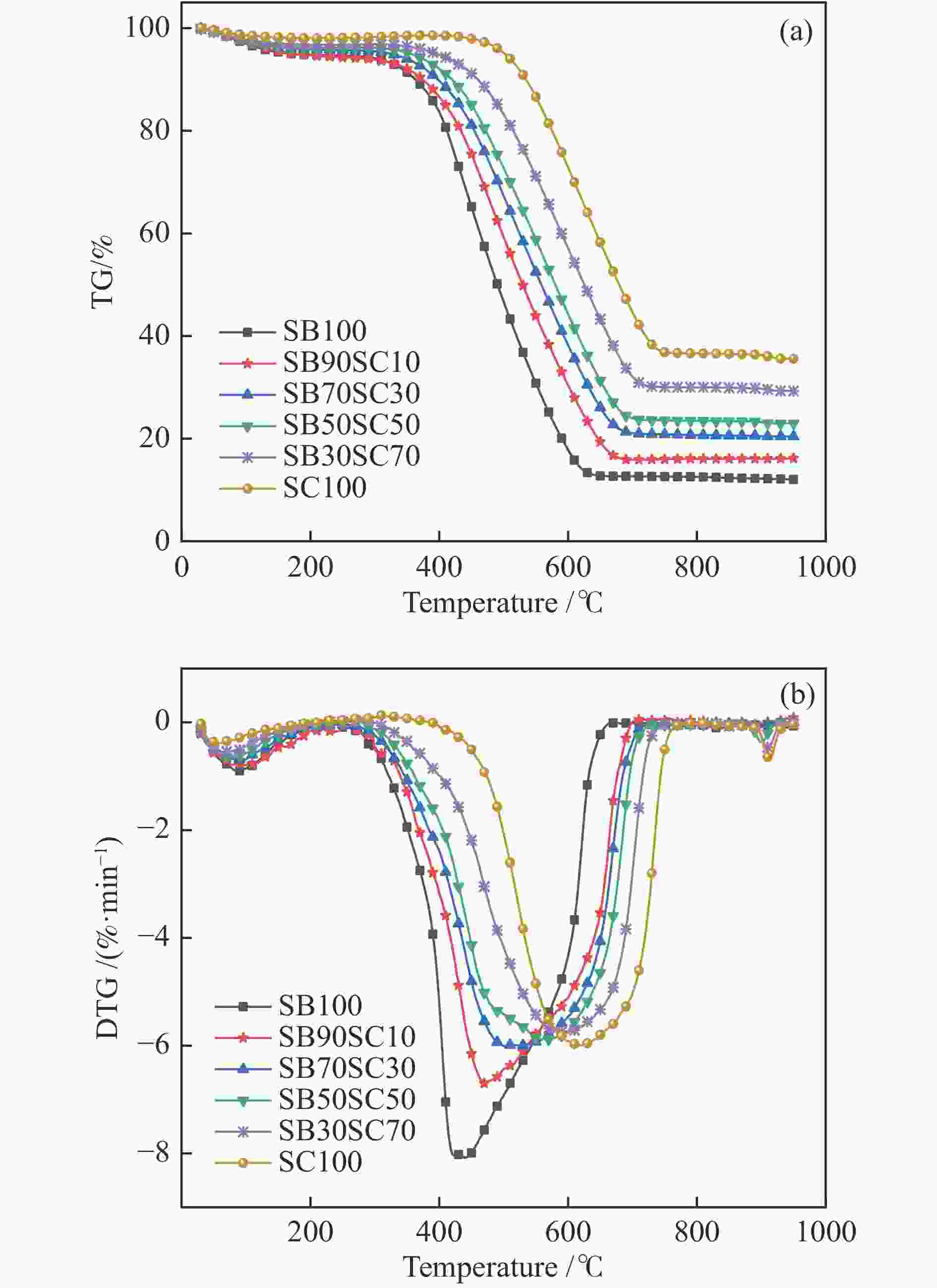
Sample Ti/°C Tm/°C Tf/°C DTGmax/(%·min−1) DTGave/(%·min−1) CCI/10−7 Rw/103 SB 363.6 439.8 640.6 8.08 5.62 5.36 4.34 SC10%SB90% 377.6 470.1 670.6 6.70 5.03 3.52 3.24 SC30%SB70% 391.3 501.9 693.2 6.01 4.65 2.63 2.63 SC50%SB50% 410.1 569.6 711.0 5.88 4.52 2.22 2.16 SC70%SB30% 451.1 599.9 713.6 5.76 4.64 1.84 1.82 SC 510.0 620.6 750.2 5.99 4.81 1.48 1.63 Table 3 Combustion parameters of 30% semi-coke blend in different O2 concentration
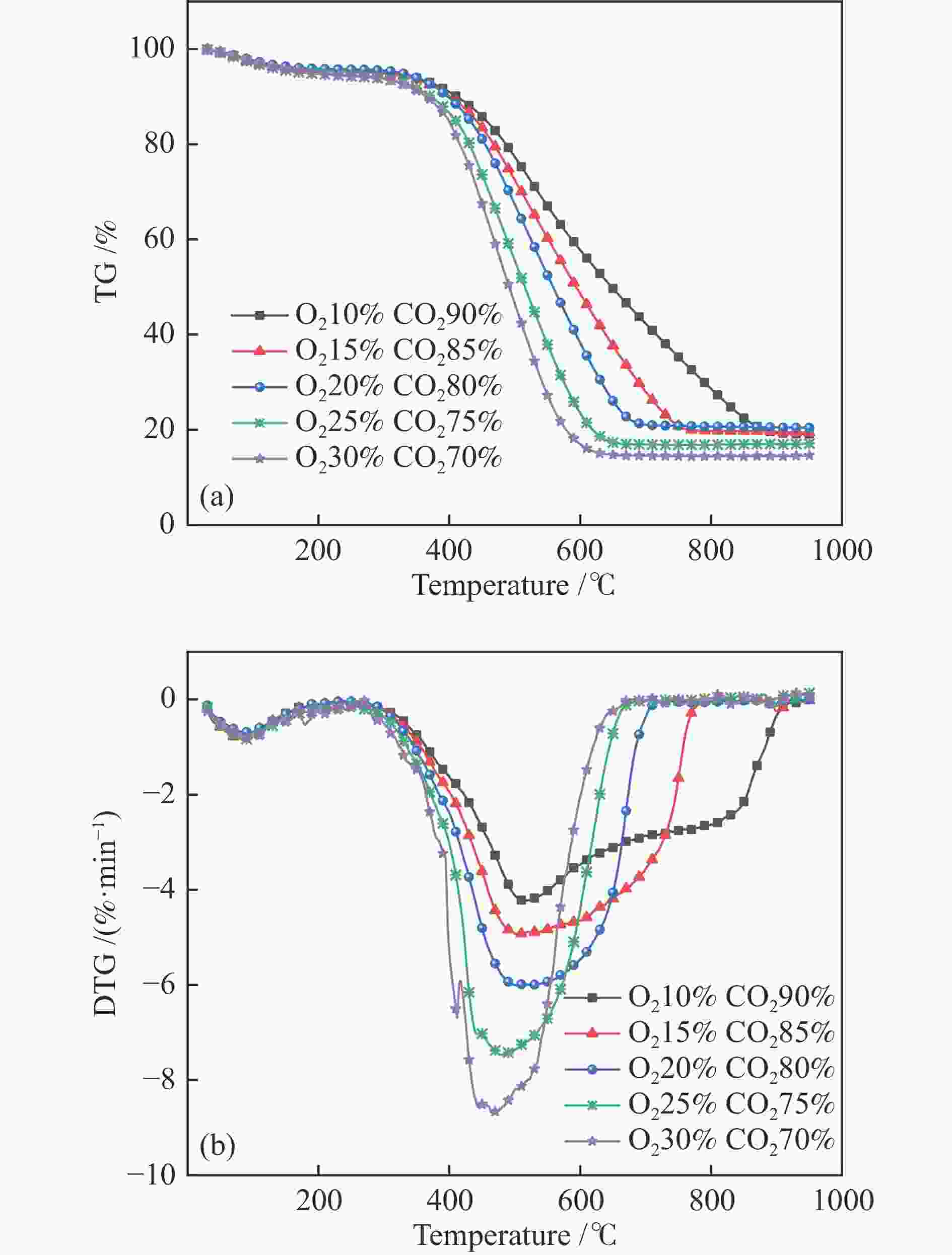
O2 content w/% Ti/°C Tm/°C Tf/°C DTGmax/(%·min−1) DTGave/(%·min−1) CCI/10−7 Rw/103 10 454.1 515.0 869.3 4.24 3.13 0.74 1.56 15 417.2 504.3 747.5 4.93 4.11 1.56 2.01 20 391.3 501.9 677.6 6.01 4.85 2.81 2.63 25 355.2 482.0 631.2 7.47 5.34 5.01 3.75 30 330.9 465.0 609.6 8.68 5.54 7.21 4.84 Table 4 Activation energies (Ea) for the samples using different iso-conversional approaches
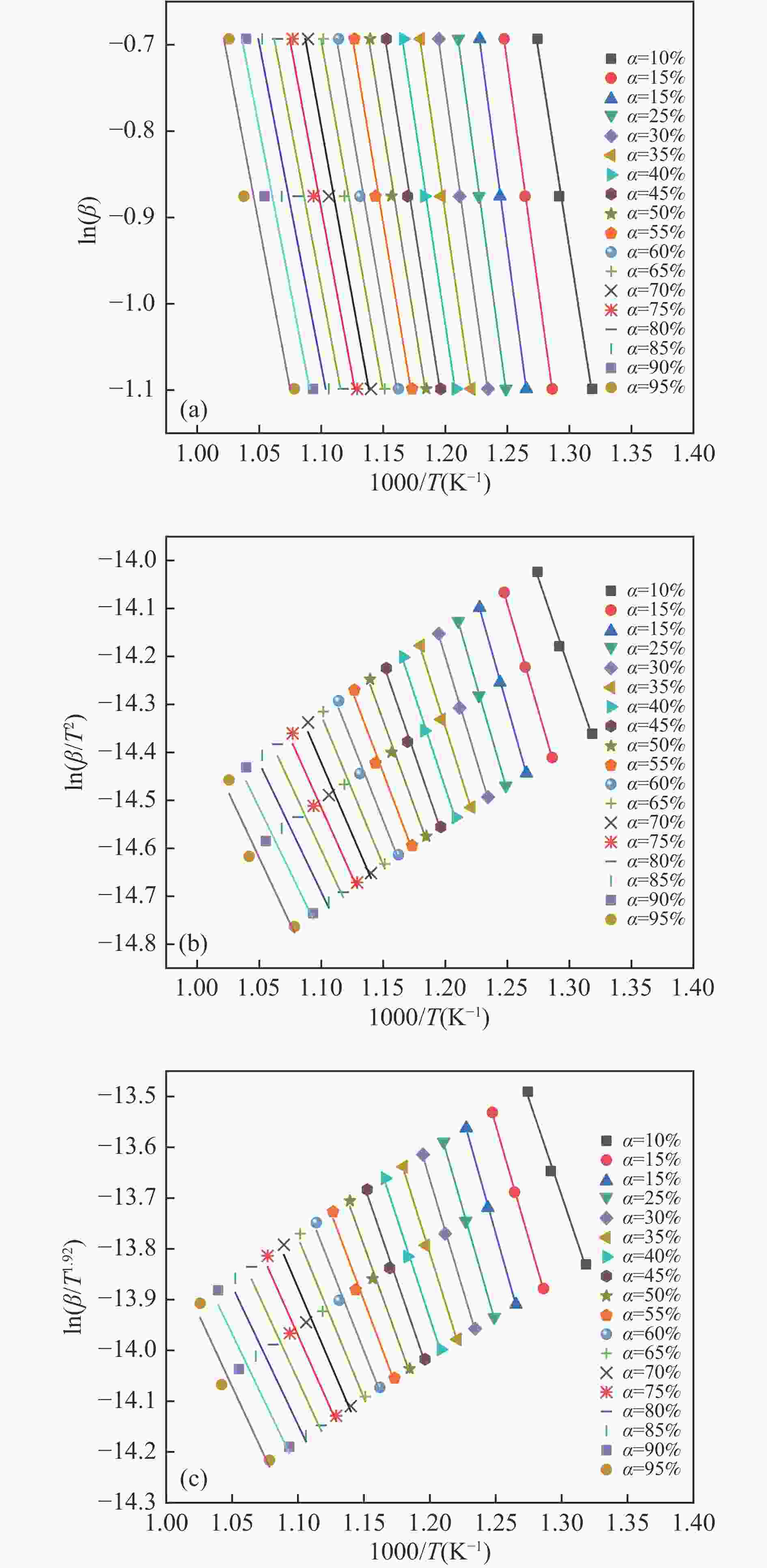
Sample α/% FWO KAS Starink k0 Ea/(kJ·mol−1) R Ea/(kJ·mol−1) R Ea/(kJ·mol−1) R SB 10 43.17 0.9635 35.21 0.9856 35.59 0.9857 2.12 15 89.92 0.9572 83.83 0.9843 84.19 0.9843 3.50×104 20 119.84 0.9623 115.05 0.9654 115.40 0.9655 1.40×107 25 90.61 0.9856 84.14 0.9872 84.51 0.9872 3.72×104 30 75.85 0.9874 68.46 0.9676 68.86 0.9677 1.75×103 35 67.43 0.9877 59.44 0.9689 59.85 0.9692 2.96×102 40 62.06 0.9877 53.63 0.9788 54.05 0.9788 93.06 45 58.30 0.9869 49.51 0.9826 49.94 0.9826 40.66 50 54.95 0.9868 45.81 0.9704 46.25 0.9704 19.25 55 51.58 0.9826 42.10 0.9859 42.55 0.9860 9.03 60 48.42 0.9894 38.60 0.9901 39.06 0.9881 4.39 65 45.93 0.9902 35.80 0.9887 36.27 0.9887 2.45 70 43.96 0.9853 33.54 0.9886 34.02 0.9886 1.53 75 42.11 0.9827 31.39 0.9888 31.88 0.9887 0.97 80 39.93 0.9643 28.90 0.9864 29.40 0.9865 0.57 85 37.93 0.9604 26.59 0.9684 27.10 0.9684 0.35 90 36.16 0.9615 24.50 0.9653 25.02 0.9654 0.22 95 35.06 0.9623 23.08 0.9614 23.61 0.9615 0.16 Average 57.96 48.87 49.31 SB70SC30 10 65.23 0.9974 57.92 0.9865 58.30 0.9867 1.18×102 15 87.68 0.9846 81.00 0.9814 81.38 0.9823 8.97×103 20 100.17 0.9954 93.83 0.9855 94.21 0.9834 9.59×104 25 89.26 0.9681 82.17 0.9937 82.57 0.9965 1.12×104 30 74.39 0.9634 66.35 0.9614 66.77 0.9693 5.86×102 35 65.83 0.9917 57.17 0.9618 57.60 0.9643 1.03×102 40 60.51 0.9925 51.39 0.9756 51.84 0.9784 34.27 45 56.49 0.9745 46.99 0.9672 47.45 0.9639 14.65 50 53.45 0.9986 43.62 0.9896 44.09 0.9793 7.60 55 50.93 0.9740 40.80 0.9763 41.28 0.9814 4.37 60 48.95 0.9674 38.53 0.9804 39.02 0.9876 2.80 65 47.21 0.9689 36.52 0.9795 37.02 0.9746 1.88 70 45.75 0.9855 34.8 0.9788 35.31 0.9789 1.33 75 44.66 0.9987 33.46 0.9874 33.97 0.9901 1.02 80 43.94 0.9954 32.50 0.9884 33.02 0.9885 0.84 85 44.17 0.9825 32.53 0..9798 33.06 0.9841 0.84 90 46.57 0.9734 34.79 0.9756 35.33 0.9785 1.34 95 53.78 0.9655 42.04 0.9602 42.58 0.9603 5.66 average 59.94 50.36 50.82 SC 10 72.22 0.9961 63.12 0.9946 63.59 0.9946 25.88 15 82.71 0.9997 73.86 0.9996 74.32 0.9996 1.36×102 20 84.94 0.9997 75.99 0.9996 76.46 0.9996 1.89×102 25 83.43 0.9997 74.22 0.9996 74.70 0.9996 1.45×102 30 80.88 0.9991 71.37 0.9988 71.86 0.9988 93.36 35 78.06 0.9983 68.25 0.9976 68.75 0.9976 57.69 40 75.20 0.9969 65.08 0.9956 65.59 0.9957 35.36 45 72.56 0.9953 62.16 0.9931 62.67 0.9932 22.44 50 70.00 0.9934 59.31 0.9901 59.84 0.9903 14.39 55 67.80 0.9909 56.85 0.9860 57.38 0.9863 9.78 60 65.36 0.9887 54.13 0.9823 54.67 0.9826 6.37 65 63.37 0.9856 51.89 0.9770 52.44 0.9775 4.47 70 61.55 0.9825 49.83 0.9714 50.38 0.9720 3.22 75 59.89 0.9796 47.92 0.9659 48.48 0.9667 2.37 80 58.50 0.9752 46.30 0.9577 46.87 0.9587 1.83 85 57.25 0.9692 44.81 0.9465 45.39 0.9478 1.44 90 56.48 0.9623 43.82 0.9434 44.41 0.9450 1.23 95 56.41 0.9400 43.54 0.9426 44.14 0.9446 1.17 average 69.26 58.47 59.00 Table 5 Thermodynamic parameters of the samples
Sample α /% ΔH/(kJ·mol−1) ΔG/(kJ·mol−1) ΔS/(kJ·mol−1) SB 10 30.60 197.65 −252.80 15 78.89 192.92 −172.56 20 109.95 191.19 −122.95 25 78.99 192.90 −172.38 30 63.26 194.03 −197.89 35 54.19 194.80 −212.79 40 48.32 195.36 −222.52 45 44.13 195.79 −229.51 50 40.37 196.21 −235.85 55 36.59 196.67 −242.25 60 33.02 197.14 −248.36 65 30.16 197.55 −253.32 70 27.82 197.90 −257.38 75 25.60 198.26 −261.29 80 23.04 198.70 −265.84 85 20.64 199.15 −270.15 90 18.47 199.59 −274.10 95 16.93 199.91 −276.90 SB70SC30 10 53.04 205.69 −219.86 15 75.84 203.77 −184.24 20 88.52 202.92 −164.77 25 76.80 203.68 −182.75 30 60.92 204.91 −207.37 35 51.68 205.76 −221.92 40 45.84 206.37 −231.20 45 41.37 206.88 −238.38 50 37.93 207.31 −243.94 55 35.04 207.69 −248.65 60 32.70 208.01 −252.49 65 30.62 208.32 −255.92 70 28.83 208.59 −258.89 75 27.41 208.81 −261.27 80 26.36 208.97 −263.01 85 26.30 208.97 −263.09 90 28.43 208.58 −259.46 95 35.51 207.51 −247.72 SC 10 57.28 257.96 −234.00 15 67.86 256.84 −220.37 20 69.89 256.64 −217.77 25 68.05 256.81 −220.11 30 65.13 257.08 −223.83 35 61.94 257.40 −227.92 40 58.71 257.74 −232.07 45 55.72 258.06 −235.93 50 52.82 258.39 −239.71 55 50.29 258.69 −243.00 60 47.52 259.03 −246.64 65 45.22 259.33 −249.67 70 43.09 259.62 −252.48 75 41.12 259.89 −255.10 80 39.43 260.13 −257.35 85 37.87 260.36 −259.43 90 36.81 260.52 −260.86 95 36.43 260.56 −261.35 -
[1] LIU S Q, ZHANG Y J, TUO K Y, WANG L P, CHEN G. Structure, electrical conductivity, and dielectric properties of semi-coke derived from microwave-pyrolyzed low-rank coal[J]. Fuel Process Technol,2018,178:139−147. doi: 10.1016/j.fuproc.2018.05.028 [2] ZHENG S W, HU Y J, WANG Z Q, CHENG X X. Experimental investigation on ignition and burnout characteristics of semi-coke and bituminous coal blends[J]. J Energy Inst,2020,93(4):1373−1381. doi: 10.1016/j.joei.2019.12.007 [3] XIE K C, LI W Y, ZHAO W. Coal chemical industry and its sustainable development in china[J]. Energy,2010,35(11):4349−4355. doi: 10.1016/j.energy.2009.05.029 [4] HU L L, ZHANG Y, CHEN D G, ZHANG M, WU Y X, ZHANG H. Experimental study on the combustion and NOx emission characteristics of a bituminous coal blended with semi-coke[J]. Appl Therm Eng,2019,160:113993. doi: 10.1016/j.applthermaleng.2019.113993 [5] ZHANG J P, WANG C A, JIA X W, WANG P Q, CHE D F. Experimental study on combustion and NO formation characteristics of semi-coke[J]. Fuel,2019,258:11618. [6] GONG Z Q, LIU Z C, ZHOU T, LU Q G, SUN Y K. Combustion and NO emission of Shenmu char in a 2 MW circulating fluidized bed[J]. Energy Fuels,2015,29(2):1219−1226. doi: 10.1021/ef502768w [7] YAO Y, ZHU J G, LU Q G. Experimental study on nitrogen transformation in combustion of pulverized semi-coke preheated in a circulating fluidized bed[J]. Energy Fuels,2015,29(6):3985−3991. doi: 10.1021/acs.energyfuels.5b00791 [8] LV Z M, XIONG X H, YU S L, TAN H Z, XIANG B X, HUANG J, PENG J H, LI P. Experimental investigation on NO emission of semi-coke under high temperature preheating combustion technology[J]. Fuel,2021,283:119293. doi: 10.1016/j.fuel.2020.119293 [9] LI J B, ZHU M B, ZHANG Z Z, ZHANG K, SHEN G Q, ZHANG D K. The mineralogy, morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong sub-bituminous coal and a bituminous coal in a drop tube furnace[J]. Fuel Process Technol,2016,149:176−186. doi: 10.1016/j.fuproc.2016.04.021 [10] LIAO X J, SINGH S, YANG H P, WU C F. A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene[J]. Fuel,2021,287:19355. [11] AREEPRASERT C, SCALA F, COPPOLA A, URCIUOLO M, CHIRONE R, CHANYAVANICH P, YOSHIKAWA K. Fluidized bed co-combustion of hydrothermally treated paper sludge with two coals of different rank[J]. Fuel Process Technol,2016,144:230−238. doi: 10.1016/j.fuproc.2015.12.033 [12] BUYUKADA M. Investigation of thermal conversion characteristics and performance evaluation of co-combustion of pine sawdust and sub-bituminous coal using TGA, artificial neural network modeling and likelihood method[J]. Bioresour Technol,2019,287:121461. doi: 10.1016/j.biortech.2019.121461 [13] LIAO Y F, MA X Q. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge[J]. ApEn,2010,87(11):3526−3532. [14] MUTHURAMAN M, NAMIOKA T, YOSHIKAWA K. Characteristics of co-combustion and kinetic study on hydrothermally treated municipal solid waste with different rank coals: A thermogravimetric analysis[J]. ApEn,2010,87(1):141−148. [15] LIU H P, LIANG W X, QIN H, WANG Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk[J]. Appl Therm Eng,2016,109:653−662. doi: 10.1016/j.applthermaleng.2016.08.125 [16] LIU H P, LIANG W X, QIN H, WANG Q. Thermal behavior of co-combustion of oil shale semi-coke with torrefied cornstalk[J]. Appl Therm Eng,2016,109:413−422. doi: 10.1016/j.applthermaleng.2016.08.084 [17] WANG Q, XU H, LIU H P, JIA C X, ZHAO W Z. Co-combustion performance of oil shale semi-coke with corn stalk[J]. Energy Procedia,2012,17:861−868. doi: 10.1016/j.egypro.2012.02.180 [18] WANG Q, ZHAO W Z, LIU H P, JIA C X, LI S H. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion[J]. ApEn,2011,88(6):2080−2087. [19] YANG Y, LU X F, WANG Q H. Investigation on the co-combustion of low calorific oil shale and its semi-coke by using thermogravimetric analysis[J]. Energy Convers Manage,2017,136:99−107. doi: 10.1016/j.enconman.2017.01.006 [20] LIU Z, LI J B, ZHU M M, WANG Q H, LU X F, ZHANG Y Y, ZHANG Z Z, ZHANG D K. Investigation into scavenging of sodium and ash deposition characteristics during co-combustion of Zhundong sub-bituminous coal with an oil shale semi-coke of high aluminosilicate in a circulating fluidized bed[J]. Fuel,2019,257:116099. doi: 10.1016/j.fuel.2019.116099 [21] HU Z J, LIU Y R, XU H, ZHU J M, WU S L, SHEN Y S. Co-combustion of semicoke and coal in an industry ironmaking blast furnace: Lab experiments, model study and plant tests[J]. Fuel Process Technol,2019,196:106165. doi: 10.1016/j.fuproc.2019.106165 [22] SUN L T, YAN Y H, SUN R, ZHU W K, YUAN M F, QI H L, WU J Q. Effects of bluff-body cone angle on turbulence-chemistry interaction behaviors in large-scale semicoke and bituminous coal co-combustion[J]. Fuel Process Technol,2021,221:106915. doi: 10.1016/j.fuproc.2021.106915 [23] YAO H F, HE B S, DING G C, TONG W X, KUANG Y C. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal[J]. Appl Therm Eng,2019,156:708−721. doi: 10.1016/j.applthermaleng.2019.04.115 [24] WANG P Q, WANG C A, YUAN M B, WANG C W, ZHANG J P, DU Y B, TAO Z C, CHE D F. Experimental evaluation on co-combustion characteristics of semi-coke and coal under enhanced high-temperature and strong-reducing atmosphere[J]. ApEn,2020,260:114203. [25] ZHANG J P, JIA X W, WANG C A, ZHAO N, WANG P Q, CHE D F. Experimental investigation on combustion and NO formation characteristics of semi-coke and bituminous coal blends[J]. Fuel,2019,247:87−96. doi: 10.1016/j.fuel.2019.03.045 [26] ZHU S J, LYU Q G, ZHU J G, WU H X, WU G L. Effect of air distribution on NOx emissions of pulverized coal and char combustion preheated by a circulating fluidized bed[J]. Energy Fuels,2018,32(7):7909−7915. doi: 10.1021/acs.energyfuels.8b01366 [27] NIU S L, LU C M, HAN K H, ZHAO J L. Thermogravimetric analysis of combustion characteristics and kinetic parameters of pulverized coals in oxy-fuel atmosphere[J]. JTAC,2009,98:267−274. [28] XU C B, YANG J J, HE L, WEI W X, YANG Y, YIN X D, YANG W J, LIN A J. Carbon capture and storage as a strategic reserve against China's CO2 emissions[J]. Environ Dev,2021,37:100608. doi: 10.1016/j.envdev.2020.100608 [29] DESSI F, MUREDDU M, FERRARA F, FERMOSO J, ORSINI A, SANNA A, PETTINAU A. Thermogravimetric characterisation and kinetic analysis of Nannochloropsis sp. and Tetraselmis sp. microalgae for pyrolysis, combustion and oxy-combustion[J]. Energy,2021,217:119394. doi: 10.1016/j.energy.2020.119394 [30] SEDDIGHI S, CLOUGH P T, ANTHONY E J, HUGHES R W, LU P. Scale-up challenges and opportunities for carbon capture by oxy-fuel circulating fluidized beds[J]. ApEn,2018,232:527−542. [31] LIU Q W, SHI Y, ZHONG W Q, YU A B. Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2 capture: A review of recent advances[J]. Chin J Chem Eng,2019,27(10):2261−2272. doi: 10.1016/j.cjche.2019.07.013 [32] LI A J, CHEN Z, LIAO Y H, LIU Y H. A synthetical evaluation of developing low-carbonized coal-fired power technologies in China[J]. IJHE,2017,42(32):20857−20867. [33] RUAN R H, TAN H Z, WANG X B, HU Z F. Evolution of particulate matter in the post-combustion zone of Zhundong sub-bituminous coal[J]. Fuel,2020,281:118780. doi: 10.1016/j.fuel.2020.118780 [34] S. P S P, G S, JOSHI V V. Thermogravimetric analysis of hazardous waste: Pet-coke, by kinetic models and Artificial neural network modeling[J]. Fuel,2021,287:119470. doi: 10.1016/j.fuel.2020.119470 [35] HU M, CHEN Z H, GUO D B, LIU C X, XIAO B, HU Z Q, LIU S M. Thermogravimetric study on pyrolysis kinetics of Chlorella pyrenoidosa and bloom-forming cyanobacteria[J]. Bioresour Technol,2015,177:41−50. doi: 10.1016/j.biortech.2014.11.061 [36] NIU S L, LIU M Q, LU C M, LI H, HUO M J. Thermogravimetric analysis of carbide slag[J]. JTAC,2013,115:73−79. [37] GONZALEZ D L, LOPEZ M F, VALVERDE J L, SILVA L S. Kinetic analysis and thermal characterization of the microalgae combustion process by thermal analysis coupled to mass spectrometry[J]. ApEn,2014,114:227−237. [38] NIU S L, YU H W, ZHAO S, ZHANG X Y, LI X M, HAN K H, LU C M, WANG Y Z. Apparent kinetic and thermodynamic calculation for thermal degradation of stearic acid and its esterification derivants through thermogravimetric analysis[J]. Renewable Energy,2019,133:373−381. doi: 10.1016/j.renene.2018.10.045 [39] XIAO H M, MA X Q, LAI Z Y. Isoconversional kinetic analysis of co-combustion of sewage sludge with straw and coal[J]. ApEn,2009,86(9):1741−1745. [40] YUAN X S, HE T, CAO H L, YUAN Q X. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods[J]. Renewable Energy,2017,107:489−496. doi: 10.1016/j.renene.2017.02.026 [41] MAIA A A D, DE Morais L C. Kinetic parameters of red pepper waste as biomass to solid biofuel[J]. Bioresour Technol,2016,204:157−163. doi: 10.1016/j.biortech.2015.12.055 [42] FAN Y J, YANG B L, ZHANG B, WU Z Q, SUN Z Y, SHANG J X. Synergistic effects from fast co-pyrolysis of lignin with low-rank coal: On-line analysis of products distribution and fractal analysis on co-pyrolysis char[J]. J Energy Inst,2021,97:152−160. doi: 10.1016/j.joei.2021.04.009 [43] XIE C D, LIU J Y, ZHANG X C, XIE W M, SUN J, CHANG K L, KUO J H, XIE W H, LIU C, SUN S Y, BUYUKADA M, EVRENDILEK F. Co-combustion thermal conversion characteristics of textile dyeing sludge and pomelo peel using TGA and artificial neural networks[J]. ApEn,2018,212:786−795. [44] ZHOU C C, LIU G J, WANG X D, QI C C, HU Y H. Combustion characteristics and arsenic retention during co-combustion of agricultural biomass and bituminous coal[J]. Bioresour Technol,2016,214:218−224. doi: 10.1016/j.biortech.2016.04.104 [45] BOTELHO T, COSTA M, WILK M, MAGDZIARZ A. Evaluation of the combustion characteristics of raw and torrefied grape pomace in a thermogravimetric analyzer and in a drop tube furnace[J]. Fuel,2018,212:95−100. doi: 10.1016/j.fuel.2017.09.118 [46] CHEN J C, XIE C D, LIU J Y, HE Y, XIE W M, ZHANG X C, CHANG K L, KUO J H, SUN J, ZHENG L, SUN S Y, BUYUKADA M, EVRENDILEK F. Co-combustion of sewage sludge and coffee grounds under increased O2/CO2 atmospheres: Thermodynamic characteristics, kinetics and artificial neural network modeling[J]. Bioresour Technol,2018,250:230−238. doi: 10.1016/j.biortech.2017.11.031 [47] SHADDIX C R, MOLINA A. Particle imaging of ignition and devolatilization of pulverized coal during oxy-fuel combustion[J]. Proc Combust Inst,2009,32(2):2091−2098. doi: 10.1016/j.proci.2008.06.157 [48] RIAZA J, GIL M V, ALVAREZ L, PEVIDA C, PIS J J, RUBIERA F. Oxy-fuel combustion of coal and biomass blends[J]. Energy,2012,41(1):429−435. doi: 10.1016/j.energy.2012.02.057 [49] XU Y L, CHEN B L. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis[J]. Bioresour Technol,2013,146:485−493. doi: 10.1016/j.biortech.2013.07.086 [50] KIM H S, Kim J H. Kinetics and thermodynamics of microwave-assisted drying of paclitaxel for removal of residual methylene chloride[J]. Process Biochem,2017,56:163−170. doi: 10.1016/j.procbio.2017.02.007 [51] KOTHARI R, PATHAK V V, PANDEY A, AHMAD S, SRIVASTVA C, TYAGI V V. A novel method to harvest Chlorella sp. via low cost bioflocculant: Influence of temperature with kinetic and thermodynamic functions[J]. Bioresour Technol,2017,225:84−89. doi: 10.1016/j.biortech.2016.11.050 [52] DENG S H, WANG X B, TAN H Z, MIKULCIC F, LI Z F, DUIC N. Thermogravimetric study on the Co-combustion characteristics of oily sludge with plant biomass[J]. Thermochim Acta,2016,633:69−76. doi: 10.1016/j.tca.2016.03.006 [53] SEZER S, KARTAL F, OZVEREN U. The investigation of co-combustion process for synergistic effects using thermogravimetric and kinetic analysis with combustion index[J]. Therm Sci Eng Prog,2021,23:100889. doi: 10.1016/j.tsep.2021.100889 [54] GIL MV, RIAZA J, ALVAREZ L, PEVIDA C, PIS J J, RUBIERA F. Kinetic models for the oxy-fuel combustion of coal and coal/biomass blend chars obtained in N2 and CO2 atmospheres[J]. Energy,2012,48(1):510−518. doi: 10.1016/j.energy.2012.10.033 [55] CHEN J C, HE Y, LIU J Y, LIU C, XIE W M, KUO J H, ZHANG X C, LI S P, LIANG J L, SUN S Y, BUYAKADA M, EVRENDILEK F. The mixture of sewage sludge and biomass waste as solid biofuels: Process characteristic and environmental implication[J]. Renew Energ,2019,139:707−717. doi: 10.1016/j.renene.2019.01.119 [56] LI J B, ZHU M M, ZHANG Z Z, ZHANG K, SHEN G Q, ZHANG D K. Effect of coal blending and ashing temperature on ash sintering and fusion characteristics during combustion of Zhundong sub-bituminous coal[J]. Fuel,2017,195:131−142. doi: 10.1016/j.fuel.2017.01.064 [57] QIAO L, DENG C B, LU B, WANG Y S, WANG X F, DENG H Z, ZHANG X. Study on calcium catalyzes coal spontaneous combustion[J]. Fuel,2022,307:121884. doi: 10.1016/j.fuel.2021.121884 [58] ZHAO Y J, FENG D D, LI B W, SUN S Z, ZHANG S. Combustion characteristics of char from pyrolysis of Zhundong sub-bituminous coal under O2/steam atmosphere: Effects of mineral matter[J]. Int J Greenh Gas Control,2019,80:54−60. doi: 10.1016/j.ijggc.2018.12.001 -




 下载:
下载: