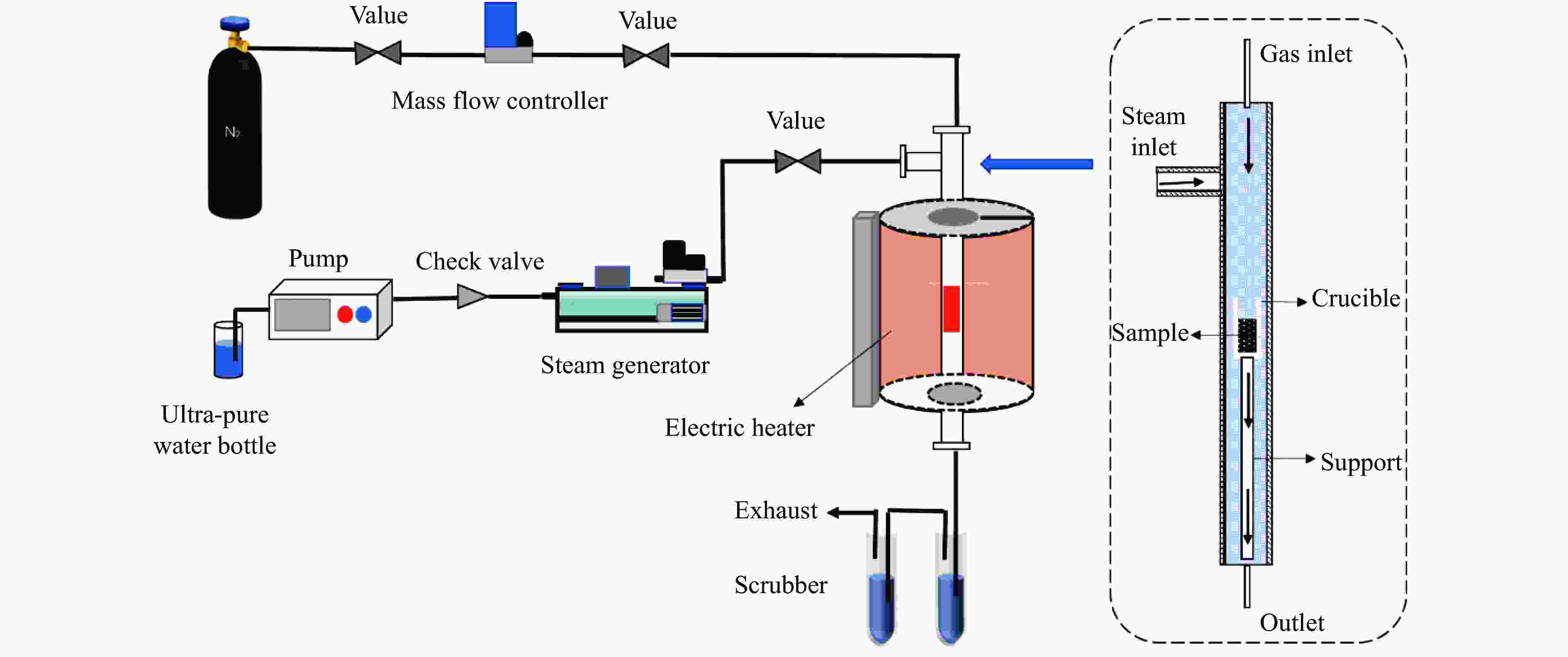
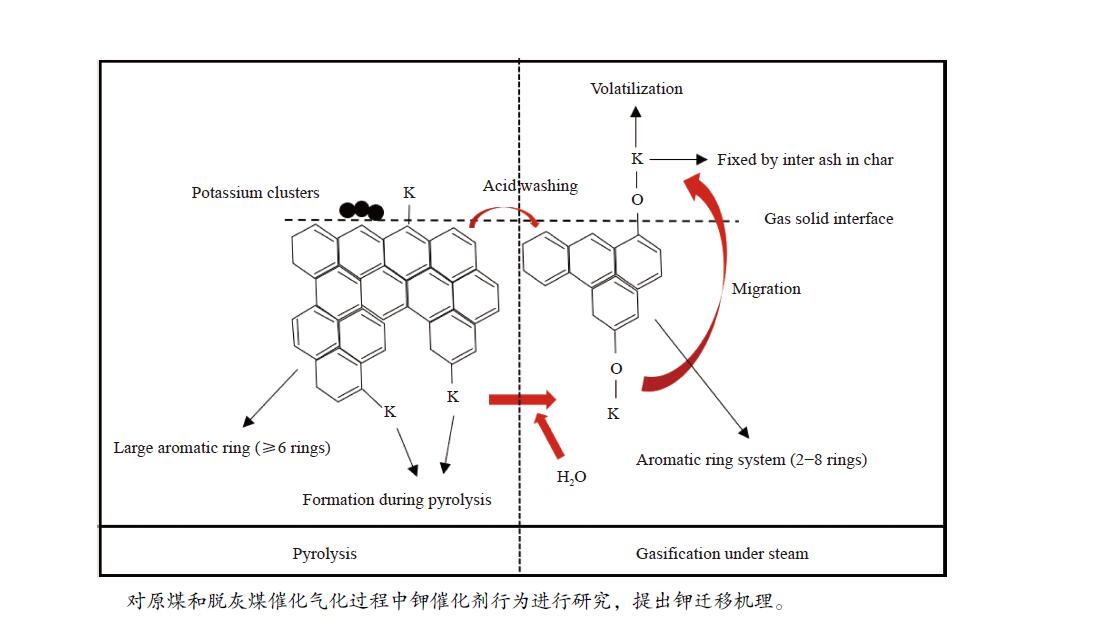
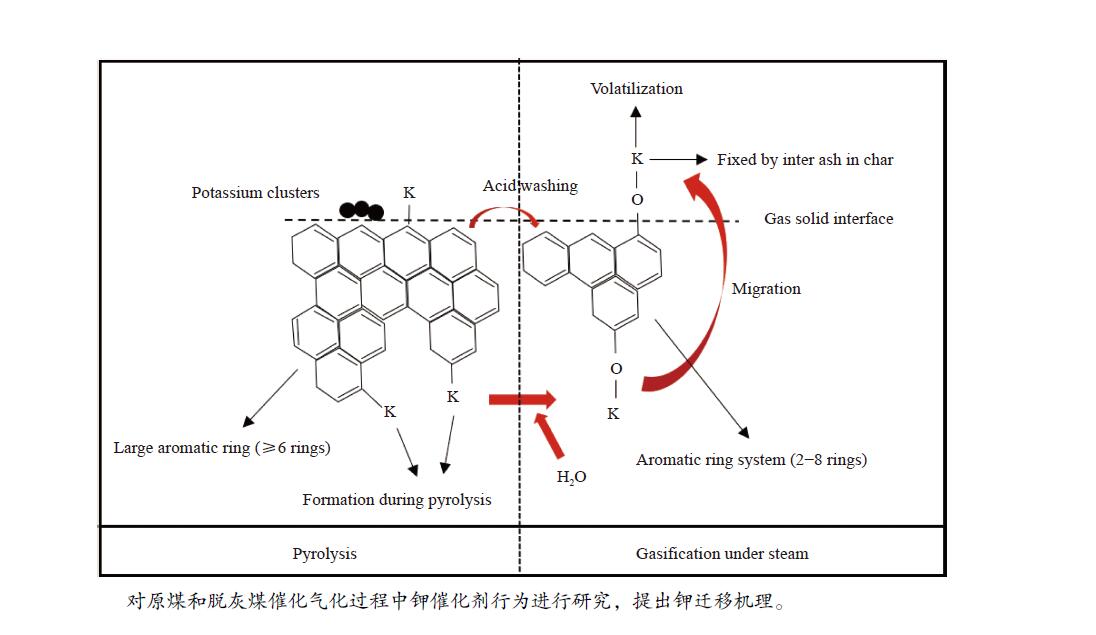
Migration behavior of potassium under condition of steam gasification of Yulin coal
-
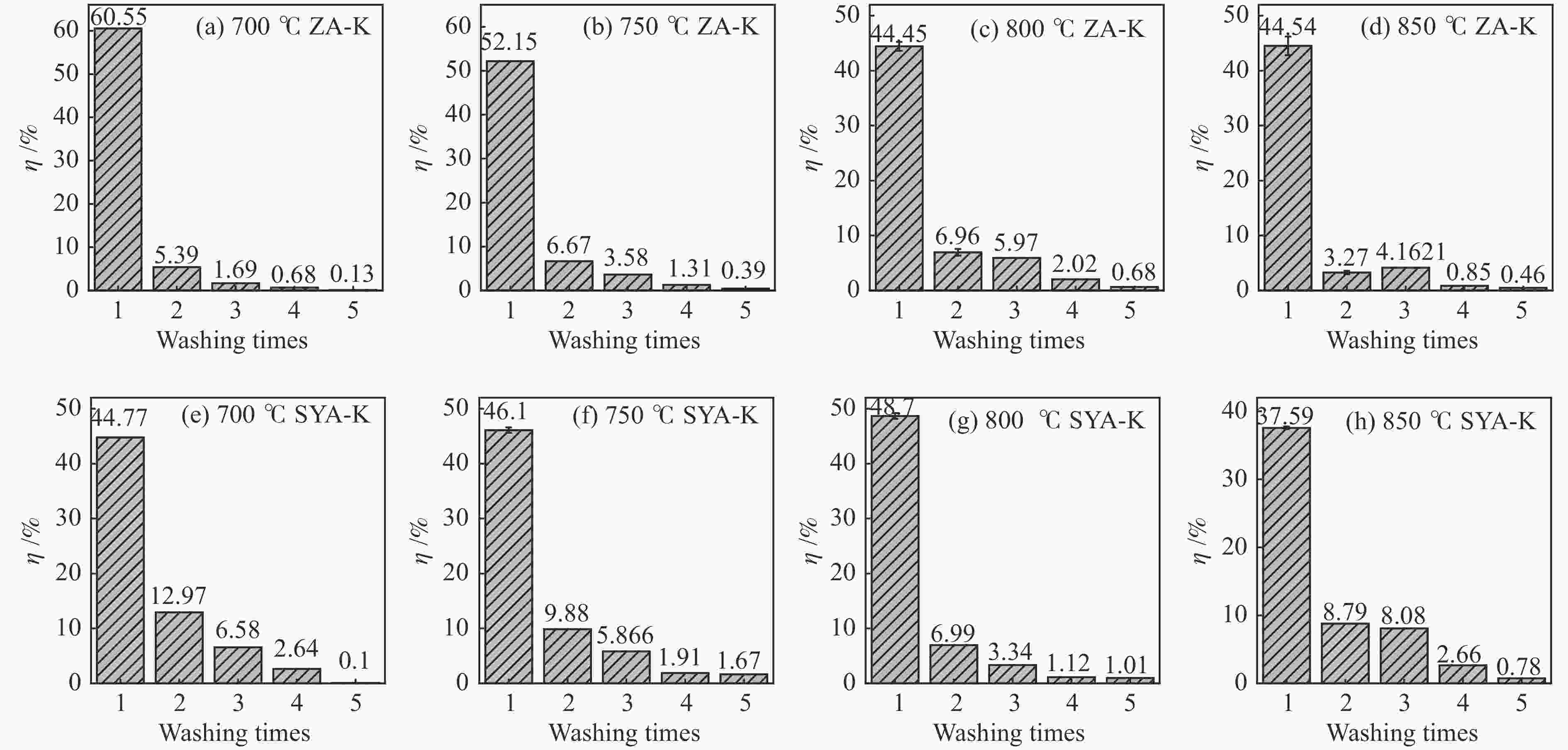
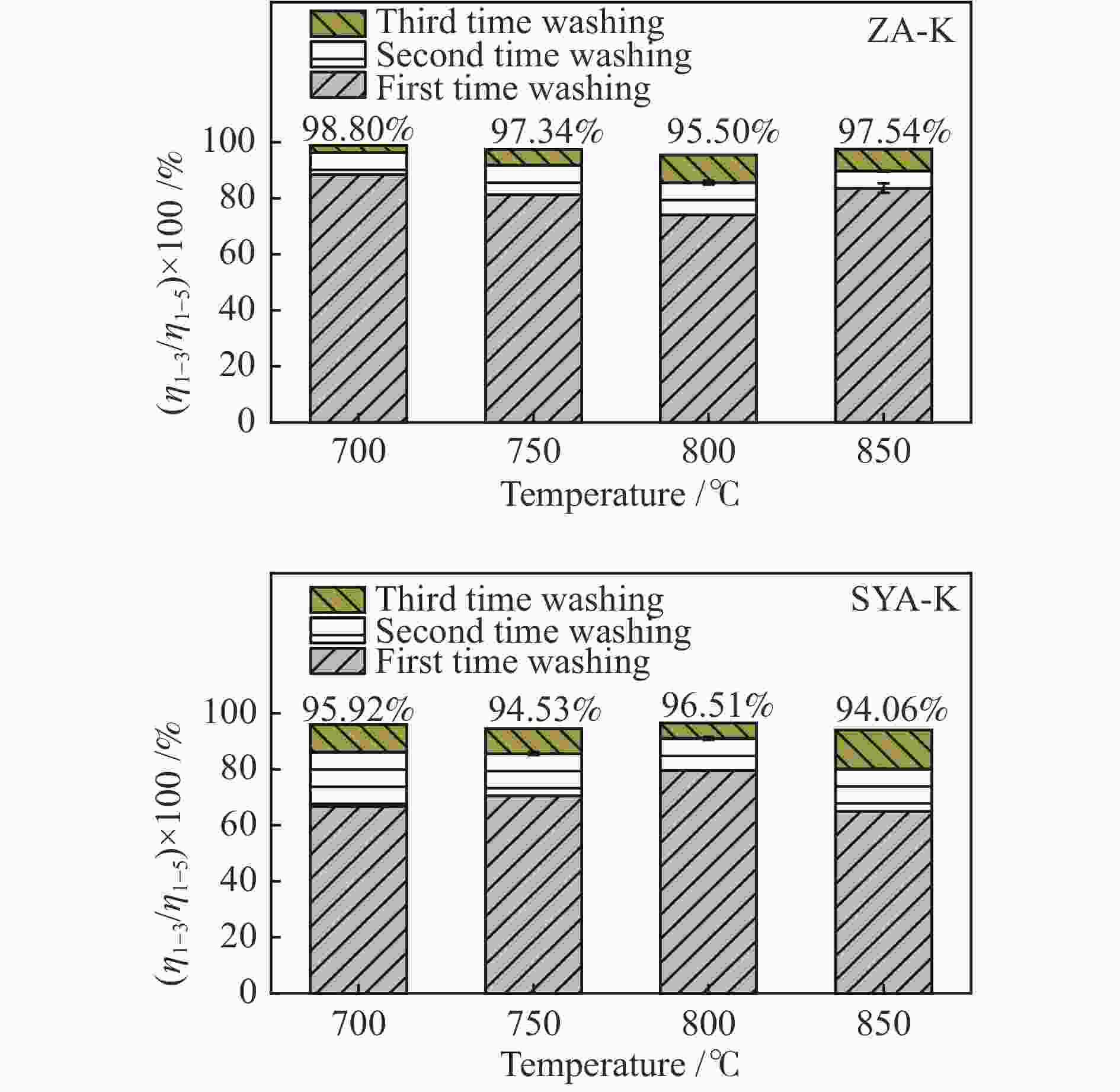
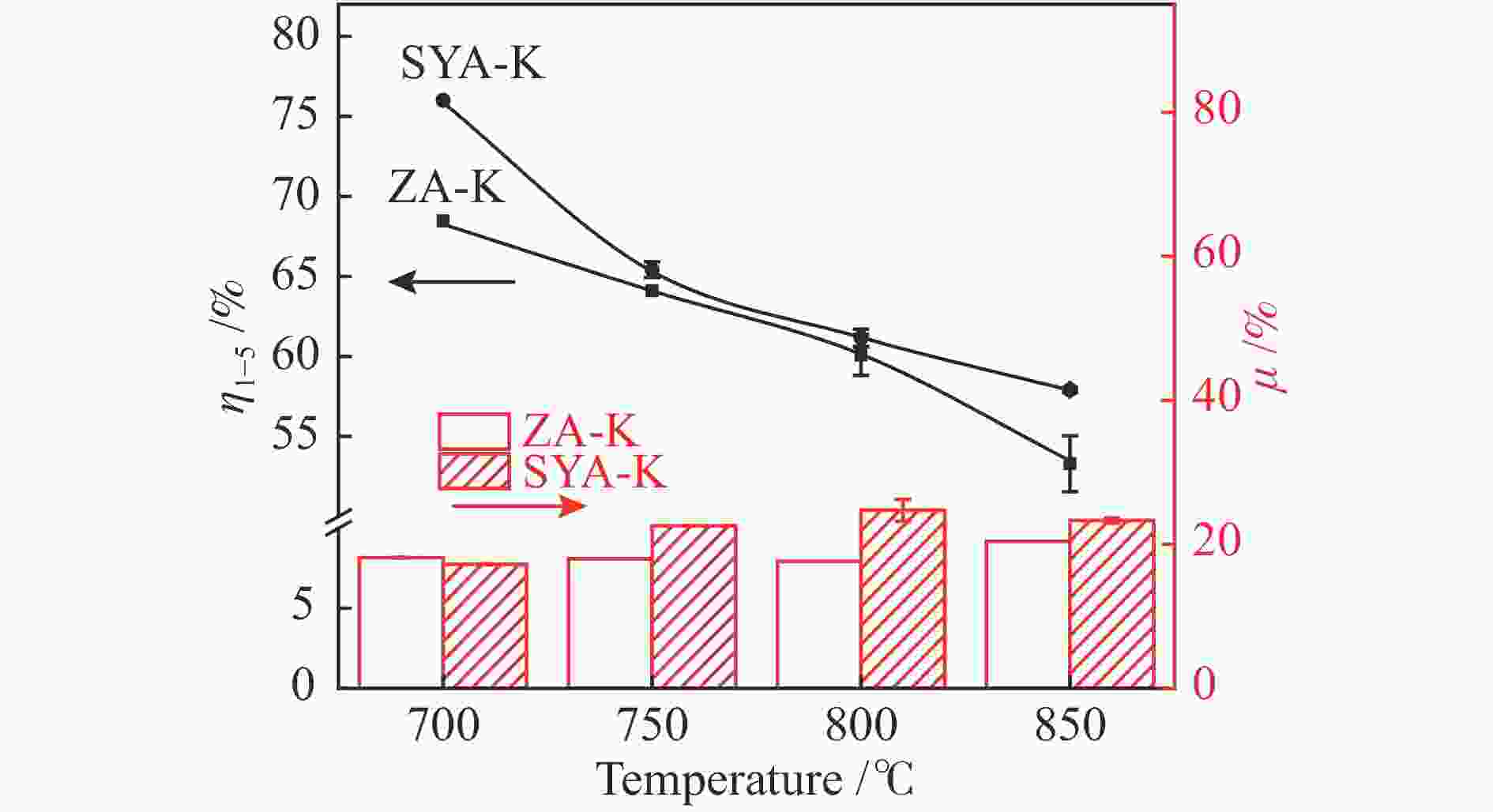
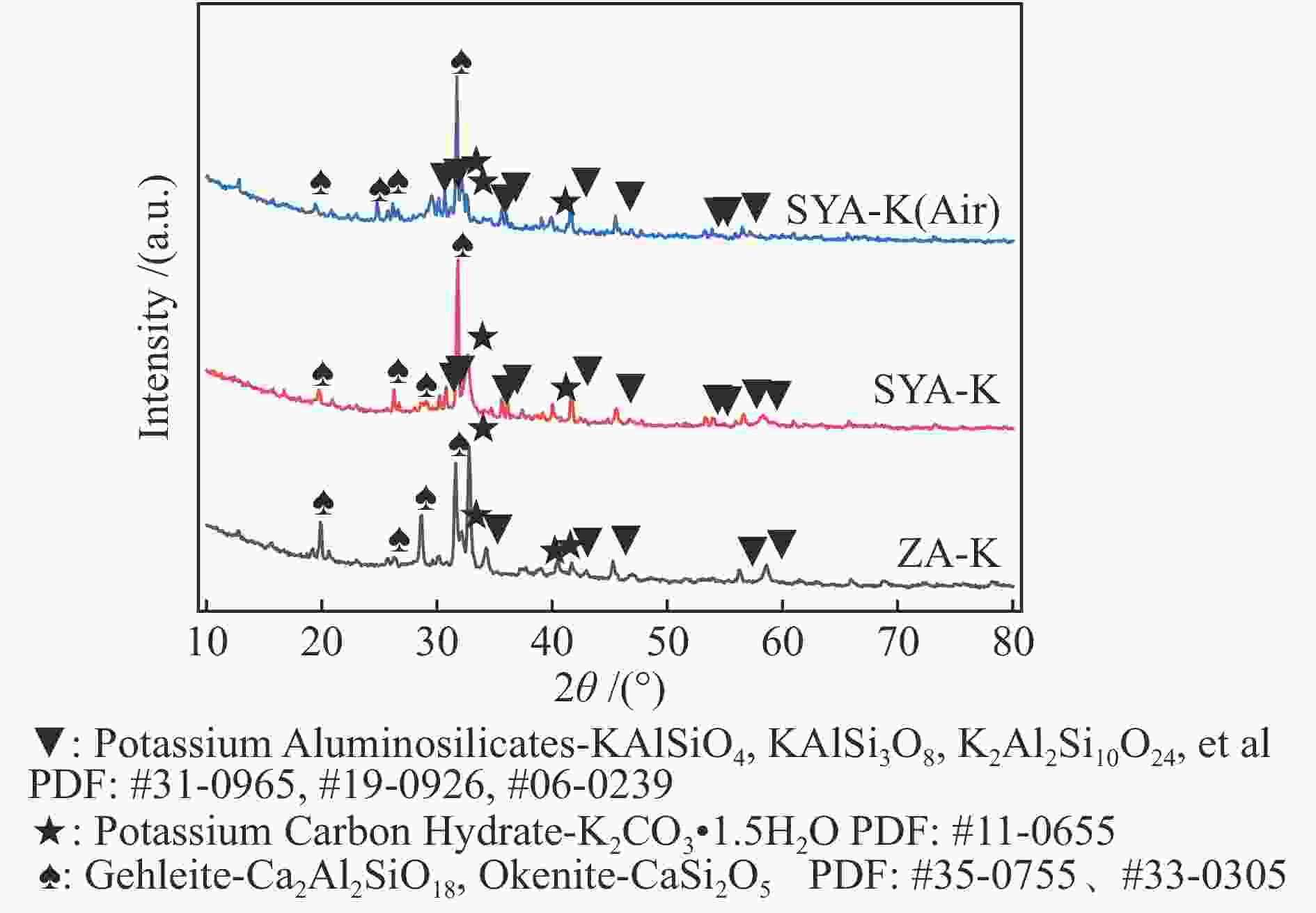
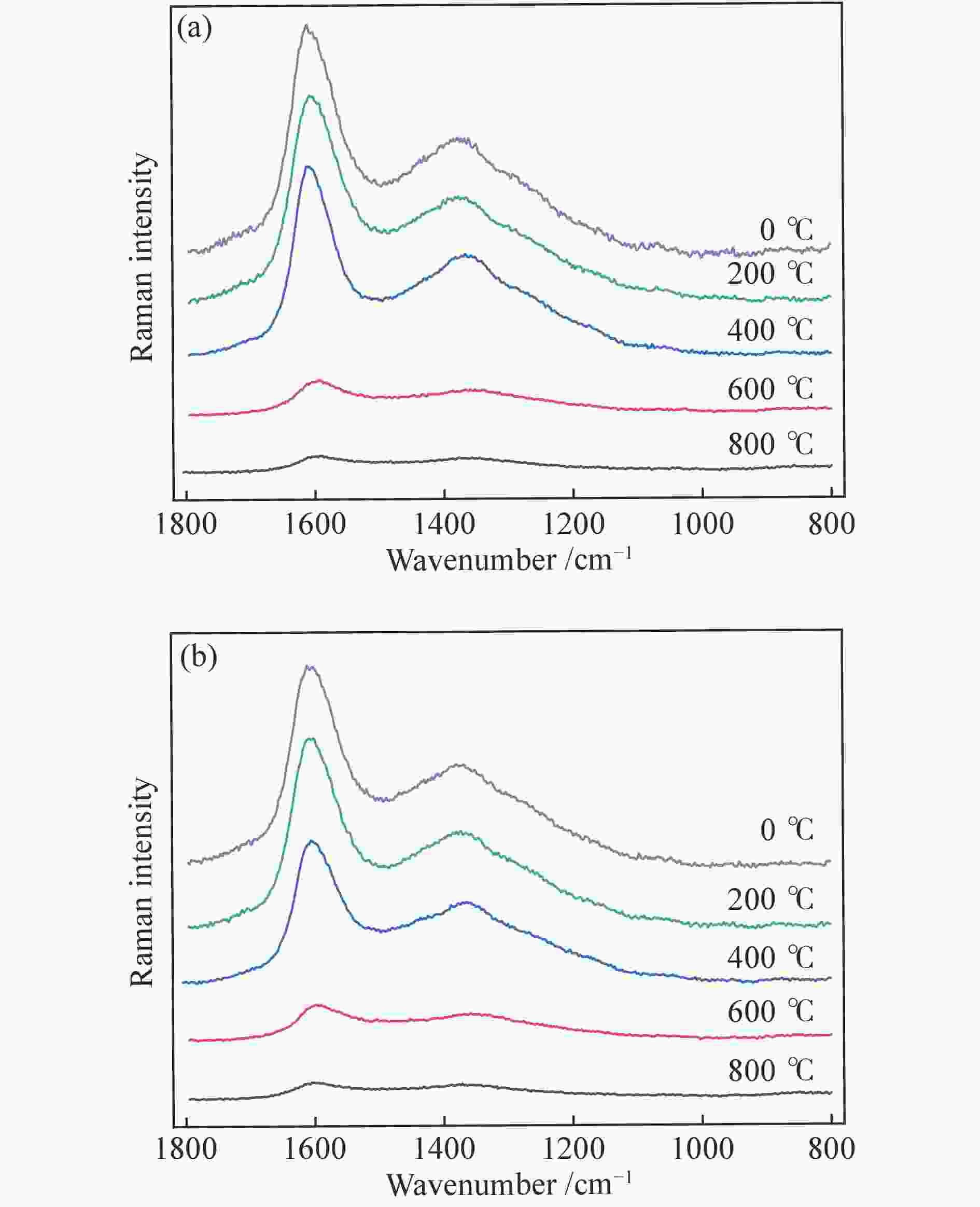
摘要: 本研究利用固定床反应装置、原子吸收光谱、X射线衍射法(XRD)考察负载碳酸钾的榆林煤(ZA-K)、负载碳酸钾的榆林脱灰煤(ZA-THK)、负载碳酸钾的模拟灰(采用SiO2、Al2O3、CaO、Fe2O3四种氧化物配置)气化反应后的钾迁移行为,采用傅里叶红外光谱、拉曼光谱,探究ZA-K及ZA-THK在热解过程中的结构演变对钾迁移行为的影响;实验结果表明,温度越高,气化反应残渣中水溶性钾回收效率越低;三次水洗可以回收总水溶性钾的94.06%−98.80%;不溶性钾的生成是因为钾与煤灰中硅铝生成钾的硅铝酸盐物相;ZA-THK比ZA-K中的钾在气化反应过程中更容易挥发,在700−850 ℃下,ZA-THK中的钾挥发比ZA-K高出10.28%−44.92%。主要原因是ZA-K中的灰分会将负载的钾固定在煤灰中;也是酸洗脱灰使煤的芳香聚合度降低,煤中出现更多的小环芳香结构(2−8环)。Abstract: A fixed bed reactor and atomic absorption spectroscopy were used to investigate potassium recovery efficiency of Yulin coal loaded with potassium carbonate (ZA-K), Yulin demineralized coal loaded with potassium carbonate (ZA-THK) and synthetic ash (Configurations of four oxides: SiO2, Al2O3, CaO, Fe2O3) loaded with potassium carbonate after reaction. Fourier infrared spectroscopy and Raman spectroscopy were used to study influence of structural evolution of ZA-K and ZA-THK on migration of potassium during pyrolysis. The results show that the yield of water-soluble potassium decreases with increasing temperature. Three times water washing could recover 94.06%−98.80% of the total water-soluble potassium. Formation of insoluble potassium is due to the phase of potassium aluminosilicate formed by potassium, silicon and aluminum in the coal ash. Potassium is easier to volatilize from ZA-THK than that from ZA-K. At 700−850 ℃ potassium in ZA-THK is volatilized 10.28%−44.92% higher than that of ZA-K, resulting from that the ash in ZA-K would fix the loaded potassium in coal ash. Another reason may be caused by decrease in the degree of aromatic polymerization of ZA-THK through demineralization process, leading to more small-ring aromatic structures (2−8 rings) appearing in the coal.
-
表 1 ZA和ZA-TH的工业分析及元素分析
Table 1 Proximate and ultimate analyses of ZA and ZA-TH
Sample Proximate analysis wd/% Ultimate analysis wdaf/% A V FC C H O* N S ZA 10.96 37.29 51.75 67.85 5.13 24.52 0.84 1.66 ZA-TH 1.44 29.58 68.98 66.76 5.43 25.60 0.63 1.58 *: by difference; d-dry basis;daf-dry and ash free basis 表 2 ZA的灰成分分析
Table 2 Ash composition of ZA
Sample SiO2 Al2O3 CaO Fe2O3 MgO K2O w/% 48.88 19.21 12.19 9.87 1.61 1.80 表 3 SYA的化学组成
Table 3 Chemical composition of SYA
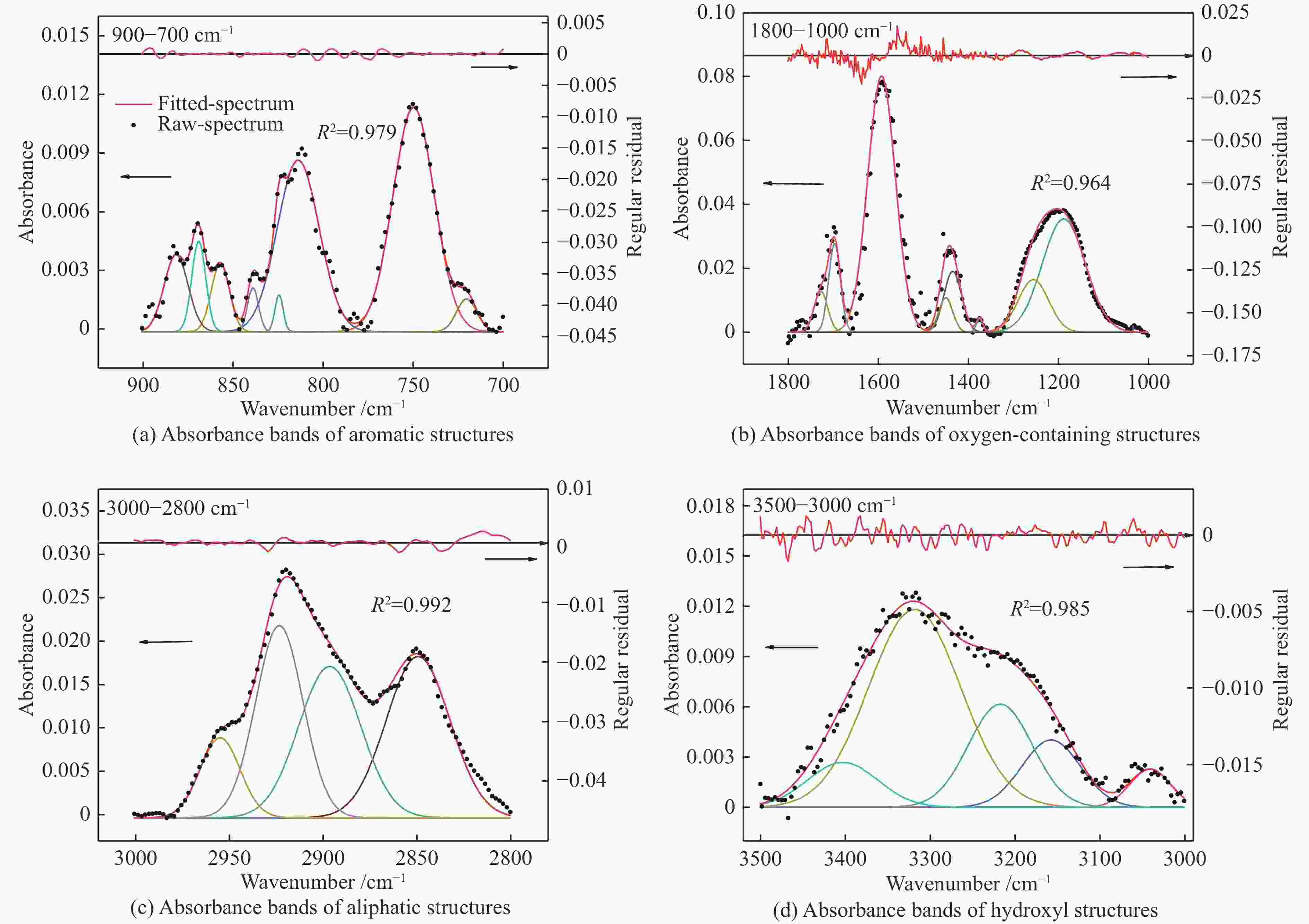
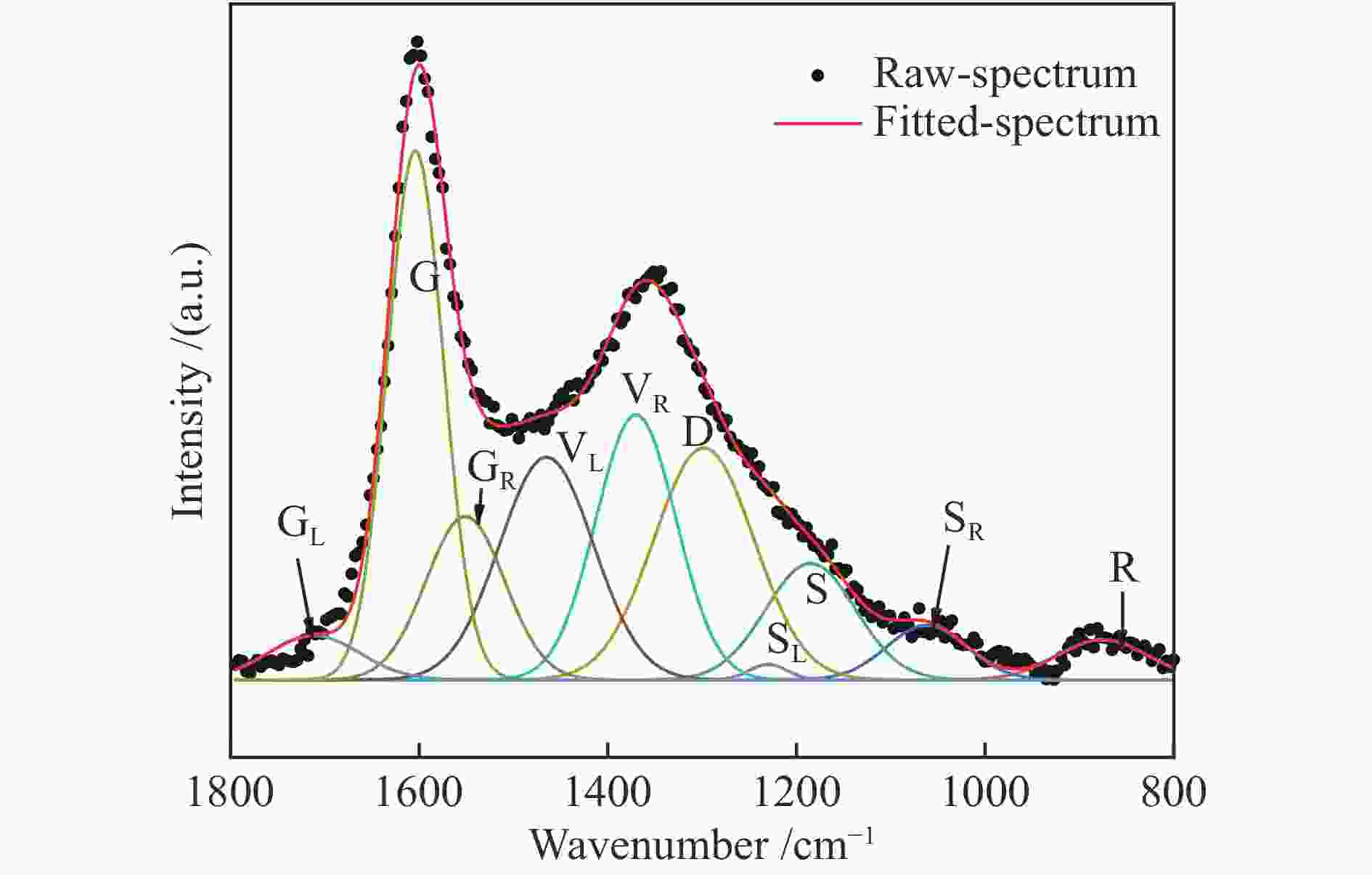
Sample SiO2 Al2O3 CaO Fe2O3 w/% 54.22 21.31 13.52 10.95 表 4 ZA-TH的红外光谱拟合峰参数
Table 4 Infrared spectrum fitting peak parameters of ZA-TH
Peak# Center/cm−1 Hight Width/cm−1 Assignment Area 1 3403.1 0.0027 97.2 stretching vibration of hydrogen-bond 0.2745 2 3318.3 0.0118 127.8 stretching vibration of −OH, −NH 1.6069 3 3217.3 0.0062 88.5 stretching vibration of hydrogen-bond 0.5799 4 3157.5 0.0040 81.7 stretching vibration of hydrogen-bond 0.3495 5 3040.9 0.0022 56.3 stretching vibration of CH in aromatic structures 0.1305 6 2955.0 0.0092 25.2 asymmetric stretching vibration of CH3 0.2464 7 2923.4 0.0221 29.8 stretching vibration of CH in alkanes 0.7005 8 2896.5 0.0174 39.0 stretching vibration of CH in alkanes 0.7227 9 2849.4 0.0186 40.0 symmetric stretching vibration of CH2 in alkanes 0.7892 10 1727.9 0.0128 35.8 stretching vibration of C=O in conjugated esters 0.4869 11 1697.6 0.0277 30.4 stretching vibration of C=O in carboxylic acids 0.8960 12 1592.9 0.0801 72.3 stretching vibration of C=C in aromatic rings 6.1682 13 1434.8 0.0190 46.2 asymmetric deformation vibration of CH3 0.9361 14 1375.0 0.0044 16.8 asymmetric stretching vibration of C−O−C in aromatic ethers 0.0784 15 1255.5 0.0165 79.3 stretching vibration of C−OH in phenols 1.3938 16 1188.0 0.0354 112.6 stretching vibration of C−OH in phenols 4.2467 17 881.7 0.0040 15.6 out-of-plane deformation vibration of =C−H in aromatic structures with isolated aromatic hydrogens (1H) 0.0655 18 869.2 0.0046 9.1 out-of-plane deformation vibration of =C−H in aromatic structures with isolated aromatic hydrogens (1H) 0.0447 19 857.1 0.0035 11.9 out-of-plane deformation vibration of =C−H in aromatic structures with two adjacent hydrogens per ring (2H) 0.0442 20 839.0 0.0022 7.2 out-of-plane deformation vibration of =C−H in aromatic structures with two adjacent hydrogens per ring (2H) 0.0172 21 824.5 0.0019 5.4 out-of-plane deformation vibration of =C−H in aromatic structures with two adjacent hydrogens per ring (2H) 0.0108 22 813.9 0.0088 27.4 out-of-plane deformation vibration of =C−H in aromatic structures with three adjacent hydrogens per ring (3H) 0.2558 23 749.8 0.0115 27.1 out-of-plane deformation vibration of =C−H in aromatic structures with three adjacent hydrogens per ring (3H) 0.3319 24 720.3 0.0017 13.2 plane swing vibration of alkane (CH2)n ≥ 4 0.0236 表 5 ZA和ZA-TH的红外结构参数
Table 5 Infrared structure parameters of ZA and ZA-TH
Sample ƒa A(CH2)/A(CH3) I ‘C’ DOC ZA 0.945 4.612 0.235 0.202 0.146 ZA-TH 0.946 2.843 0.323 0.183 0.128 -
[1] 武恒, 李克忠, 吴松怡, 赵锐君, 刘雷, 王会芳. 钾钠复合催化剂对煤气化反应的影响[J]. 现代化工,2020,40(11):109−112.WU Heng, LI Ke-zhong, WU Song-yi, ZHAO Rui-jun, LIU Lei, WANG Hui-fang. Effect of K2CO3-Na2CO3 compound catalyst on coal gasification[J]. Mod Chem Ind,2020,40(11):109−112. [2] LI W, YU Z, GUAN G. Catalytic coal gasification for methane production: A Review[J]. Carbon Res Convers,2021,4:89−99. doi: 10.1016/j.crcon.2021.02.001 [3] ARNOLD R A, HILL J M. Catalysts for gasification: A review[J]. Sustainable Energy Fuels,2019,3:656−672. doi: 10.1039/C8SE00614H [4] WANG J, JIANG M, YAO Y, ZHANG Y, CAO J. Steam gasification of coal char catalyzed by K2CO3 for enhanced production of hydrogen without formation of methane[J]. Fuel,2009,88(9):1572−1579. [5] YUAN X Z, NAMKUNG H, KANG T, KIM H. K2CO3-catalyzed steam gasification of Indonesian low-rank coal for H2-rich gas production in a fixed bed reactor[J]. Energy Technol,2015,3(5):527−534. doi: 10.1002/ente.201402198 [6] ARASH K, MURRAY R G. Effectiveness and mobility of catalysts for gasification of bitumen coke[J]. Fuel,2011,90(1):120−125. doi: 10.1016/j.fuel.2010.07.032 [7] CHEN S G, YANG R T. Unified mechanism of alkali and alkaline earth catalyzed gasification reactions of carbon by CO2 and H2O[J]. Energy Fuels,1997,11(2):421−427. doi: 10.1021/ef960099o [8] WIGMANS T, GOEBEL J C, MOULIJN J A. The influence of pretreatment conditions on the activity and stability of sodium and potassium catalysts in carbon-steam reactions[J]. Carbon,1983,21(3):295−301. doi: 10.1016/0008-6223(83)90094-5 [9] SHADMAN F, SAMS D A, PUNJAK W A. Significance of the reduction of alkali carbonates in catalytic carbon gasification[J]. Fuel,1987,66(12):58−63. [10] SABER J M, KESTER K B, FALCONER J L, BROWN L F. A mechanism for sodium oxide catalyzed CO2 gasification of carbon[J]. J Catal,1988,109(2):329−346. doi: 10.1016/0021-9517(88)90216-3 [11] KOPYSCINSKI J, RAHMAN M, GUPTA R, MIMS C A, HILL J M. K2CO3 catalyzed CO2 gasification of ash-free coal. Interactions of the catalyst with carbon in N2 and CO2 atmosphere[J]. Fuel,2014,117(1):1181−1189. [12] HUHN F, KLEIN J, JUNTGEN H. Investigations on the alkali-catalysed steam gasification of coal: Kinetics and interactions of alkali catalyst with carbon[J]. Fuel,1983,62(2):196−199. doi: 10.1016/0016-2361(83)90197-7 [13] FENG D, ZHAO Y, ZHANG Y, XU H H, ZHANG L Y, SUN S Z. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification[J]. Fuel,2018,212(15):523−532. [14] LU T, MAO Y, WANG H, LIU L, LI K. Effect of pre-treatment on catalytic coal gasification characteristics of sub-bituminous coal[J]. J Energy Inst,2021,96:173−180. doi: 10.1016/j.joei.2021.03.013 [15] 陈杰, 陈凡敏, 王兴军, 于广锁, 王辅臣. 煤催化气化过程中钾催化剂回收的实验研究[J]. 化学工程,2012,40(6):68−71. doi: 10.3969/j.issn.1005-9954.2012.06.017CHEN Jie, CHEN Fan-min, WANG Xing-jun, YU Guang-suo, WANG Fu-chen. Experimental study on potassium catalyst recovery in coal catalytic gasification[J]. Chem Eng,2012,40(6):68−71. doi: 10.3969/j.issn.1005-9954.2012.06.017 [16] YUAN X, FAN S, CHOI S W, KIM H T, LEE K B. Potassium catalyst recovery process and performance evaluation of the recovered catalyst in the K2CO3-catalyzed steam gasification system[J]. Appl Energy,2017,195(1):850−860. [17] 梅艳钢, 王志青, 高松平, 郑洪岩, 张郃, 房倚天. 碱金属与碱土金属在煤炭热转化过程中的影响研究进展[J]. 燃料化学学报,2020,48(4):386−393.MEI Yan-gang, WANG Zhi-qing, GAO Song-ping, ZHANG He, FANG Yi-tian. Research progress of the influence of alkali metals and alkaline earth metals on coal thermal chemical conversion[J]. J Fuel Chem Technol,2020,48(4):386−393. [18] LI X, HAYASHI J, LI C. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal[J]. Fuel, 2006, 85(10/11): 1700−1707. [19] YE C P, YANG Z J, LI W Y, RONG H L, FENG J. Effect of adjusting coal properties on HulunBuir lignite pyrolysis[J]. Fuel Process Technol,2017,156:415−20. doi: 10.1016/j.fuproc.2016.10.002 [20] XIE X, LIU L, LIN D, QIU P. Influence of different state alkali and alkaline earth metal on chemical structure of Zhundong coal char pyrolyzed at elevated pressures[J]. Fuel,2019,254:1−11. [21] XIE X, ZHAO Y, QIU P H, LIN D, QIAN J, HOU H M, PEI J. Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks[J]. Fuel,2018,216(15):521−530. [22] XUE Q H, LIU X F, NIE B S, SONG D Z. FTIR and Raman spectroscopy characterization of functional groups in various rank coals[J]. Fuel,2017,206(15):555−563. [23] 江国栋. 低阶煤热解反应动力学实验与模型研究[D]. 西安: 西北大学, 2019.JIANG Guo-dong. Experimental and model research on pyrolysis reaction kinetics of low-rank coal[D]. Xi'an: Northwest University, 2019. [24] 王瀚姣, 杜美利, 薛文海, 刘忠诚. 酸洗对黄陵富油煤结构和动力学特征的影响[J]. 煤炭转化,2021,44(4):38−42.WANG Han-jiao, DU Mei-li, XUE Wen-hai, LIU Zhong-cheng. Effects of pickling on structure and Kinetic characteristic of Huangling oil-rich coal[J]. Coal Convers,2021,44(4):38−42. [25] 毛燕东. 煤催化气化过程中矿物质变迁规律及结渣机理研究[D]. 天津: 天津大学, 2017.MAO Yan-dong. Minerals transformation and slagging mechanism of coal ash in catalytic coal gasification process[D]. Tianjin: Tianjin University, 2017. [26] VARGAS S, FRANDSEN F J, DAM J K. Rheological properties of high-temperature melts of coal ashes and other silicates[J]. Prog Energy Combust Sci,2001,27(3):237−429. doi: 10.1016/S0360-1285(00)00023-X [27] 王恩德, 付建飞, 王丹丽. 结晶学与矿物学实验教程[M]. 北京: 地质出版社, 2014.WANG En-de, FU Jian-fei, WANG Dan-li. Experimental Course of Crystallography and Mineralogy[M]. Beijing: Geological Publishing House, 2014. [28] FERRARI AC, ROBERTSON J. Interpretation of Raman spectra of disordered and amorphous carbon[J]. Phys Rev B,2000,61(20):14095−14107. doi: 10.1103/PhysRevB.61.14095 [29] LI X, HAYASHI J, LI C. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part VII. Raman spectroscopic study on the changes in char structure during the catalytic gasification in air[J]. Fuel,2006,85(10-11):1509−1517. doi: 10.1016/j.fuel.2006.01.011 [30] ZHANG J, ZHANG R, BI J. Effect of catalyst on coal char structure and its role in catalytic coal gasification[J]. Catal Commun,2016,79(5):1−5. [31] UMEMOTO S, KAJITANI S, HARA S. Modeling of coal char gasification in coexistence of CO2 and H2O considering sharing of active sites[J]. Fuel,2013,103:14−21. doi: 10.1016/j.fuel.2011.11.030 [32] CHEN S G, YANG R T. Mechanism of alkali and alkaline earth catalyzed gasification of graphite by CO2 and H2O studied by electron microscopy[J]. J Catal,1992,138(1):12−23. doi: 10.1016/0021-9517(92)90003-Z -




 下载:
下载: