Research of hydrogen action during pre-sulfidation of direct coal liquefaction catalyst
-
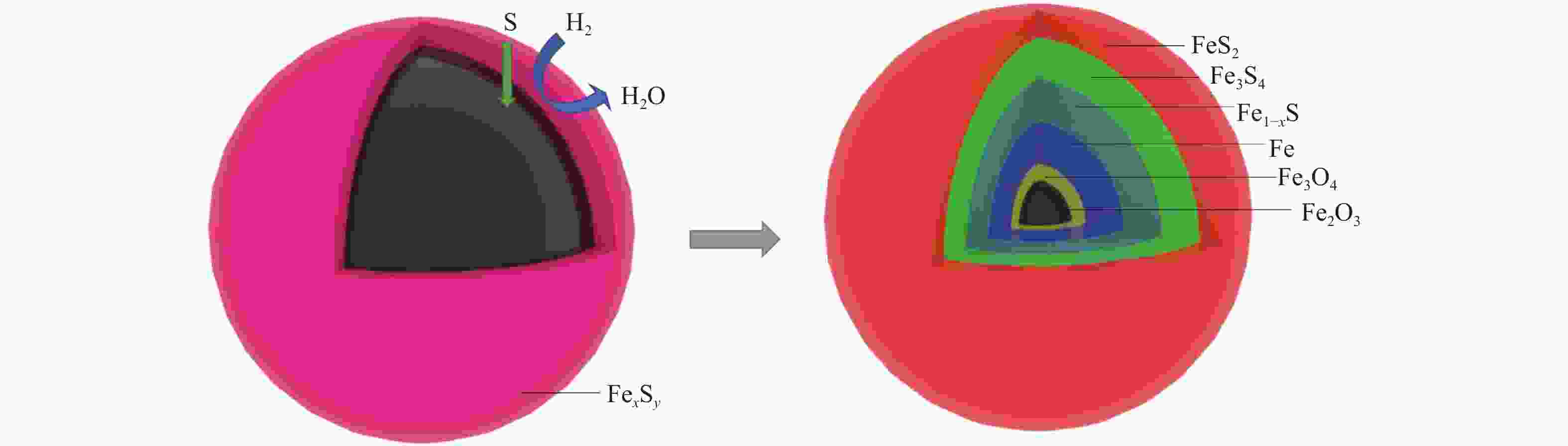
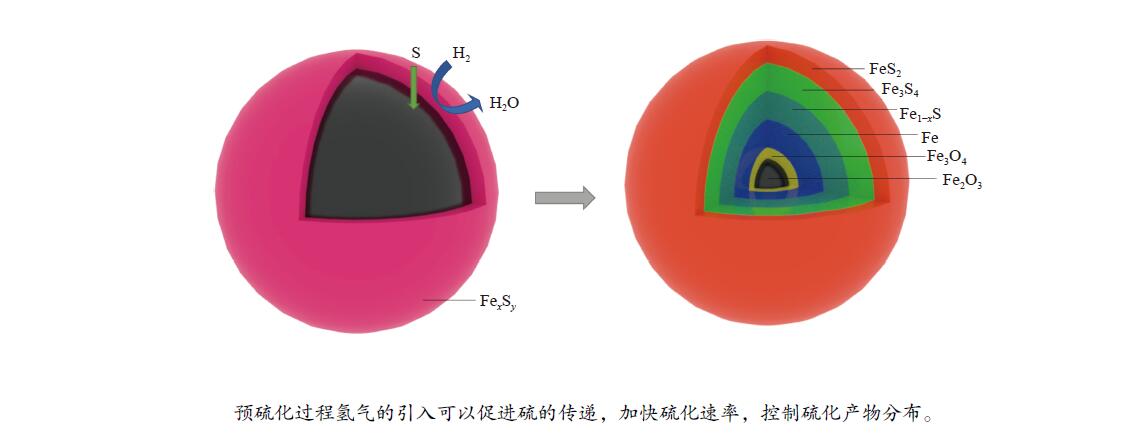
摘要: 在不同温度、不同氢气分压下预硫化制备了系列硫铁催化剂,并在5 MPa 1% H2S-H2气氛、360 ℃下研究了其催化萘加氢活性。借助XRD、MES、SEM-EDS、ICP和GC-MS等表征手段,研究了不同温度下氢气在预硫化过程中的作用。结果表明,预硫化过程中氢气的引入有利于硫的传递,从而促进了硫化。在不同温度下均有利于活性相Fe1−xS生成,但不同预硫化温度下氢气的作用有所不同。50 ℃预硫化时,氢气的引入可以有利于硫的传递,使得催化活性有所上升,但该温度下依然存在大量单质Fe和单质S;150 ℃预硫化时催化活性最好,单质Fe含量下降没有单质S生成,随着氢气分压的增大,萘加氢转化率由60.6%增加至69.1%;300 ℃预硫化时氢气将催化剂表面的硫还原至低价态,不利于硫的传递,在催化剂颗粒内部观察到晶态的Fe3O4,进而催化活性有不同程度的下降。Abstract: A series of iron sulfide catalysts were prepared by pre-sulfidation at different temperatures and different hydrogen partial pressures, and their catalytic naphthalene hydrogenation activities were studied under 5 MPa 1% H2S-H2 atmosphere at 360 ℃. By means of XRD, MES, SEM-EDS, ICP and GC-MS, the effect of hydrogen on the pre-sulfidation process at different temperatures was studied. The results show that the introduction of hydrogen during the pre-sulfidation process facilitates the transfer of sulfur, thereby promoting the sulfidation. The effect of hydrogen is different at different pre-sulfidation temperatures. When pre-sulfidation at 50 ℃, the introduction of hydrogen can be beneficial to the transfer of sulfur, which increases the catalytic activity, but a lot of elemental Fe and elemental S can still be observed at this temperature; when pre-sulfidation at 150 ℃, the catalytic activity is the best. The content of zero-valent Fe decreases and no pure S is observed. With the increase of hydrogen partial pressure, the hydrogenation conversion of naphthalene increases from 60.6% to 69.1%. When pre-sulfidation at 300 ℃, the catalytic activity decreases with the introduction of hydrogen. Hydrogen reduces the sulfur on the catalyst surface to low valence state and crystalline Fe3O4 is observed inside the catalyst particles, which is not conducive to the transfer of sulfur.
-
Key words:
- pre-sulfidation /
- iron-based catalyst /
- hydrogen /
- direct liquefaction catalyst
-
表 1 Mössbauer谱的拟合参数
Table 1 Parameters of Mössbauer spectrum
Name Site IS/(mm·s−1) QS/(mm·s−1) H(T) Hexagonal pyrrhotite 1 0.74 0.01 33.01 2 0.72 0.02 30.44 3 0.71 0.02 28.18 4 0.74 0.01 25.63 Monoclinic pyrrhotite 1 0.85(1) −0.035(4) 33.4(1) 2 0.86(2) 0.084(10) 31.4(2) 3 0.81(1) −0.086(10) 27.1(1) 4a 0.83(1) 0.084(15) 24.4(2) 4b 0.82(1) 0.166(15) 20.7(2) Greigite octahedral 0.66 − 32.7 tetrahedral 0.38 − 31.9 Troilite − 0.89 −0.14 32.8 Pyrite − 0.43(1) 0.66(1) − Maghemite x 0.47 −0.01 51.0 y 0.34 −0.03 48.1 Magnetite A 0.42 0.06 51.6 B 0.99 0.89 51.0 Highly dispersed Fe − 0.02 −0.03 25.0 α-Fe − 0.09 0.04 33.8 表 2 Cat-50-p的Mössbauer谱解析
Table 2 Mössbauer spectrum results of Cat-50-p
p/% Content/% FeS2 Fe3S4 Fe1−xS FeS Fe2O3 Fe3O4 Fe Fe3+
(spm)Fe2+
(spm)0 0.0 3.7 15.1 7.6 40.0 22.1 8.7 2.0 1.0 5 0.0 6.4 21.7 4.0 37.2 16.5 10.6 2.3 1.2 20 0.0 8.4 25.5 1.6 28.0 15.4 16.7 3.5 0.9 45 0.0 8.5 25.5 0.4 32.6 13.2 15.8 3.1 0.9 90 0.0 17.8 31.6 3.2 17.6 4.3 20.0 3.9 1.8 95 0.0 16.3 31.7 5.1 12.7 2.6 24.9 5.3 1.4 表 3 Cat-50-p的(S/Fe)atom和单质硫质量
Table 3 (S/Fe) atom and elemental sulfur quality results for Cat-50-p
p/% 0 5 20 45 90 95 S/Fe 0.87 0.88 0.89 0.96 1.14 1.16 S/(${\rm{g}}\cdot {\rm{g}}_{{\rm{cat}}}^{-1} $) 0.0155 0.0125 0.0094 0.0079 0.0068 0.0056 表 4 Cat-50-p的XPS拟合
Table 4 XPS fitting results of Cat-50-p
Content/% Fe(0) Fe(Ⅱ)−S Fe(Ⅲ)−S Fe(Ⅱ)−O Fe(Ⅲ)−O ${\rm{S}}_{2}^{{2-}} $ S2− S/S8 Cat-50-0 0.0 2.3 0.9 6.4 90.4 7.1 78.9 14.0 Cat-50-20 0.0 7.9 7.5 7.7 76.9 16.7 80.5 2.8 Cat-50-45 0.0 16.5 18.4 10.1 55.1 32.1 67.9 0.0 Cat-50-95 0.0 22.7 32.6 15.2 29.5 33.9 66.1 0.0 表 5 Cat-150-p的Mössbauer谱解析
Table 5 Mössbauer spectrum results of Cat-150-p
p/% Content/% FeS2 Fe3S4 Fe1−xS FeS Fe2O3 Fe3O4 Fe 0 21.6 14.4 23.9 0.0 24.2 11.0 5.0 5 18.9 16.0 22.7 3.4 21.9 12.6 4.5 20 16.5 16.5 25.8 3.2 25.3 7.4 5.3 45 14.8 22.4 28.6 0.3 16.5 7.6 9.8 90 15.2 19.0 42.6 2.7 2.1 6.3 12.2 95 15.6 20.6 43.8 2.5 2.8 4.3 10.4 表 6 Cat-150-p的(S/Fe)atom
Table 6 (S/Fe) atom results for Cat-150-p
p/% 0 5 20 45 90 95 S/Fe 0.92 0.94 0.96 1.03 1.19 1.23 表 7 Cat-150-p的XPS拟合结果
Table 7 XPS fitting results of Cat-150-p
Content/% Fe(0) Fe(Ⅱ)−S Fe(Ⅲ)−S Fe(Ⅱ)−O Fe(Ⅲ)−O ${\rm{S} }_{2}^{{2-} } $ S2− S/S8 Cat-150-0 0.0 25.2 5.3 22.1 47.4 38.6 60.2 1.3 Cat-150-20 0.0 24.3 15.1 21.3 39.3 36.0 64.0 0.0 Cat-150-45 0.0 24.7 11.7 18.6 45.0 31.6 68.4 0.0 Cat-150-95 0.0 28.5 23.5 21.7 26.3 37.9 62.1 0.0 表 8 Cat-300-p的Mossbauer谱解析
Table 8 Mössbauer spectrum results of Cat-300-p
p/% Content/% FeS2 Fe3S4 Fe1−xS FeS Fe2O3 Fe3O4 Fe 0 20.9 9.4 26.2 0.0 28.2 12.9 2.4 5 5.7 10.8 40.2 4.2 24.9 14.2 0.0 20 2.2 4.7 55.5 4.1 16.6 14.1 2.8 45 3.0 9.7 57.3 1.3 9.8 15.2 3.8 90 1.7 6.9 63.4 5.8 8.8 10.5 2.9 95 6.7 7.9 53.2 5.8 13.2 9.3 3.9 表 9 Cat-300-p的(S/Fe)atom结果
Table 9 (S/Fe) atom results for Cat-300-p
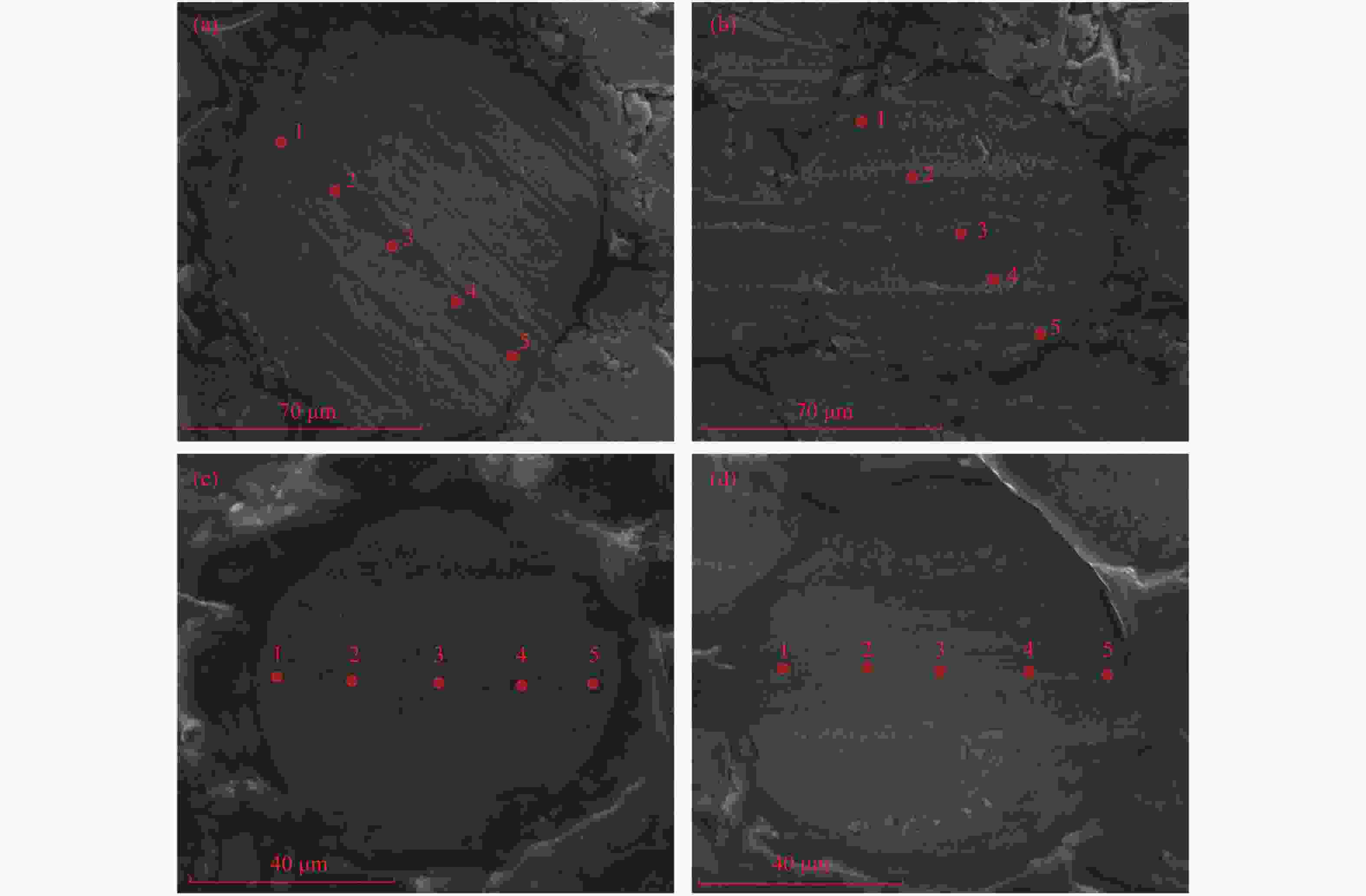
p/% 0 5 20 45 90 95 S/Fe 0.95 0.87 0.84 0.90 0.90 0.91 表 10 Cat-300-95的SEM-EDS的(O/S)wt
Table 10 (O/S) wt results of SEM-EDS of Cat-300-95
Site 1 Site 2 Site 3 Site 4 Site 5 a 0.59 0.65 0.79 0.70 0.61 b 0.68 0.60 0.71 1.44 0.66 c 0.74 0.77 0.94 0.92 0.88 d 0.22 0.34 3.52 0.47 0.69 表 11 Cat-300-p的XPS拟合
Table 11 XPS fitting results of Cat-300-p
Content/% Fe(0) Fe(Ⅱ)−S Fe(Ⅲ)−S Fe(Ⅱ)−O Fe(Ⅲ)−O ${\rm{S} }_{2}^{{2-} } $ S2− S/S8 Cat-300-0 0.0 23.7 13.1 13.3 49.9 45.3 54.7 0.0 Cat-300-20 0.0 23.7 11.9 18.8 45.6 31.6 68.4 0.0 Cat-300-45 0.0 26.0 24.0 22.2 27.9 20.6 79.4 0.0 Cat-300-95 0.0 29.8 25.2 21.5 23.5 19.5 80.5 0.0 表 12 不同温度下分压为0和95时的Mössbauer谱解析
Table 12 Mössbauer spectrum results of 0 and 95 in different temperature
Content/% FeS2 Fe3S4 Fe1−xS FeS Fe2O3 Fe3O4 Fe Fe3+
(spm)Fe2+
(spm)Cat-50-0 0.0 3.7 15.1 7.6 40.0 22.1 8.7 2.0 1.0 Cat-50-95 0.0 16.3 31.7 5.1 12.7 2.6 24.9 5.3 1.4 Cat-150-0 21.6 14.4 23.9 0.0 24.2 11.0 5.0 − − Cat-150-95 15.6 20.6 43.8 2.5 2.8 4.3 10.4 − − Cat-300-0 20.9 9.4 26.2 0.0 28.2 12.9 2.4 − − Cat-300-95 6.7 7.9 53.2 5.8 13.2 9.3 3.9 − − 表 13 不同温度下分压为0和95时的(S/Fe)atom结果
Table 13 (S/Fe) atom results for 0 and 95 in different temperature
Cat-50-0 Cat-50-95 Cat-150-0 Cat-150-95 Cat-300-0 Cat-300-95 S/Fe 0.87 1.16 0.82 1.23 0.95 0.91 表 14 不同温度下分压为0和95时的XPS拟合
Table 14 XPS fitting results of 0 and 95 in different temperature
Content/% Fe(0) Fe(Ⅱ)−S Fe(Ⅲ)-S Fe(Ⅱ)−O Fe(Ⅲ)−O ${\rm{S} }_{2}^{{2-} } $ S2− S/S8 Cat-50-0 0.0 2.3 0.9 6.4 90.4 7.1 78.9 14.0 Cat-50-95 0.0 22.7 32.6 15.2 29.5 33.9 66.1 0.0 Cat-150-0 0.0 25.2 5.3 22.1 47.4 38.6 60.2 1.3 Cat-150-95 0.0 28.5 23.5 21.7 26.3 37.9 62.1 0.0 Cat-300-0 0.0 23.7 13.1 13.3 49.9 45.3 54.7 0.0 Cat-300-95 0.0 29.8 25.2 21.5 23.5 19.5 80.5 0.0 -
[1] LIU Z, SHI S, LI Y. Coal liquefaction technologies—Development in China and challenges in chemical reaction engineering[J]. Chem Eng Sci,2010,65(1):12−17. doi: 10.1016/j.ces.2009.05.014 [2] WILLIAMS R H, LARSON E D. A comparison of direct and indirect liquefaction technologies for making fluid fuels from coal[J]. Energy Sustain Dev,2003,7(4):103−129. doi: 10.1016/S0973-0826(08)60382-8 [3] PETRAKIS L, GRANDY D W. Formation and behaviour of coal free radicals in pyrolysis and liquefaction conditions[J]. Nature,1981,289(5797):476−477. doi: 10.1038/289476a0 [4] PETRAKIS L, GRANDY D W, JONES G L. Use of in-situ electron paramagnetic resonance to assess formation of free radicals and their role in the hydroliquefaction of coal[J]. Fuel,1983,62(9):1066−1069. doi: 10.1016/0016-2361(83)90142-4 [5] POUTSMA M L. Free-radical thermolysis and hydrogenolysis of model hydrocarbons relevant to processing of coal[J]. Energy Fuels,1990,4(2):113−131. doi: 10.1021/ef00020a001 [6] KEOGH R A, DAVIS B H. Comparison of liquefaction pathways of a bituminous and subbituminous coal[J]. Energy Fuels,1994,8(2):289−293. doi: 10.1021/ef00044a001 [7] VASIREDDY S, MORREALE B, CUGINI A, SONG C, SPIVEY J J. Clean liquid fuels from direct coal liquefaction: chemistry, catalysis, technological status and challenges[J]. Energy Environ Sci,2011,4(2):311−345. doi: 10.1039/C0EE00097C [8] DADYBURJOR D B, FOUT T E, ZONDLO J W. Ferric-sulfide-based catalysts made using reverse micelles: Effect of preparation on performance in coal liquefaction[J]. Catal Today,2000,63(1):33−41. doi: 10.1016/S0920-5861(00)00443-0 [9] STRUCK R T, ZIELKE C W. Hydrocracking of coal to light distillate with molten zinc chloride[J]. Fuel,1981,60(9):795−800. doi: 10.1016/0016-2361(81)90140-X [10] KANEKO T, TAZAWA K, KOYAMA T, SATOU K, SHIMASAKI K, KAGEYAMA Y. Transformation of iron catalyst to the active phase in coal liquefaction[J]. Energy Fuels,1998,12(5):897−904. doi: 10.1021/ef9702310 [11] NAKAO Y, YOKOYAMA S, MAEKAWA Y, KAERIYAMA K. Coal liquefaction by colloidal iron sulphide catalyst[J]. Fuel,1984,63(5):721−722. doi: 10.1016/0016-2361(84)90176-5 [12] SHAH N, ZHAO J, HUGGINS F E, HUFFMAN G P. In situ XAFS spectroscopic studies of direct coal liquefaction catalysts[J]. Energy Fuels,1996,10(2):417−420. doi: 10.1021/ef950157q [13] HU H, BAI J, ZHU H, CHEN G. Catalytic liquefaction of coal with highly dispersed Fe2S3 impregnated in-situ[J]. Energy Fuels,2001,15(4):830−834. doi: 10.1021/ef000227f [14] LIU Z, YANG J, ZONDLO J W, STILLER A H. In situ impregnated iron-based catalysts for direct coal liquefaction[J]. Fuel,1996,75(1):51−57. doi: 10.1016/0016-2361(95)00226-X [15] 任相坤, 房鼎业, 金嘉璐, 高晋生. 煤直接液化技术开发新进展[J]. 化工进展,2010,29(2):198−204.REN Xiang-kun, FANG Ding-ye, JIN Jia-lu, GAO Jin-sheng. New proceed achieved in the direct coal liquefaction[J]. Chem Ind Eng Prog,2010,29(2):198−204. [16] 王仲义, 闫作杰, 单敏, 陈平平, 童健. 器外预硫化加氢裂化催化剂开工技术应用总结[J]. 炼油技术与工程,2021,51(1):4.WANG Zhong-yi, YAN Zuo-jie, SHAN Min, CHEN Ping-ping, DONG Jian. Application summary of Start-up technology of ex-situ presulfiding Hydrocracking catalyst[J]. Pet Refin Eng,2021,51(1):4. [17] 张黎, 范文青, 肖文灿, 徐琳, 刘长坤. 加氢催化剂预硫化技术探讨[J]. 广东化工,2020,47(12):2.ZHANG Li, FAN Wen-qing, XIAO Wen-chan, XU Lin, LIU Chang-kun. Study on the technology of pre-sulfurization for hydrogented catalyst[J]. Guangdong Chem Ind,2020,47(12):2. [18] 孟祥彬, 高善彬, 胡胜, 于春梅, 孙发明, 刘李佳. 以单质硫为硫化介质的加氢催化剂间歇釜器外预硫化工艺[J]. 化工进展,2015,34(7):77−81.MENG Xiang-bin, GAO Shan-bin, HU Sheng, YU Chun-mei, SUN Fa-ming, LIU Li-jia. Presulfidation with sulphur for hydrogenation catalyst in a batch reactor[J]. Chem Ind Eng Prog,2015,34(7):77−81. [19] 高善彬, 董群, 迟克彬, 谭明伟, 刘彦峰, 孟祥彬. 以H2S为硫化介质的加氢催化剂间歇釜器外预硫化工艺[J]. 化工进展,2010,29(9):1654−1657+1665.GAO Shan-bin, DONG Qun, CHI Ke-bin, TAN Ming-wei, LIU Yan-feng, MENG Xiang-bin. Presulfidation with H2S for hydrogenation catalyst by batch reactor[J]. Chem Ind Eng Prog,2010,29(9):1654−1657+1665. [20] 姚军. 器内和器外预硫化技术[J]. 当代化工研究,2016,(4):2.YAO Jun. Technology for in-situ presufurzation and ex-situ presufiding[J]. Modern Chem Res,2016,(4):2. [21] NARKIEWICZ M R, MATHEWS J P. Improved low-volatile bituminous coal representation: Incorporating the molecular-weight distribution[J]. Energy Fuels,2008,22(5):3104−3111. doi: 10.1021/ef700779j [22] LI L, HOU Y, WU W, LIANG S, REN S. Behaviors of tetralin and 9, 10-dihydroanthracene as hydrogen donor solvents in the hydrogenolysis of coal-related model compounds[J]. Fuel Process Technol,2019,191:202−210. doi: 10.1016/j.fuproc.2019.04.005 [23] NIU B, JIN L, LI Y, SHI Z, HU H. Isotope analysis for understanding the hydrogen transfer mechanism in direct liquefaction of Bulianta coal[J]. Fuel,2017,203:82−89. doi: 10.1016/j.fuel.2017.04.079 [24] 陈晨, 李海杰, 白杨, 冯富祥, 田磊, 杨勇, 刘源, 郭强. 预硫化温度对煤直接液化催化剂组分转变及其催化性能的影响[J]. 燃料化学学报,2022,50(1):54−62. doi: 10.1016/S1872-5813(21)60118-4CHEN Chen, LI Hai-jie, BAI Yang, FENG Fu-xiang, TIAN Lei, YANG Yong, LIU yuan, GUO Qiang. Effect of sulfidation temperature on component transformation and catalytic performance of direct coal liquefaction catalyst[J]. J Fuel Chem Technol,2022,50(1):54−62. doi: 10.1016/S1872-5813(21)60118-4 -





 下载:
下载: