Study on catalytic combustion of chlorobenzene over TiO2-supported V-W composite bimetallic catalysts
-
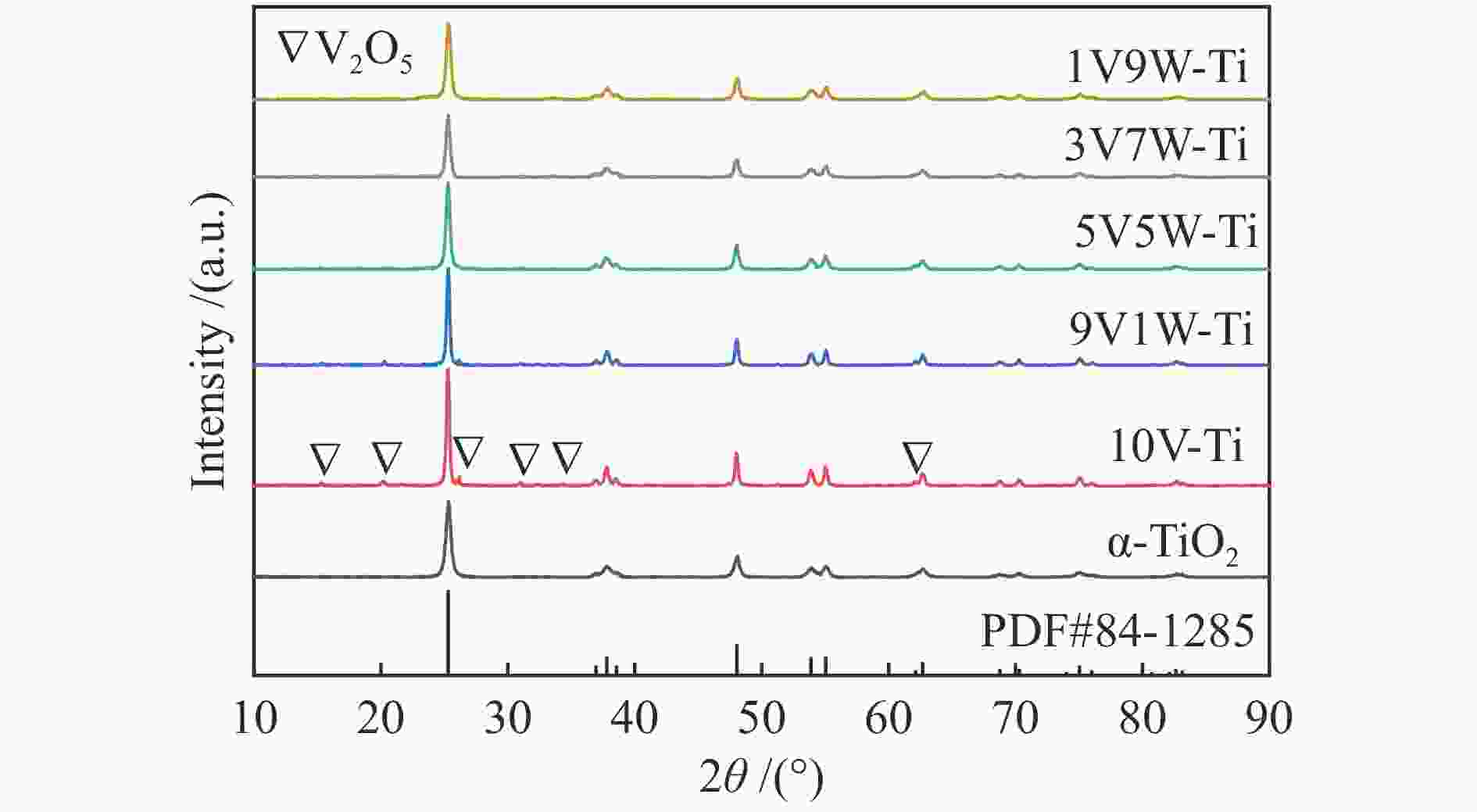
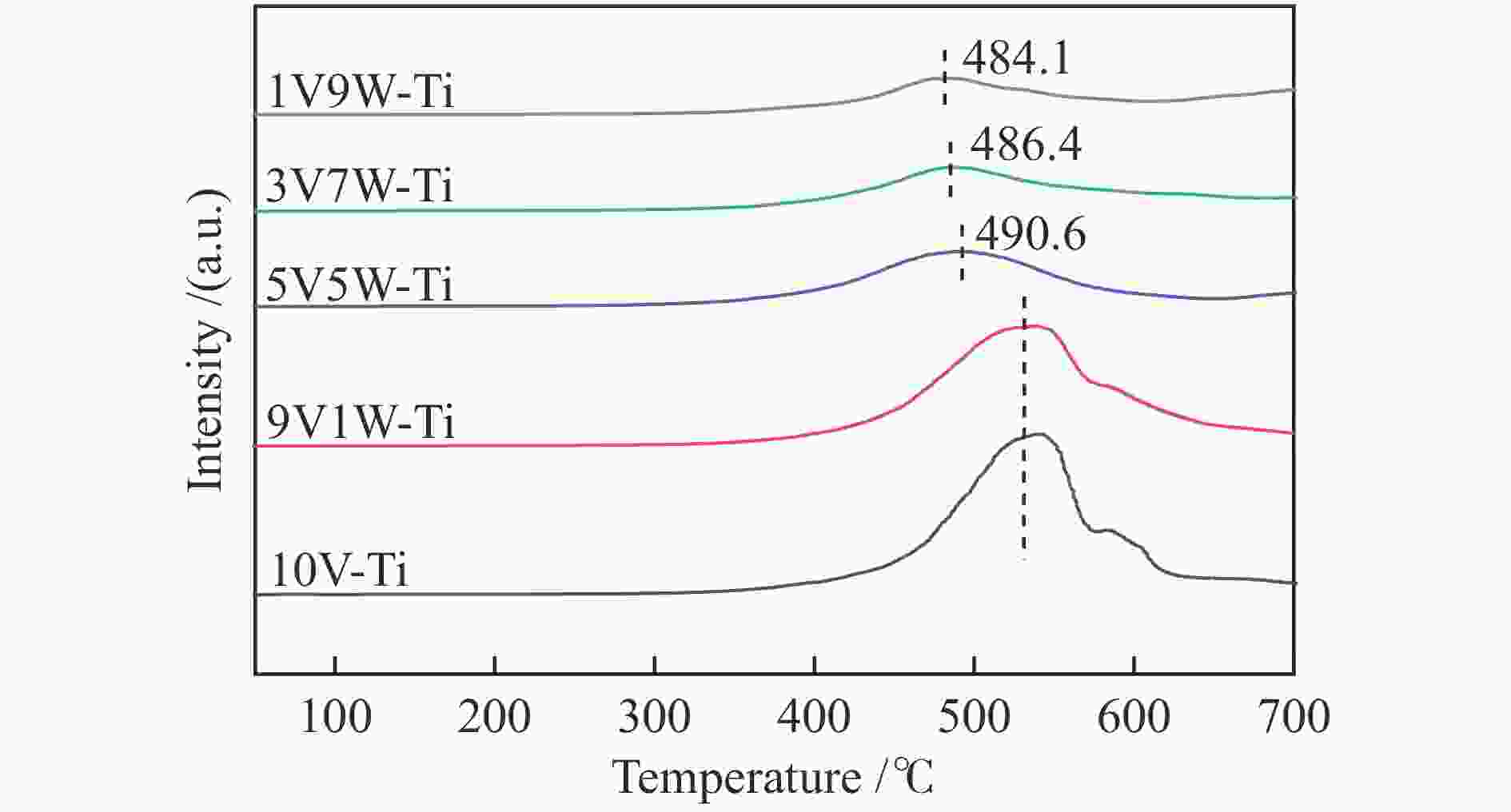
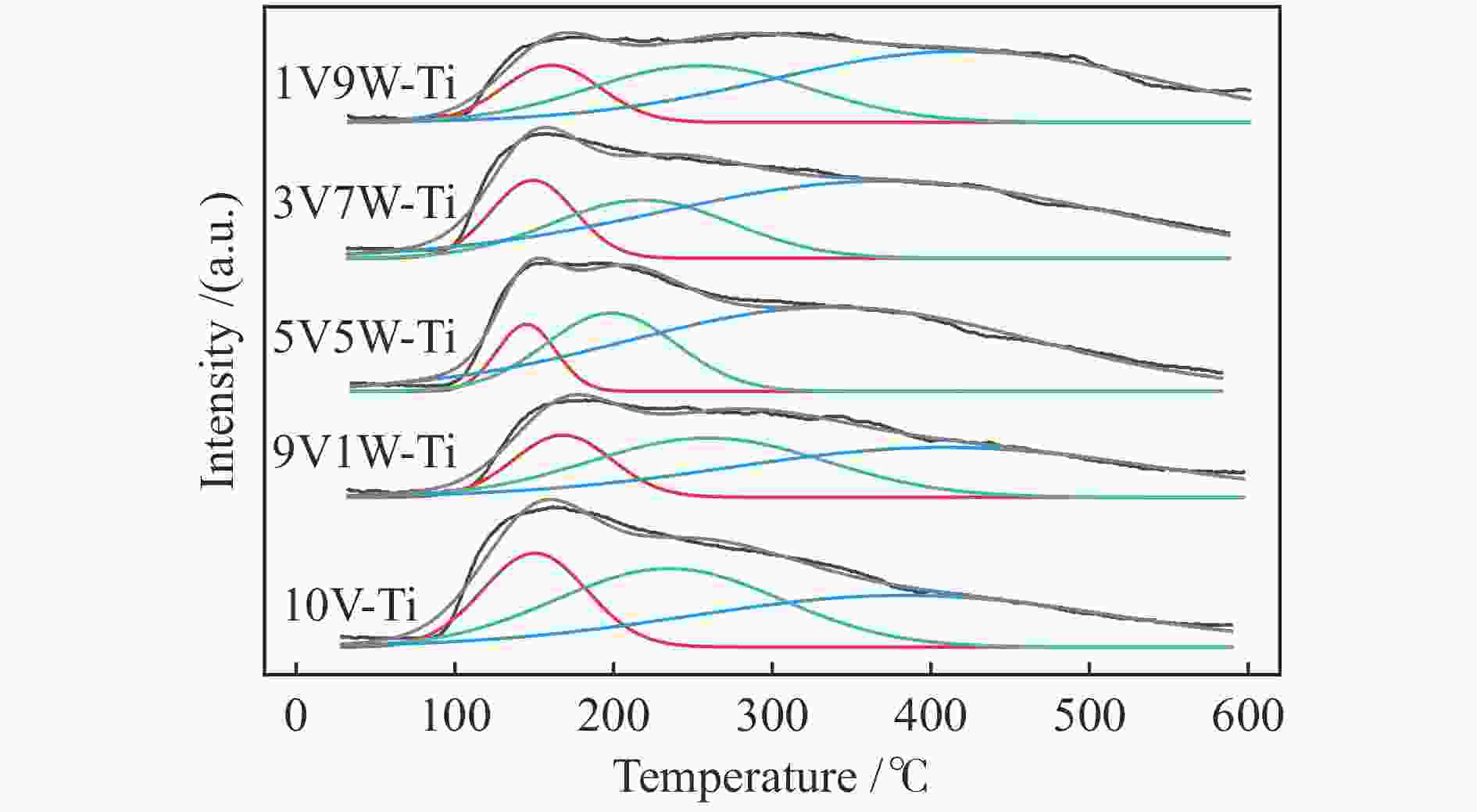
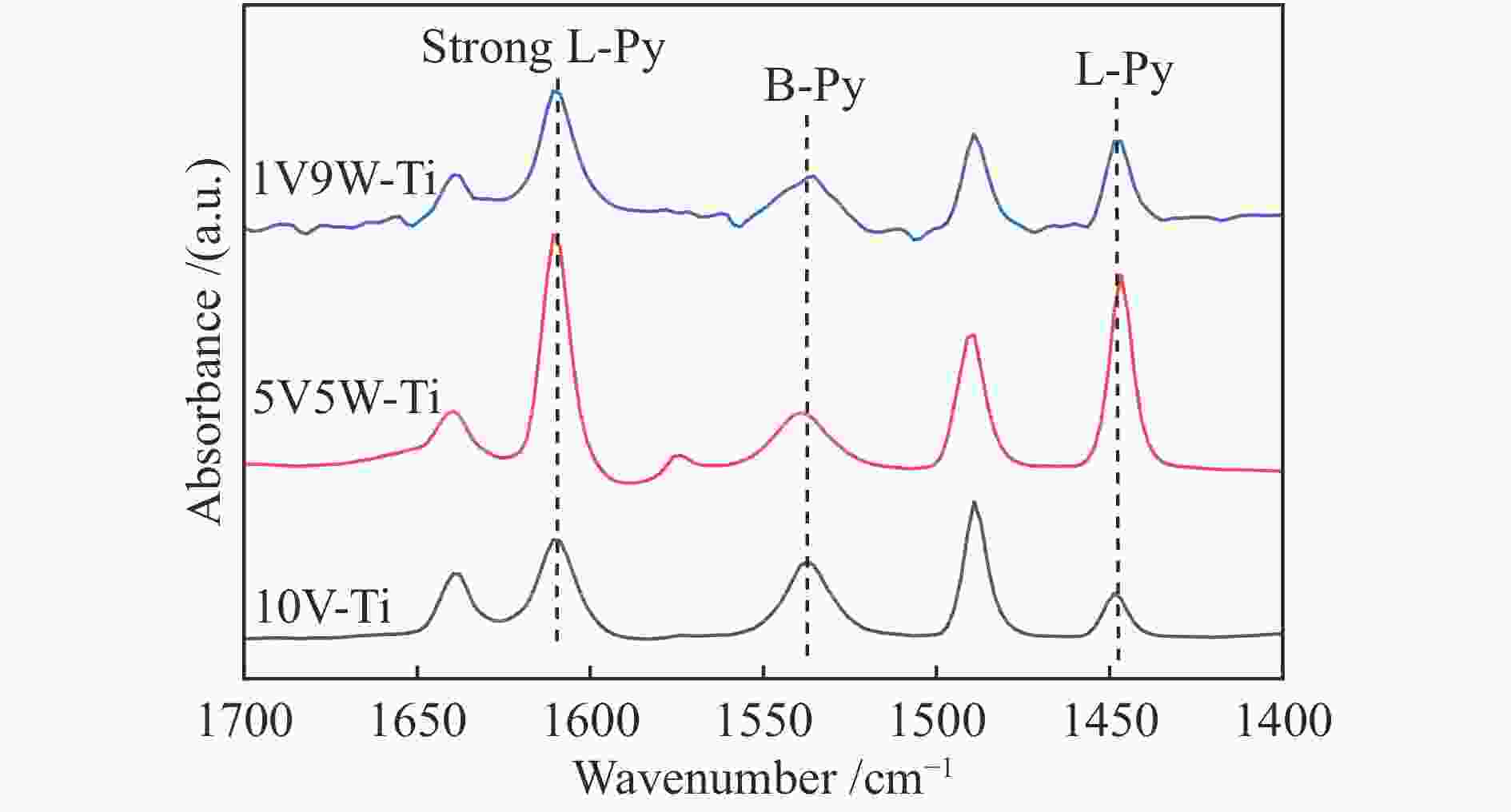
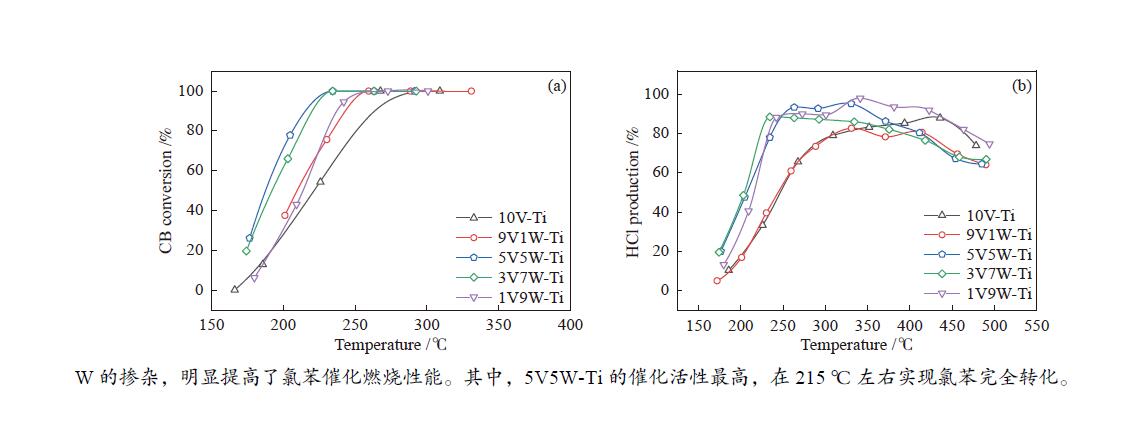
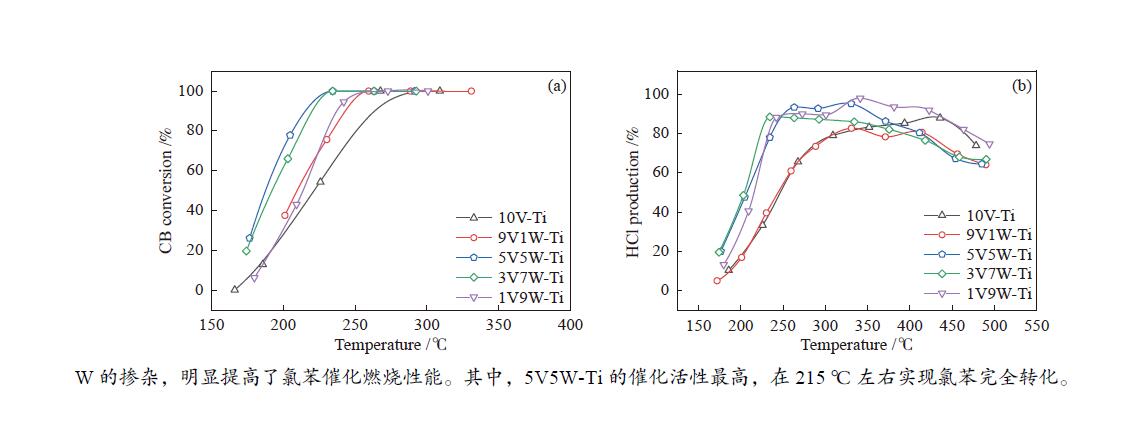
摘要: 本研究采用等体积浸渍法制备了一系列TiO2负载V-W双金属氧化物催化剂,考察了V/W比例对于氯苯催化燃烧活性和HCl选择性的影响。结果表明,适量W的掺杂(5V5W-Ti 和3V7W-Ti)提高了氯苯催化燃烧活性和HCl选择性。结合BET、XRD、XPS、H2-TPR、NH3-TPD和Py-FTIR等表征,说明其高的活性是由于其高活性组分分散度和丰富的催化剂表面吸附氧;适量W的掺杂显著增强了催化剂表面酸性,尤其是强酸和Brønsted酸,从而提高了产物中HCl的选择性。Abstract: A series of TiO2-supported V-W bimetallic catalysts were prepared by incipient wetness impregnation method. The effects of V/W ratios on the catalytic combustion performance for chlorobenzene were investigated. The results showed that proper W doping (5V5W-Ti and 3V7W-Ti) improved the catalytic combustion activity of chlorobenzene and the selectivity of HCl. In combination with BET, XRD, XPS, H2-TPR, NH3-TPD and Py-FTIR characterizations, it indicated that higher activity for chlorobenzene oxidation was attributed to the high dispersion of the active species and the abundant surface adsorbed oxygen. In addition, moderate doping of W significantly enhanced the surface acidity of catalysts, especially strong acids and Brønsted acids, and thus improved the selectivity to HCl in the products.
-
Key words:
- chlorobenzene /
- catalytic combustion /
- bimetallic catalysts /
- selectivity to HCl
-
表 1 xV(10−x)W-Ti催化剂的比表面积和孔容
Table 1 Specific surface area and pore volume of xV(10−x)W-Ti catalysts ( x =1, 3, 5, 9 and 10)
Sample SBET/(m2·g−1) Pore volume/(mL·g−1) α-TiO2 89.73 0.26 10V-Ti 33.04 0.18 9V1W-Ti 54.71 0.16 5V5W-Ti 50.35 0.20 3V7W-Ti 51.96 0.22 1V9W-Ti 69.81 0.23 表 2 10V-Ti、5V5W-Ti和1V9W-Ti催化剂的酸量
Table 2 Acid amount of 10V-Ti, 5V5W-Ti and 1V9W-Ti catalysts
Sample B acid/ (μmol·g−1) L acid/ (μmol·g−1) Total acidity/ (μmol·g−1) 1V9W-Ti 51.03 28.29 79.32 5V5W-Ti 50.83 61.21 112.03 10V-Ti 14.26 59.37 73.63 表 3 10V-Ti、5V5W-Ti和1V9W-Ti催化剂表面元素价态及不同氧物种含量
Table 3 Surface elemental valence states and oxygen species content of 10V-Ti, 5V5W-Ti and 1V9W-Ti catalysts
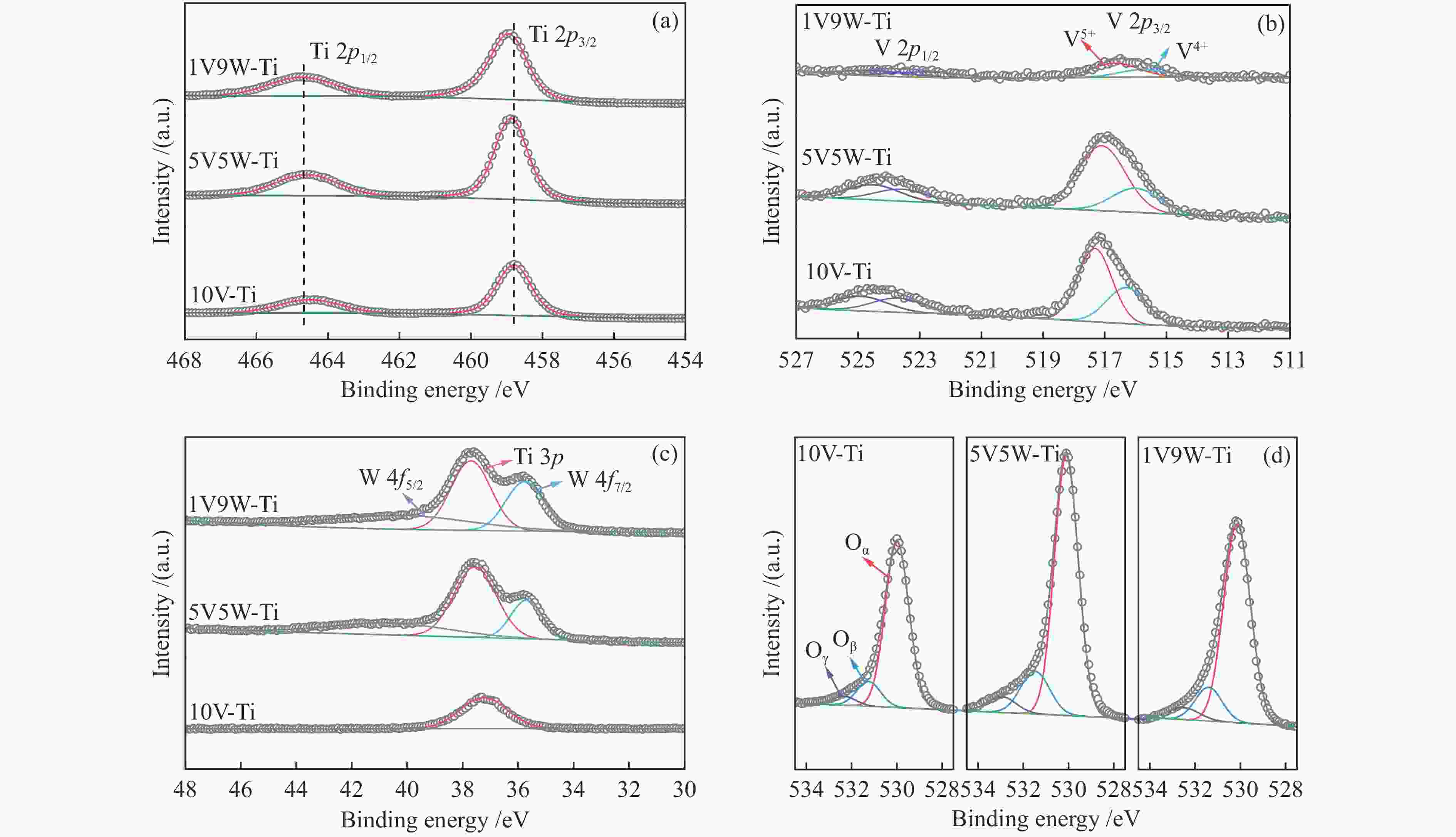
Sample V5+/(V5++V4+) Oβ/(Oα+Oβ+Oγ) Oγ/(Oα+Oβ+Oγ) 1V9W-Ti 0.65 0.14 0.06 5V5W-Ti 0.73 0.16 0.06 10V-Ti 0.64 0.12 0.05 -
[1] DELAIGLE R, DEBECKER D P, BERTINCHAMPS F, GAIGNEAUX E M. Revisiting the behaviour of vanadia-based catalysts in the abatement of (chloro)-aromatic pollutants: Towards an integrated understanding[J]. Top Catal,2009,52:501−516. doi: 10.1007/s11244-009-9181-9 [2] 关丽萍. 工业源VOCs排放特征及控制思路分析[J]. 现代化工,2018,38(10):20−22.GUAN Li-ping. Emission characteristics and control ideas of VOCs from industrial source[J]. Mod Chem Ind,2018,38(10):20−22. [3] BRINK R, KRZAN M, FEIJEN-JEURISSEN M, LOUW R, MULDER P. The role of the support and dispersion in the catalytic combustion of chlorobenzene on noble metal based catalysts[J]. Appl Catal B: Environ,2000,24(3):255−264. [4] ARANZABAL A. The reaction pathway and kinetic mechanism of the catalytic oxidation of gaseous lean TCE on Pd/alumina catalysts[J]. J Catal,2003,214(1):130−135. doi: 10.1016/S0021-9517(02)00091-X [5] WANG J, WANG X, LIU X, ZHU T, GUO Y, QI H. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts: The effects of chlorine substituents[J]. Catal Today,2015,241:92−99. doi: 10.1016/j.cattod.2014.04.002 [6] GANNOUN C, DELAIGLE R, ELOY P, DEBECKER D P, GHORBEL A, GAIGNEAUX, E M. Sol-gel derived V2O5-TiO2 mesoporous materials as catalysts for the total oxidation of chlorobenzene[J]. Catal Commun,2011,15(1):1−5. doi: 10.1016/j.catcom.2011.08.001 [7] LU S, ZHAO R, LEE C W, GULLETT B K, ZHAO Y X. Decomposition of trace chlorobenzene over V2O5-WO3/TiO2-based catalysts in simulated flue gas[J]. Int J Environ Pollut,2017,61(3/4):314. doi: 10.1504/IJEP.2017.087774 [8] 黄海凤, 宁星杰, 蒋孝佳, 顾蕾, 卢晗锋. V-M/TiO2(M = Cu、Cr、Ce、Mn、Mo)催化燃烧含氯有机废气[J]. 中国环境科学,2014,34(9):2179−2185.HUANG Hai-feng, NING Xing-jie, JIANG Xiao-jia, GU Lei, LU Han-feng. Catalytic combustion of chlorinated volatile organic compounds over V-M/TiO2(M = Cu, Cr, Ce, Mn, Mo) catalysts[J]. China Environ Sci,2014,34(9):2179−2185. [9] ALBONETTI S, BLASIOLI S, BONELLI R, MENGOU J E, SCIRÈ S, TRIFIRÒ F. The role of acidity in the decomposition of 1, 2-dichlorobenzene over TiO2-based V2O5/WO3 catalysts[J]. Appl Catal A: Gen,2008,341(1-2):18−25. doi: 10.1016/j.apcata.2007.12.033 [10] BERTINCHAMPS F, ATTIANESE A, MESTDAGH M M, GAIGNEAUX E M. Catalysts for chlorinated VOCs abatement: Multiple effects of water on the activity of VOx based catalysts for the combustion of chlorobenzene[J]. Catal Today,2006,112(1/4):165−168. doi: 10.1016/j.cattod.2005.11.043 [11] SINQUIN G, PETIT C, LIBS S, HINDERMANN J P, KIENNEMANN A. Catalytic destruction of chlorinated C2 compounds on a LaMnO3+δ perovskite catalyst[J]. Appl Catal B: Environ,2001,32(1):37−47. [12] TARALUNGA M, MIJOIN J, MAGNOUX P. Catalytic destruction of chlorinated POPs—Catalytic oxidation of chlorobenzene over PtHFAU catalysts[J]. Appl Catal B: Environ,2005,60(3/4):163−171. doi: 10.1016/j.apcatb.2005.02.024 [13] CEN W, LIU Y, WU Z, LIU J, WANG H, WENG X. Cl species transformation on CeO2(111) surface and its effects on CVOCs catalytic abatement: A first-principles investigation[J]. J Phys Chem C,2014,118(13):6758−6766. doi: 10.1021/jp411532b [14] CHIN S, PARK E, KIM M, BAE G N, JURNG J. Effect of the support material (TiO2) synthesis conditions in chemical vapor condensation on the catalytic oxidation for 1, 2-dichlorobenzene over V2O5/TiO2[J]. Powder Technol,2012,217(2):388−393. [15] WU M, UNG K C, DAI Q, WANG X. Catalytic combustion of chlorinated VOCs over VOx/TiO2 catalysts[J]. Catal Commun,2012,18:72−75. doi: 10.1016/j.catcom.2011.11.028 [16] CAO S, WANG H, YU F, SHI M, CHEN S, WENG X, LIU Y, WU Z. Catalyst performance and mechanism of catalytic combustion of dichloromethane (CH2Cl2) over Ce doped TiO2[J]. J Colloid Interf Sci,2016,463:233−241. doi: 10.1016/j.jcis.2015.10.061 [17] YAZAWA Y, YOSHIDA H, TAKAGI N, KOMAI S I, SATSUMA A, HATTORI T. Acid strength of support materials as a factor controlling oxidation state of palladium catalyst for propane combustion[J]. J Catal,1999,187:15−23. doi: 10.1006/jcat.1999.2583 [18] YAZAWA Y, YOSHIDA H, TAKAGI N, KOMAI S I, SATSUMA A, HATTORI T. Oxidation state of palladium as a factor controlling catalytic activity of Pd/SiO2-Al2O3 in propane combustion[J]. Appl Catal B: Environ,1998,19:261−266. doi: 10.1016/S0926-3373(98)00080-0 [19] ZHOU W, ZHANG Q, ZHOU Y, WEI Q, DU L, DING S, JIANG S, ZHANG Y. Effects of Ga- and P-modified USY-based NiMoS catalysts on ultra-deep hydrodesulfurization for FCC diesels[J]. Catal Today,2018,305:171−181. doi: 10.1016/j.cattod.2017.07.006 [20] JIANG Y, GAO X, ZHANG Y, WU W, SONG H, LUO Z, CEN K. Effects of PbCl2 on selective catalytic reduction of NO with NH3 over vanadia-based catalysts[J]. J Hazard Mater,2014,274:270−278. doi: 10.1016/j.jhazmat.2014.04.026 [21] LAI J K, WACHS I E. A perspective on the selective catalytic reduction (SCR) of NO with NH3 by supported V2O5-WO3/TiO2 catalysts[J]. ACS Catal,2018,8:6537−6551. doi: 10.1021/acscatal.8b01357 [22] ODENBRAND, INGEMAR, C U. CaSO4 deactivated V2O5-WO3/TiO2 SCR catalyst for a diesel power plant. Characterization and simulation of the kinetics of the SCR reactions[J]. Appl Catal B: Environ,2018,234:365−377. doi: 10.1016/j.apcatb.2018.03.063 [23] LI P, ZHAO X, JIA C J, SUN H, SUN L, CHENG X, LIU L, FAN W. ZnWO4/BiOI heterostructures with highly efficient visible light photocatalytic activity: The case of interface lattice and energy level match[J]. J Mater Chem A,2013,1(10):3421−3429. doi: 10.1039/c3ta00442b [24] XU H, CAO Y, XIE J, HU J, LI Y, JIA D. A construction of Ag-modified raspberry-like AgCl/Ag2WO4 with excellent visible-light photocatalytic property and stability[J]. Mater Res Bull,2018,102:342−352. doi: 10.1016/j.materresbull.2018.02.047 [25] CAMPOSECO R, CASTILLO S, MUGICA V, MEJÍA-CENTENO I, MARÍN J. Role of V2O5-WO3/H2Ti3O7-nanotube-model catalysts in the enhancement of the catalytic activity for the SCR-NH3 process[J]. Chem Eng J,2014,242:313−320. doi: 10.1016/j.cej.2014.01.002 [26] XU X, LI L, HUANG J, JIN H, FANG X, LIU W, ZHANG N, WANG H, WANG X. Engineering Ni3+ cations in NiO lattice at the atomic level by Li+ doping: The Roles of Ni3+ and oxygen species for CO oxidation[J]. ACS Catal,2018,8:8033−8045. doi: 10.1021/acscatal.8b01692 [27] LIMA T M, DE MACEDO V, SILVA D S A, CASTELBLANCO W N, PEREIRA C A, RONCOLATTO R E, GAWANDE M B, ZBOŘIL R, VARMA R S, URQUIETA-GONZÁLEZ E A. Molybdenum-promoted cobalt supported on SBA-15: Steam and sulfur dioxide stable catalyst for CO oxidation[J]. Appl Catal B: Environ,2020,277:119248. doi: 10.1016/j.apcatb.2020.119248 [28] ZHANG C, WANG C, ZHAN W, GUO Y, GUO Y, LU G, BAYLET A, GIROIR-FENDLER A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts[J]. Appl Catal B: Environ,2013,129:509−516. doi: 10.1016/j.apcatb.2012.09.056 [29] YANG Y, HUANG J, WANG S, DENG S, WANG B, YU G. Catalytic removal of gaseous unintentional POPs on manganese oxide octahedral molecular sieves[J]. Appl Catal B: Environ,2013,142−143:568−578. doi: 10.1016/j.apcatb.2013.05.048 [30] YANG Y, ZHANG S, WANG S, ZHANG K, WANG H, HUANG J, DENG S, WANG B, WANG Y, YU G. Ball milling synthesized MnOx as highly active catalyst for gaseous POPs removal: Significance of mechanochemically induced oxygen vacancies[J]. Environ Sci Technol,2015,49:4473−4480. doi: 10.1021/es505232f [31] HUANG X, PENG Y, LIU X, LI K, DENG Y, LI J. The promotional effect of MoO3 doped V2O5/TiO2 for chlorobenzene oxidation[J]. Catal Commun,2015,69:161−164. doi: 10.1016/j.catcom.2015.04.020 [32] CHOI J S, YOUN H K, KWAK B H, WANG Q, YANG K S, CHUNG J S. Preparation and characterization of TiO2-masked Fe3O4 nano particles for enhancing catalytic combustion of 1, 2-dichlorobenzene and incineration of polymer wastes[J]. Appl Catal B: Environ,2009,91:210−216. doi: 10.1016/j.apcatb.2009.05.026 -




 下载:
下载: