A theoretical study of H2S adsorption and dissociation mechanism on defected graphene doped with Pt
-
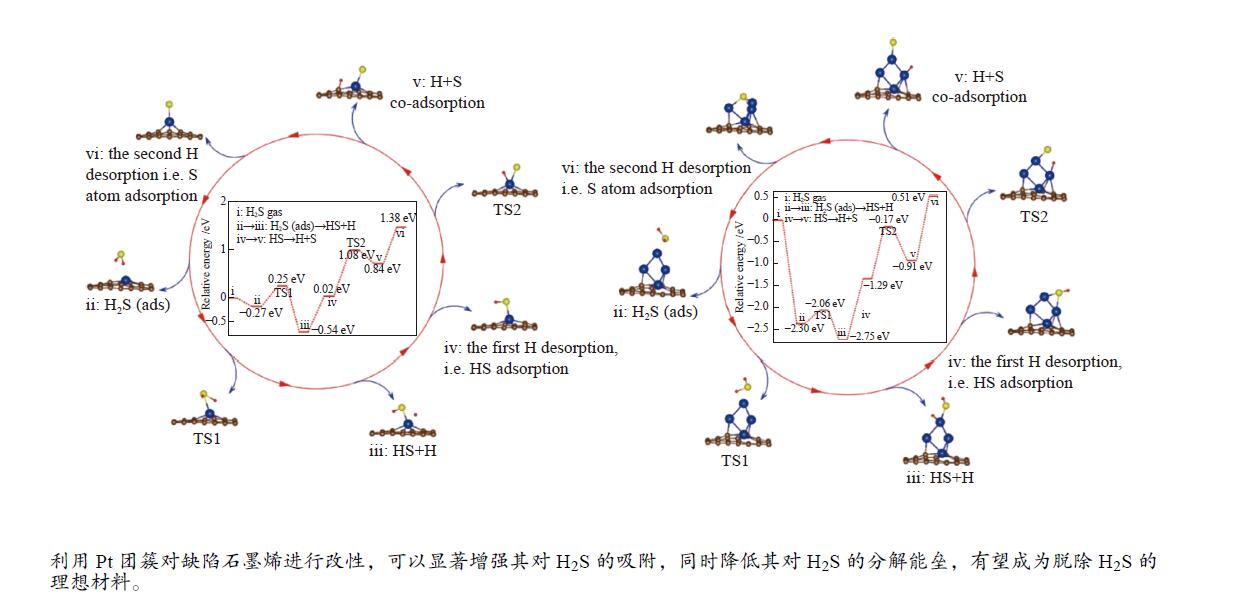
摘要: 通过第一性原理计算研究了四种Pt或Pt团簇修饰石墨烯材料的几何结构、电子结构及其对硫化氢(H2S)分子吸附、分解行为。结果表明,H2S在四种材料上均为弱的物理吸附,但H2S分解后的HS和S可以稳定吸附在材料表面。对于H2S的分解过程,考虑了三个基本过程:(I) H2S (g)→H2S (ads);(II) H2S (ads)→HS (ads) + H (ads);(III) HS (ads)→H (ads) + S (ads)。H2S在四种不同表面的第一H–S键断裂能垒分别为1.69、0.52、0.01和0.24 eV;H–S中断裂第二H–S键的能垒分别为2.34、1.08、0.81和1.12 eV。因此,H2S完全解离的控制步骤是第二个H–S键断裂过程。结合H2S吸附和分解结果研究发现,单Pt原子掺杂缺陷石墨烯有利于吸附H2S,但对解离不利;Pt团簇掺杂的空位较大的缺陷石墨烯能够轻松吸附并消除H2S分子,有望成为吸附、分解H2S气体的理想材料。Abstract: The adsorption and dissociation of hydrogen sulfide (H2S) molecule on Pt or Pt4 cluster doped graphene with different vacancy (VG), as well as their geometric and electronic structures have been investigated by density functional theory (DFT). It has found that H2S and H atom are weakly adsorbed on Pt/Pt4-VG, while HS and S atom are strongly chemisorbed on different surfaces. By using climbing nudged elastic band method (CI-NEB), three elementary processes have been studied: (I) H2S(gas)→H2S(ads); (II) H2S(ads)→HS(ads) + H(ads); (III) HS(ads) → H(ads)+ S(ads). The energy barriers to break the first H–S bond in H2S on four different surfaces (Pt-MVG, Pt-DVG, Pt4-MVG, Pt4-DVG) are 1.69, 0.52, 0.01 and 0.24 eV respectively. In contrast, the energy barriers to break the second H–S bond in HS are 2.34, 1.08, 0.81 and 1.12 eV respectively. It is suggested that the control step of the complete dissociation of H2S is the second H–S bond rupture process. The trend shown in this study reveals that single Pt atom doped defected graphene is favored for adsorption of H2S, but is disadvantage for dissociation. Pt cluster doped defected graphene with bigger vacancy can successfully adsorb and easily eliminate H2S molecule, which is expected to be the ideal material for the adsorption and dissociation.
-
Key words:
- DFT /
- defected graphene /
- Pt doped /
- hydrogen sulfide
-
图 2 不同表面Pt原子相邻C的投影态密度(PDOS) ((a)和(b))和电荷差异((c)−(f))
Figure 2 Projected density of states (PDOS) ((a) and (b)) and charge differences for C adjacent to Pt atom in different surfaces ((c)−(f)) (The Fermi level and isosurfaces are respectively set to 0 eV and 0.005 eV/Å3, charge accumulation is in blue and depletion in yellow and the color coding of atoms are the same as Figure 1, Δq stands for the total charge, negative means electrons are transferred from Pt/Pt4 to C)
表 1 MVG / DVG上Pt / Pt4掺杂的计算
Table 1 Summary of the calculated results for Pt/Pt4 doped on MVG/DVG
Pt-MVG Pt-DVG Pt4-MVG Pt4-DVG ΔEb/eV −4.57 −4.43 −7.56 −7.01 $ {\overline d _{{\rm{P}}{\rm{t}} - C}} $/Å 1.93 1.98 1.96 2.05 dPt–Pt min/Å − − 2.55 2.49 dPt–Pt, max/Å − − 2.62 2.60 $\angle $C–Pt–C/(°) 90.28 88.14 87.82 85.90 表 2 H2S和HS在Pt / Pt4掺杂石墨烯表面的吸附能( ΔEads )和电荷转移(ΔqM)
Table 2 Adsorption energy (ΔEads) and charge transfer (ΔqM) of H2S and HS adsorbed on Pt/Pt4 doped graphene surface
Pt-MVG Pt-DVG Pt4-MVG Pt4-DVG ΔEads/ eV H2S −0.85 −0.27 −1.81 −2.30 HS −3.24 −3.23 −4.35 −3.96 ΔqM/e H2S −0.13 −0.013 −0.17 −0.21 HS 0.26 0.14 0.18 0.12 -
[1] 吴博, 黄戒介, 张荣俊, 赵建涛, 陈富艳, 王洋. 活性炭(焦)低温吸附催化脱除H2S的基础研究[J]. 燃料化学学报,2009,37(3):355−359. doi: 10.3969/j.issn.0253-2409.2009.03.017WU Bo, HUANG Jie-jie, ZHANG Rong-jun, ZHAO Jian-tao, CHEN Fu-yan, WANG Yang. Adsorption and catalytic removal of hydrogen sulfide on active carbon(char) at low temperature[J]. J Fuel Chem Technol,2009,37(3):355−359. doi: 10.3969/j.issn.0253-2409.2009.03.017 [2] ATAKIIL H, WAKKER J P, GERRLTEN A W, BERG P J. Removal of H2S from fuel gases at high temperatures using MnO/g-Al2O3[J]. Fuel,1995,74(2):187−191. doi: 10.1016/0016-2361(95)92653-N [3] YANG L, CHENG Z, LIU M, WILSON L. New insights into sulfur poisoning behavior of Ni-YSZ anode from long-term operation of anode-supported SOFCs[J]. Energy Environ Sci,2010,3(11):1804−1809. [4] AITA B C, MAYER F D, MURATT D T, BRONDANI M, PUJOL S B, DENARDI L B, HOFFMANN R, DA SILVEIRA D D. Biofiltration of H2S-rich biogas using Acidithiobacillus thiooxidans[J]. Clean Technol Environ Policy,2016,18(3):689−703. doi: 10.1007/s10098-015-1043-5 [5] SUMAN H, SRIVASTAVA R, SHRIVASTAVA S, SRIVASTAVA A, JACOB A P, MALVI C S Chem. DFT analysis of H2S adsorbed zigzag and armchair graphene nanoribbons[J]. Phys Lett,2020,745:137280. [6] HUYNH NHUT H, THANH V L T, LE L T. Removal of H2S in biogas using biotrickling filter: Recent development[J]. Process Saf Environ Prot,2020,144:297−309. [7] GHOSH T K, TOLLEFSON E L. A continuous process for recovery of sulfur from natural gas containing low concentrations of hydrogen sulfide[J]. Can J Chem Eng,1986,64:960−968. [8] COX H H J, DESHUSSES M A. Co-treatment of H2S and toluene in a biotrickling filter[J]. Chem Eng J,2002,87:101−110. [9] CHEN Y, WANG J, LIU Z. Graphene and its derivative-based biosensing systems[J]. Chin J Anal Chem,2012,40(11):1772−1779. [10] SUN P, WANG K, ZHU H. Recent developments in graphene-based membranes: Structure, mass-transport mechanism and potential applications[J]. Adv Mater,2016,28:2287−2310. doi: 10.1002/adma.201502595 [11] 宋述鹏, 贾娜娜, 龚铁夫, 周和荣, 吴润. Fe, Co, Ni掺杂石墨烯表面吸附C2H4的第一性原理研究[J]. 原子与分子物理学报,2019,36(4):710−716. doi: 10.3969/j.issn.1000-0364.2019.04.028SONG Shu-peng, JIA Na-na, GONG Tie-fu, ZHOU He-rong, WU Run. First-principles study of C2H4 adsorption on Fe-, Co- and Ni-doped graphene surface[J]. J At Mol Phys,2019,36(4):710−716. doi: 10.3969/j.issn.1000-0364.2019.04.028 [12] 马玲, 马欢, 张建宁, 马良财, 张建民. 小分子吸附调控Ti掺杂石墨烯电子结构和磁性的密度泛函理论研究[J]. 原子与分子物理学报,2018,35(4):577−584. doi: 10.3969/j.issn.1000-0364.2018.04.008MA Ling, MA Huan, ZHANG Jian-ning, MA Liang-cai, ZHANG Jian-min. A DFT study on tuning electronic structure and magnetic property of Ti doped graphene by small gass adsorption[J]. J At Mol Phys,2018,35(4):577−584. doi: 10.3969/j.issn.1000-0364.2018.04.008 [13] LI J, KANG L. Non-noble metal single atom catalysts with S, N co-doped defective graphene support: A theoretical study of highly efficient acetylene hydration[J]. Mater Today Commun,2021,27:102216. [14] TANG Y, LEI Y, TONG K, YANG T, FU T, XIANG Y, ZHANG S, SI Y, GUO C. Fe, N-doped graphene-wrapped carbon black nanoparticles as highly efficient catalyst towards oxygen reduction reaction[J]. Appl Surf Sci,2021,545:148981. [15] KHODADADI Z. Evaluation of H2S sensing characteristics of metals-doped graphene and metals-decorated graphene: Insights from DFT study[J]. Phys E Low-Dimensional Syst Nanostructures,2018,99:261−268. [16] ZHANG H, LUO X, SONG H, LIN X, LU X, TANG Y. DFT study of adsorption and dissociation behavior of H2S on Fe-doped graphene[J]. Appl Surf Sci,2014,317:511−516. [17] FAYE O, EDUOK U, SZPUNAR J, SAMOURA A, BEYE A. H2S adsorption and dissociation on NH-decorated graphene: A first principles study[J]. Surf Sci,2018,668:100−106. [18] GANJI M D, SHARIFI N, ARDJMAND M, AHANGARI M G. Pt-decorated graphene as superior media for H2S adsorption: A first-principles study[J]. Appl Surf Sci,2012,261:697−704. [19] CHEN D, ZHANG X, TANG J, FANG J, LI Y, LIU H. Adsorption and dissociation mechanism of SO2 and H2S on Pt decorated graphene: a DFT-D3 study[J]. Appl Phys A Mater Sci Process,2018,124:404. [20] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio totalenergy calculations using a plane-wave basis set[J]. Phys Rev B,1996,54:11169−11186. [21] KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci,1996,6:15−50. [22] KRESSE G, FURTHMÜLLER J. Generalized gradient approximation made simple[J]. Phys Rev Lett,1996,77:3865. [23] EHRLICH S, MOELLMANN J, PECKIEN W, BREDOW T, GRIMME S. System-dependent dispersion coefficients for the DFT-D3 treatment of adsorption processes on ionic surfaces[J]. Chem Phys,2011,12:3414−3420. [24] GRIMME S, ANTONY J, EHRLICH S, KRIEG H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. Chem Phys,2010,132(15):154104. [25] GRIMME S. Semiempirical GGA-type density functional constructed with a longrange dispersion correction[J]. Comput Chem,2006,27:1787−1799. doi: 10.1002/jcc.20495 [26] HOU M, CEN W, ZHANG H, LIU J, YIN H, WEI F. Adsorption and oxidation of NO on graphene oxides: A dispersion corrected density functional theory investigation[J]. Appl Surf Sci,2015,339:55−61. [27] LI J, HOU M, CHEN Y, CEN W, CHU Y, YIN S. Enhanced CO2 capture on graphene via N, S dual-doping[J]. Appl Surf Sci,2017,399:420−425. [28] CEN W, HOU M, LIU J, YUAN S, LIU Y, CHU Y. Oxidation of SO2 and NO by epoxy groups on graphene oxides: The role of the hydroxyl group[J]. RSC Adv,2015,5(29):22802−22810. [29] LIDE D R. Handbook of Chemistry and Physics[M]. Boca Raton: CRC Press, 2003. [30] TUCKERMAN M E. Ab initio molecular dynamics: basic concepts, current trends and novel applications[J]. Journal of Physics Condensed Matter,2002,14:R1297. [31] BOM M, OPPENHEIMER R. Quantum theory of molecules[J]. Ann Phys,1927,84:457. [32] HALGREN T A, LIPSCOMN W N. The synchronous-transit method for determining reaction pathways and locating molecular transition states[J]. Chem Phys Lett,1977,49:225−232. [33] HENKELMAN G, JÓNSSON H J. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. Chem Phys,2000,113:9901−9904. [34] HENKELMAN G, UBERUAGA B P, JÓNSSON H J. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points[J]. Chem Phys,2000,113:9978−9985. [35] BO Z, GUO X, WEI X, YANG H, YAN J, CEN K. Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene[J]. Phys E Low-Dimensional Syst Nanostructures,2019,109:156−163. [36] ZHAO C, WU H. A first-principles study on the interaction of biogas with noble metal (Rh, Pt, Pd) decorated nitrogen doped graphene as a gas sensor: A DFT study[J]. Appl Surf Sci,2018,435:1199−1212. [37] GANJI M D, SHARIFI N, AHANGARI M G. Adsorption of H2S molecules on non-carbonic and decorated carbonic graphenes: A van der Waals density functional study[J]. Comput Mater Sci,2014,92:127−134. [38] HOU M, ZHANG X, YUAN S, CEN W. Double graphitic-N doping for enhanced catalytic oxidation activity of carbocatalysts[J]. Phys Chem Chem Phys,2019,21(10):5481−5488. [39] JIANG Z, QIN P, FANG T. Investigation on adsorption and decomposition of H2S on Pd (1 0 0) surface: A DFT study[J]. Surf Sci,2015,632:195−200. [40] HAMMER B, NØRSKOV J K. Electronic factors determining the reactivity of metal surfaces[J]. Sur Sci,1995,343:211−220. [41] 蒋元祺, 彭平, 文大东, 韩绍昌. 双二十面体Ag_n(n=19, 23, 24, 25)团簇低能稳态构型的TS搜索[J]. 化学学报,2013,71(10):1429−1434. doi: 10.6023/A13050470JIANG Yuan-qi, PENG Ping, WEN Da-dong, HAN Xiao-chang. A TS search for stable cofigurations of double icosahedral Agn(n=19, 23, 24, 25) clusters linked by sharing atoms[J]. Acta Chim Sinica.,2013,71(10):1429−1434. doi: 10.6023/A13050470 -





 下载:
下载: