Effect of highly dispersed Co3O4 on the catalytic performance of LaCoO3 perovskite in the combustion of lean methane
-
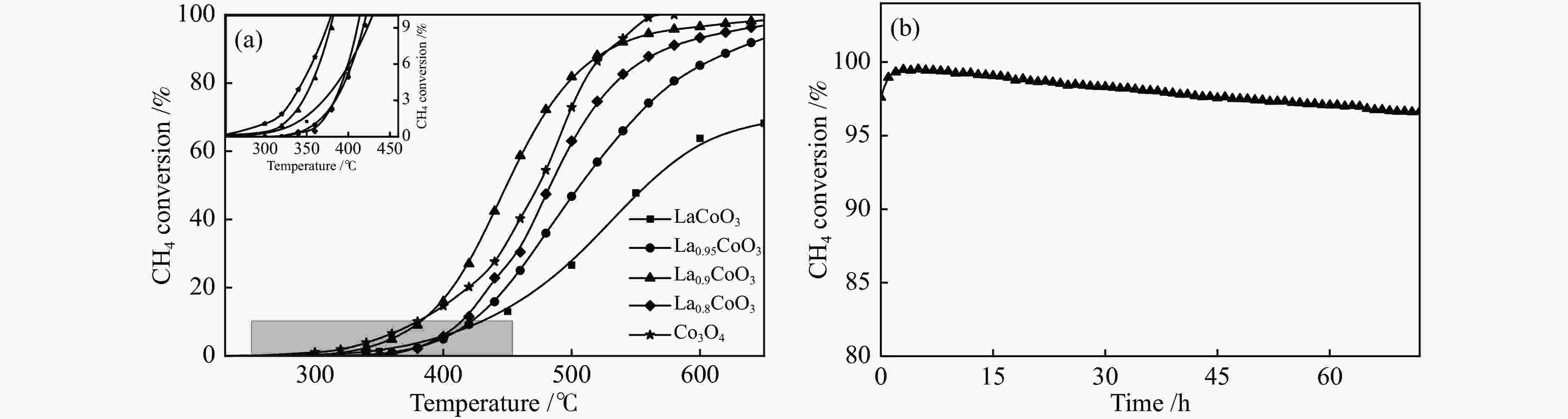
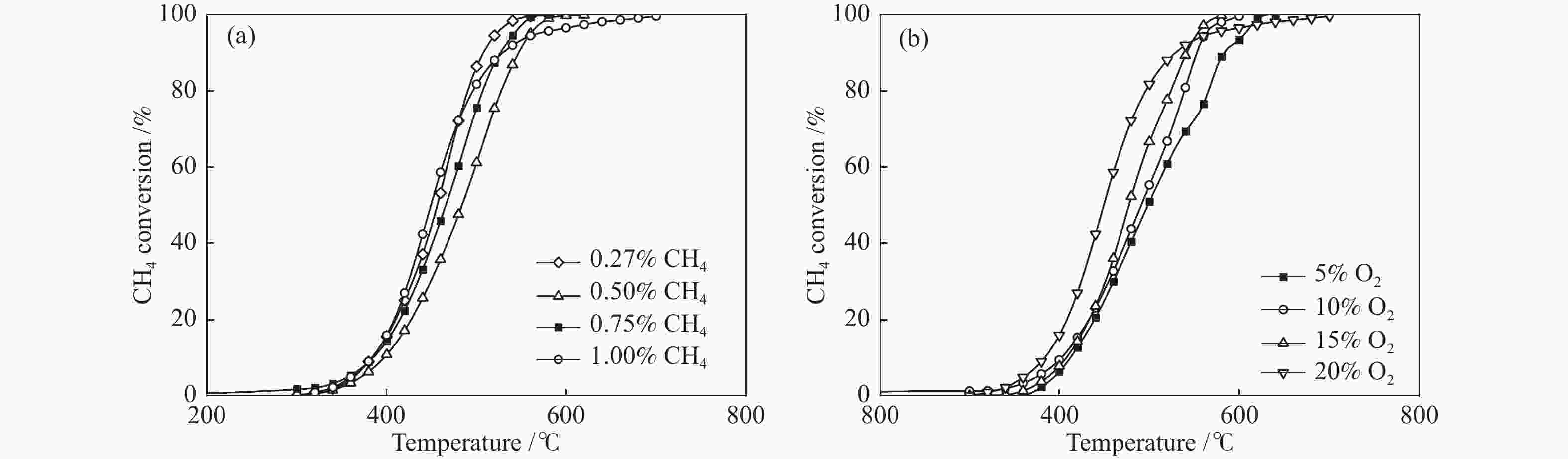
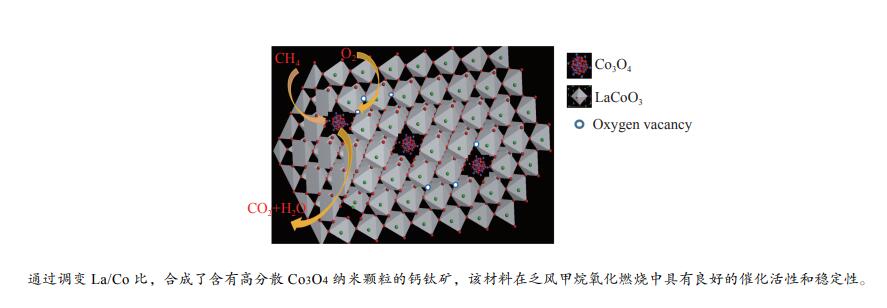
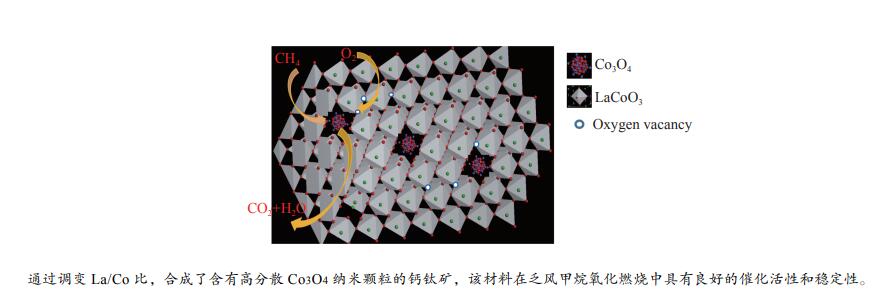
摘要: 本研究采用溶胶凝胶法,通过调变镧钴比合成了一种纳米新型钙钛矿类催化剂。利用物理吸附、ICP、XRD、H2-TPR、O2-TPD和XPS等技术对催化剂进行了表征,并对其在乏风甲烷氧化燃烧中的催化性能进行了研究。结果表明,高分散性的Co3O4纳米颗粒有利于甲烷的低温活化,且催化剂中镧钴钙钛矿体相可提供大量的晶格氧,促进高温下甲烷的催化燃烧速率和催化剂的高温稳定性。通过调变镧钴比例,可有效调变催化剂中Co3O4纳米颗粒的分散状态,进而实现催化剂低温活性和高温稳定性的有效统一。当La/Co比为0.9时,在空速为30000 mL/(gcat·h)的条件下,La0.9CoO3钙钛矿催化剂的甲烷起燃温度为382 ℃;稳定运行72 h后,甲烷转化率保持在95%以上。这些结果为今后开发低成本、高活性和高稳定性的甲烷燃烧催化剂提供了参考。Abstract: In this work, a series of nano LaCoO3 perovskite catalysts were effectively synthesized by a sol-gel method through modulating the La/Co molar ratio. These catalysts were characterized by ICP, XRD, N2 sorption, H2-TPR, O2-TPD, and XPS, and their catalytic performance in the lean methane combustion were then investigated. The results indicate that highly dispersed Co3O4 nanoparticles on the LaCoO3 perovskite catalysts are beneficial to the activation of CH4 at a low temperature, while the La-Co-perovskite bulk phase can provide a large amount of lattice oxygen, which can enhance the reaction rate of methane combustion and the catalytic stability at a high temperature. Through altering the La/Co molar ratio, the dispersion of Co3O4 nanoparticles in the La-Co-perovskite catalyst can be effectively modulated, to achieve the concurrence of low-temperature activity and high-temperature stability in the lean methane combustion. In particular, the La0.9CoO3 perovskite catalyst with a La/Co molar ratio of 0.9 exhibits excellent performance in lean methane combustion, with a light-off temperature of 382 ℃ at a space velocity of 30000 mL/(gcat·h), the light-off temperature of methane is 382 ℃, and the methane conversion rate is still maintained above 95% after 72 h of stable operation, indicating that the highly dispersed Co3O4 nanoparticles were beneficial to the low-temperature activation of CH4, and the lanthanum-cobalt-perovskite bulk phase in the catalyst could provide a large amount of lattice oxygen, which promotes the catalytic combustion rate of CH4 and the high-temperature stability of the catalyst under high-temperature conditions. By modulating the lanthanum-cobalt ratio, the dispersion state of Co3O4 nanoparticles in the catalyst can be effectively modulated, and then the effective unification of low-temperature activity and high-temperature stability of the catalyst can be achieved, which guides the future development of low-cost, high-activity and high-stability catalysts for methane catalytic combustion.
-
Key words:
- catalytic combustion of lean methane /
- LaCoO3 /
- Co3O4 /
- high dispersion /
- lattice oxygen
-
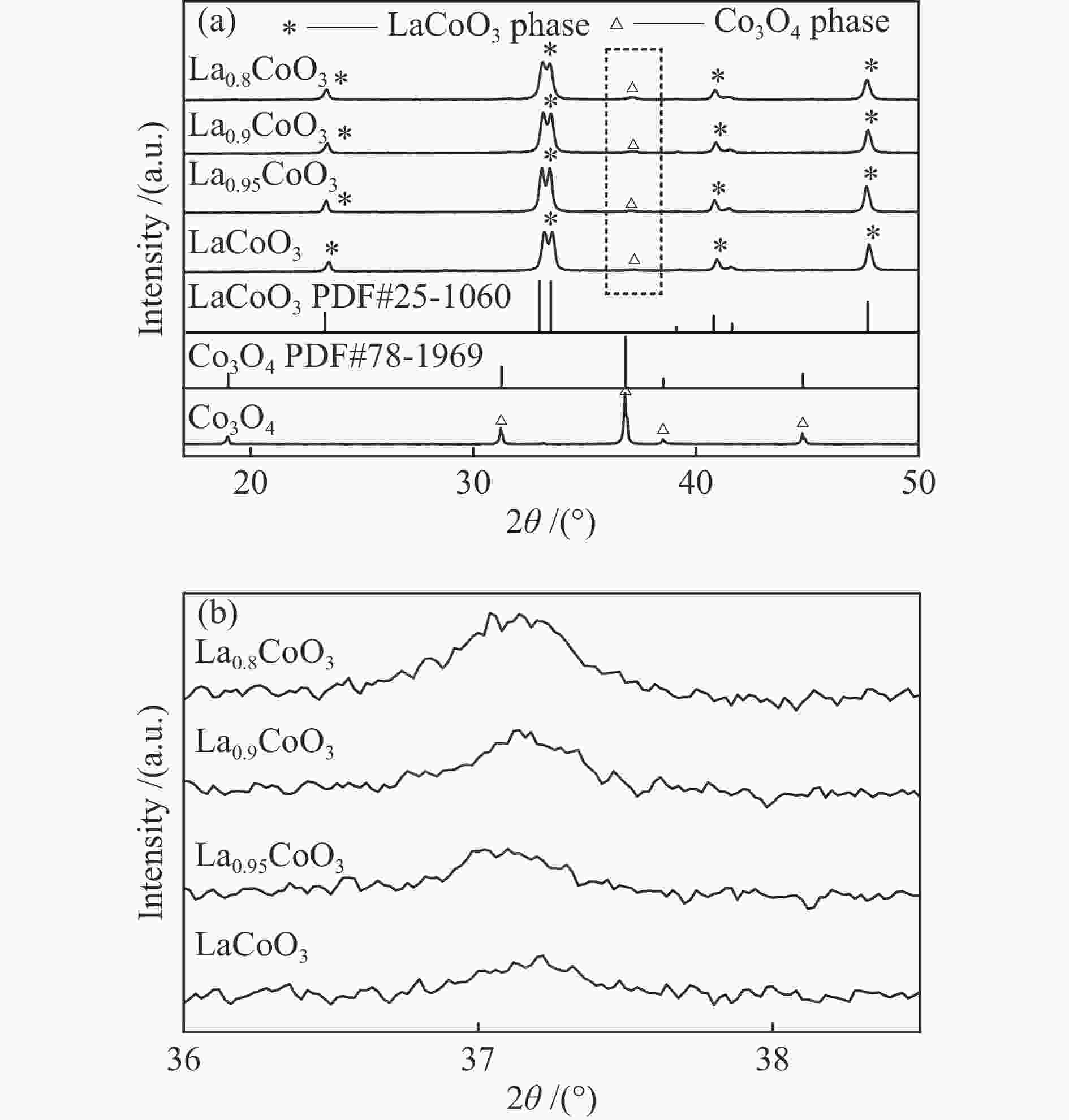
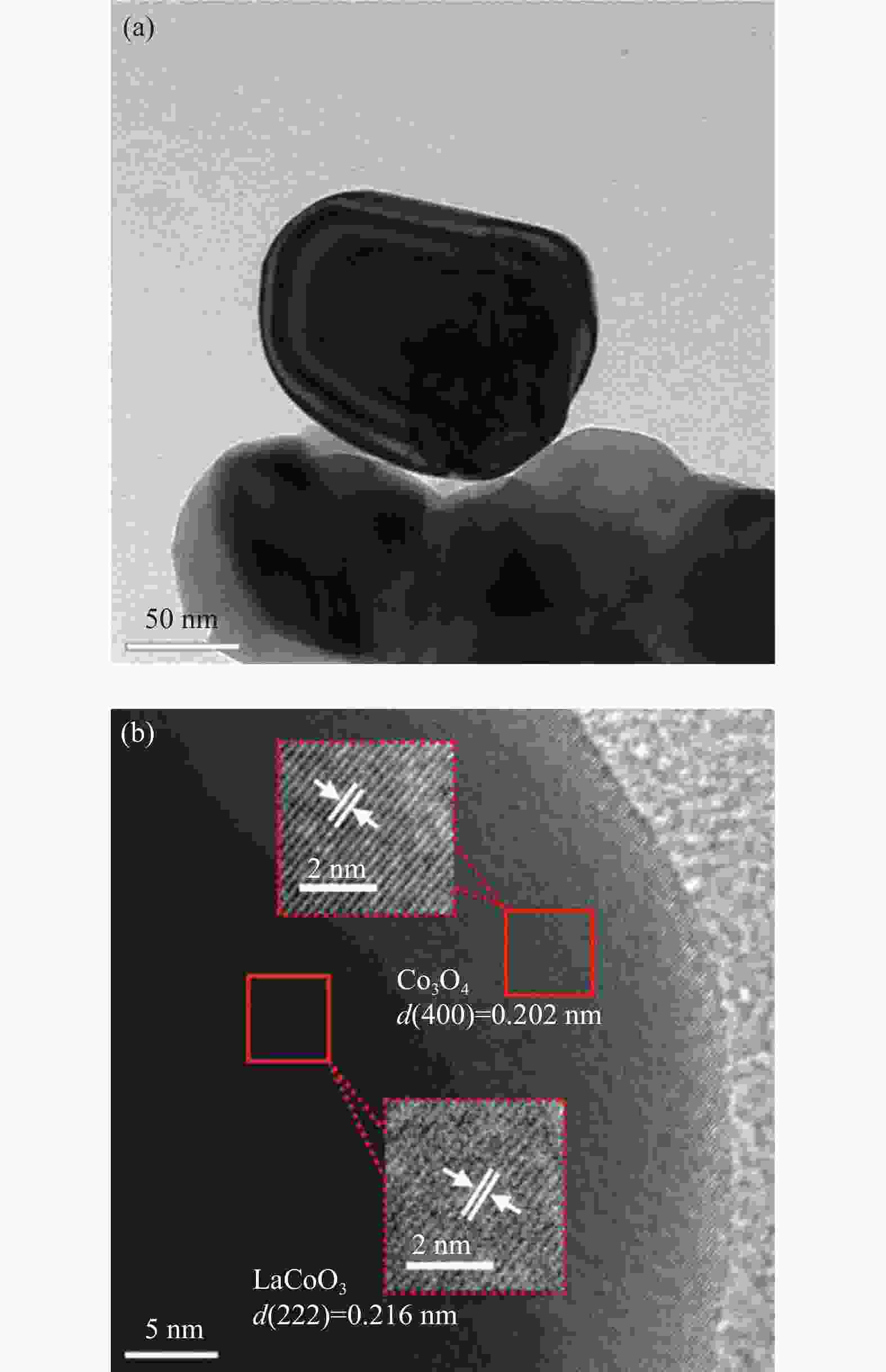
表 1 镧钴系列催化剂的物性参数
Table 1 Physical properties of various LaxCoO3 catalysts
Sample La/Co molar ratio SBET/
(m2·g−1)vpore/
(cm3·g−1)dCo3O4-XRD
/nmLaCoO3 0.917 4.436 0.034 27.76 La0.95CoO3 0.906 4.311 0.121 27.59 La0.9CoO3 0.879 5.504 0.037 28.78 La0.8CoO3 0.781 5.466 0.066 21.05 Co3O4 − 4.276 0.055 79.68 notes: La/Co molar ratio wasdetermined by ICP; the specific surface area ( SBET ) and pore volume ( vpore) were derived from N2 sorption isotherms; the particle size ( dCo3O4 ) was calculated by Scherrer’s formula 表 2 镧钴系列催化剂的Co 2p3/2谱图拟合数据
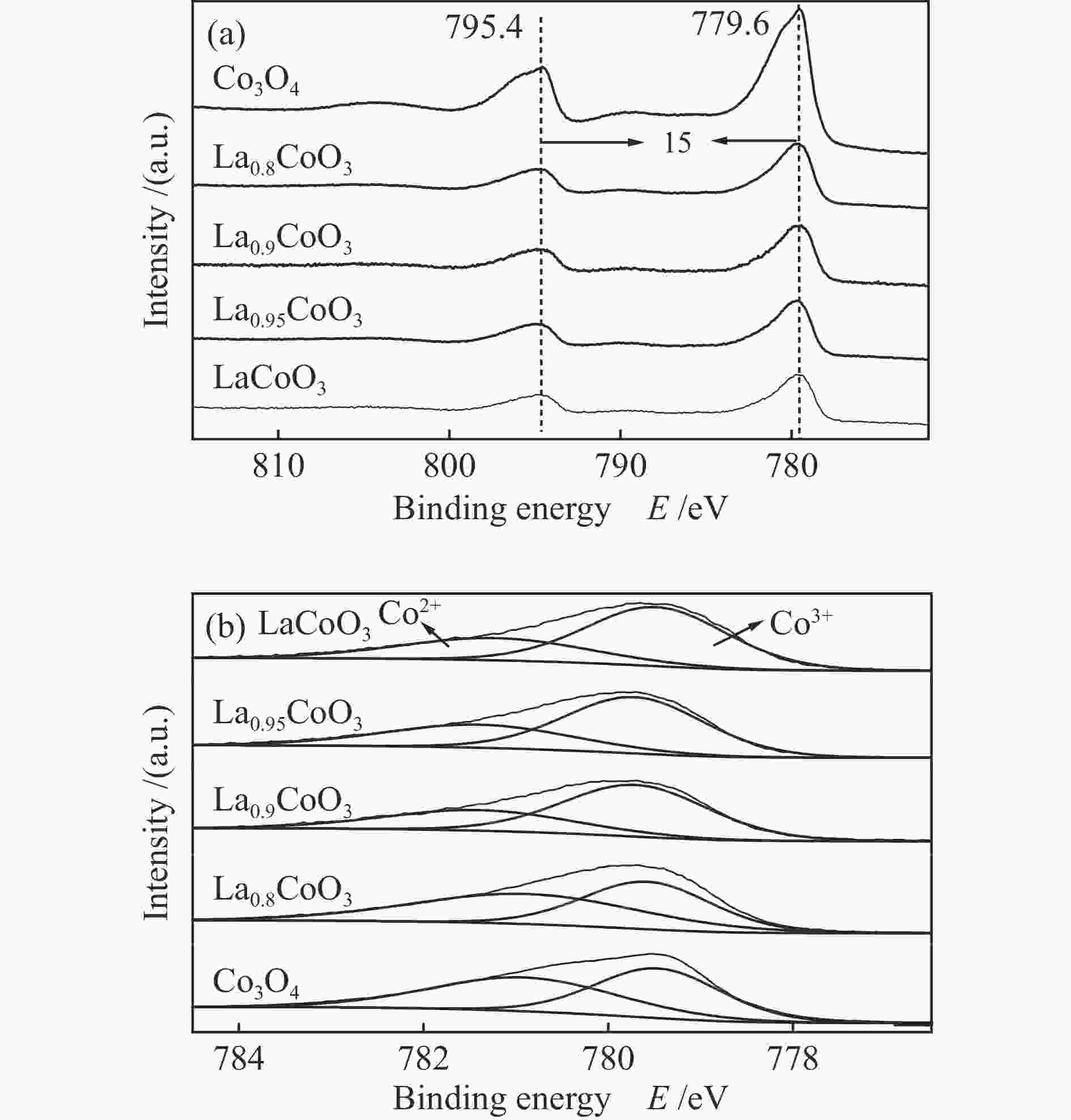
Table 2 Co 2p3/2 spectra fitting results of various LaxCoO3 samples
Sample Co3 + Co2 + Co3 + /Co2 + BE/eV Fraction/% BE/eV Fraction/% LaCoO3 779.53 61 781.29 39 1.56 La0.95CoO3 779.76 60.16 781.47 39.84 1.51 La0.9CoO3 779.67 47.78 781.08 52.22 0.92 La0.8CoO3 779.61 43.71 780.99 56.29 0.78 Co3O4 779.52 45.06 781.02 54.94 0.82 表 3 镧钴系列催化剂的O 1s谱图拟合数据
Table 3 Fitted results of O 1s XPS spectra of various LaxCoO3 samples
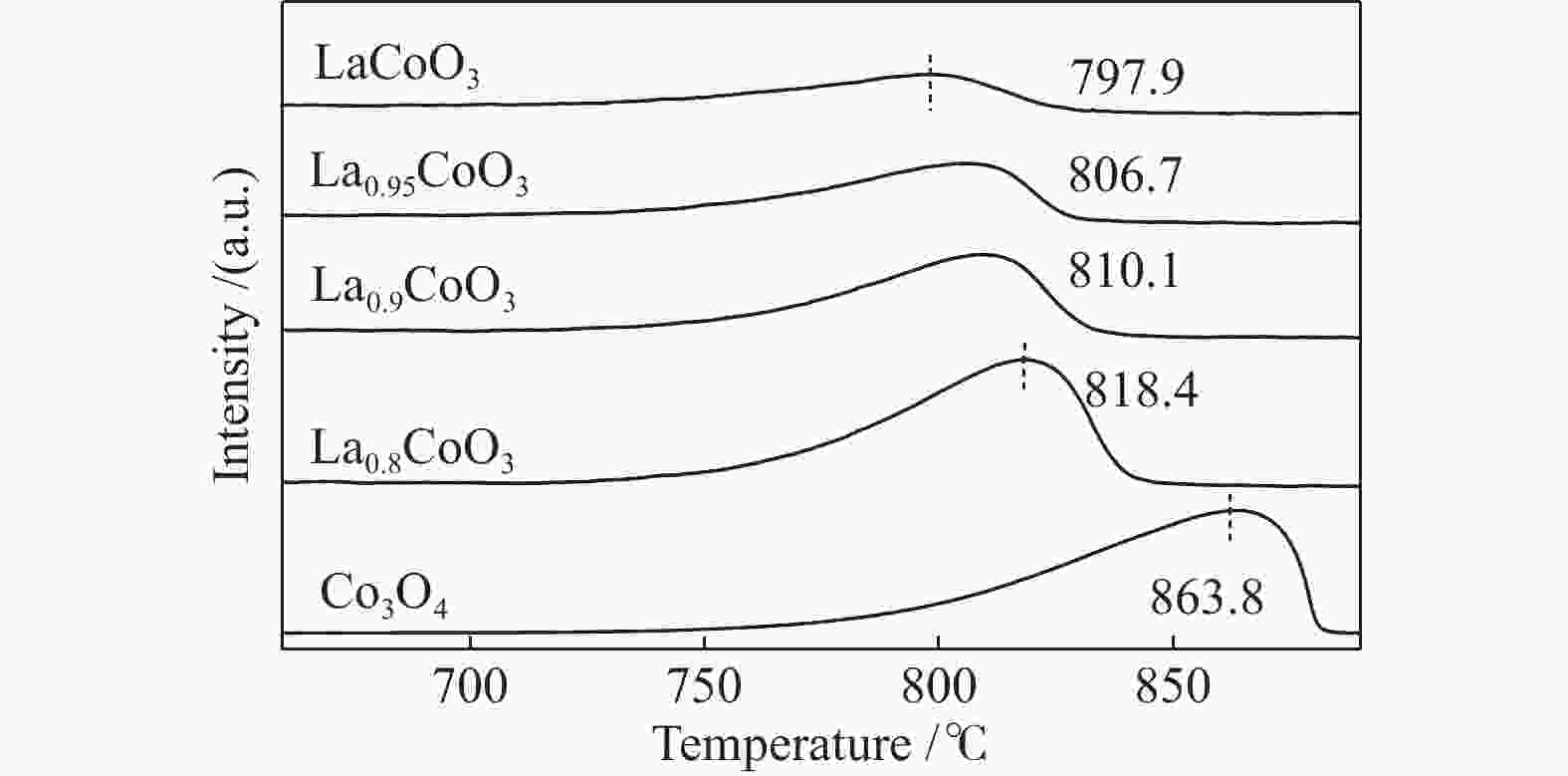
Sample Olatt Osurf Osurf/Olatt BE/eV Fraction/% BE/eV Fraction/% LaCoO3 528.59 36.92 530.98 63.08 1.71 La0.95CoO3 528.9 39.3 531.27 60.7 1.54 La0.9CoO3 528.85 48.1 531.3 51.9 1.08 La0.8CoO3 528.84 48.49 531.25 51.51 1.06 Co3O4 529.81 47.53 531.15 52.47 1.10 表 4 镧钴系列催化剂的H2-TPR拟合数据
Table 4 Fitted H2-TPR results of various LaxCoO3 samples
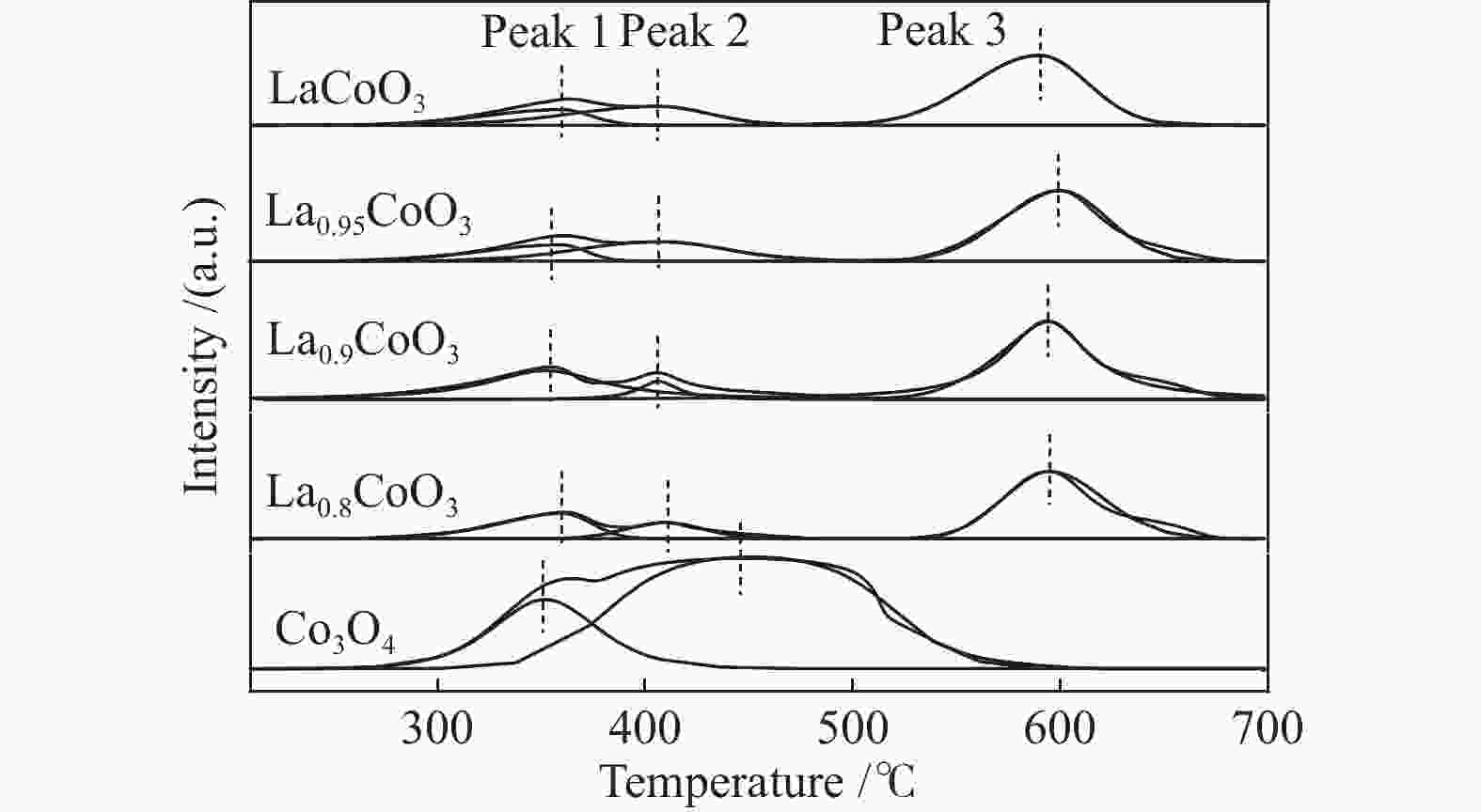
Sample Peak 1
Co3 + →Co2 + (Co3O4)Peak 2
Co2 + →Co0(Co3O4)
Co3 + →Co2 + (LaCoO3)Peak 3
Co2 + →Co0(LaCoO3)Co3O4/LaCoO3 tred/℃ percentage/% tred/℃ percentage/% tred/℃ percentage/% LaCoO3 359.26 13.39 401.57 21.24 588.94 65.37 0.0149 La0.95CoO3 353.94 14.19 406.89 24.26 599.33 61.55 0.0623 La0.9CoO3 352 32.17 406.5 7.2 593.58 60.63 0.0747 La0.8CoO3 359.26 23.15 409.37 11.91 594.26 64.94 0.0199 Co3O4 350.56 22.52 447.7 77.48 − − − notes: tred refers to the temperature corresponding to the maximum value of the reduction peak and area percentage refers to the percentage of the reduction peak area to the total reduction peak area; According to the stoichiometric number, the relative content of Co3O4 and LaCoO3 is calculated by the reduction peak area in H2-TPR; that is, Co3O4/LaCoO3 = Speak-1/(0.5× Speak-3) 表 5 甲烷催化燃烧代表性催化剂性能对比
Table 5 Comparison of representative catalysts in their performance in methane combustion
Number Catalyst Preparation
methodMethane initial
concentration/%GHSV/
(mL·gcat−1·h−1)Temp./℃ Stability test Ref. t10 t50 t90 temp. (℃)-
time (h)conv./% 1 La0.9CoO3 sol-gel method 1 30000 382 449 530 640−72 96 this work 2 Pd/Al2O3 impregnation 1 12000 298 346 401 370−20 68 [36] 3 BaMnAl11O19−d coprecipitation 0.5 18000 545 640 − − − [37] 4 Co3O4 coprecipitation 1 18000 285 373 455 360−30 80 [38] 5 LaCoO3 template method 3 30000 370 485 578 650−70 82 [11] 6 LaMnO3 template method 3 30000 405 480 570 650−70 98 [11] 7 MnOx/LaMnO3 acid etching method 2.5 30000 351 441 519 − − [39] 8 La0.8Sr0.2CoO3 acid etching method 1 44000 385 491 595 600−24 90 [40] -
[1] ZHANG B, CHEN G Q. Methane emissions in China 2007[J]. Renewable Energy,2014,30:886−902. doi: 10.1016/j.rser.2013.11.033 [2] PFEFFERLE L D, PFEFFERLE W C. Catalysis in combustion[J]. Catal Rev,1987,29(2/3):219−267. doi: 10.1080/01614948708078071 [3] ANIL B, JACQUELINE M G, OLIVIA L, MICHAEL B. Low-temperature activity and PdO-PdOx transition in methane combustion by a PdO-PdOx/γ-Al2O3 catalyst[J]. Catalysts,2018,8(7):266−284. doi: 10.3390/catal8070266 [4] CASTELLAZZI P, GROPPI G, FORZATTI P, ALEXANDRE B, MARÉCOT P, DUPREZ D. Role of Pd loading and dispersion on redox behaviour and CH4 combustion activity of Al2O3 supported catalysts[J]. Catal Today,2010,155(1/2):18−26. doi: 10.1016/j.cattod.2009.02.029 [5] WANG J H, CHEN H, HU Z C, YAO M F, LI Y D. A review on the Pd-based three-way catalyst[J]. Catal Rev,2015,57(1):79−144. doi: 10.1080/01614940.2014.977059 [6] TIAN M, WANG X, LIU X, WANG A, ZHANG T. Fe-substituted Ba-hexaaluminates oxygen carrier for carbon dioxide capture by chemical looping combustion of methane[J]. AIChE J,2016,62(3):792−801. doi: 10.1002/aic.15135 [7] ZHENG J, YU J, JIE C, XIAO T, M O JONES, HAO Z, EDWARDS PP. Catalytic combustion of methane over mixed oxides derived from Co-Mg/Al ternary hydrotalcites[J]. Fuel Process Technol,2010,91(1):97−102. doi: 10.1016/j.fuproc.2009.08.023 [8] CHEN Z, WANG S, LIU W, GAO X, GAO D, WANG M, WANG S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion[J]. Appl Catal A: Gen,2016,525:94−102. doi: 10.1016/j.apcata.2016.07.009 [9] IABLOKOV V, BARBOSA R, POLLEFEYT G, VAN D. I, CHENAKIN S, KRUSE N. Catalytic CO oxidation over well-defined cobalt oxide nanoparticles: Size-reactivity correlation[J]. ACS Catal,2015,5(10):5714−5718. doi: 10.1021/acscatal.5b01452 [10] PAREDES J R, DÍAZ E, DÍEZ F V, ORDONEZ S. Combustion of methane in lean mixtures over bulk transition-metal oxides: Evaluation of the activity and self-deactivation[J]. Energy Fuels,2008,23(1):86−93. [11] GUO G, LIAN K, WANG L, GU, F, HAN D, WANG Z. High specific surface area LaMO3 (M = Co, Mn) hollow spheres: synthesis, characterization and catalytic properties in methane combustion[J]. RSC Adv,2014,4(102):699−707. [12] GAO Z, WANG R. Catalytic activity for methane combustion of the perovskite-type La1−xSrxCoO3−δ oxide prepared by the urea decomposition method[J]. Appl Catal B: Environ,2010,98(3/4):147−53. doi: 10.1016/j.apcatb.2010.05.023 [13] LI B, YANG Q, PENG Y, CHEN J, DENG L, WANG D, HONG X, LI J. Enhanced low-temperature activity of LaMnO3 for toluene oxidation: The effect of treatment with an acidic KMnO4[J]. Chem Eng J,2019,366:92−99. doi: 10.1016/j.cej.2019.01.139 [14] SI W, WANG Y, SHEN Z, HU F, LIN J. A facile method for in situ preparation of the MnO2/LaMnO3 catalyst for the removal of toluene[J]. Environ Sci Technol,2016,50(8):4572−4578. doi: 10.1021/acs.est.5b06255 [15] NIE L, WANG J, TAN Q. In-situ preparation of macro/mesoporous NiO/LaNiO3 pervoskite composite with enhanced methane combustion performance[J]. Catal Commun,2017,97:1−4. doi: 10.1016/j.catcom.2017.04.010 [16] JYA B, HL A, BO H A, LJ A, YONG X A, DL A. Acidic H2O2 treatment of LaCoO3 towards highly dispersed Co3O4 nanoparticles with excellent catalytic performance for C3H8 combustion[J]. Catal Commun,2020,135:105830. [17] LUO Y J, WANG K C, ZUO J C, QIAN Q R, XU Y X, LIU X P, XUE H, CHEN Q H. Selective corrosion of LaCoO3 by NaOH: structural evolution and enhanced activity for benzene oxidation[J]. Catal Sci Technol,2017,7(2):496−501. doi: 10.1039/C6CY02489K [18] WANG S, XUE G, LIANG J, MENG, J. Effect of tourmaline additive on the crystal growth and activity of LaCoO3 for catalytic combustion of methane[J]. J Rare Earths,2014,32(9):855−859. doi: 10.1016/S1002-0721(14)60153-8 [19] WANG S, XU X, ZHU J, TANG, D, ZHAO, Z. Effect of preparation method on physicochemical properties and catalytic performances of LaCoO3 perovskite for CO oxidation[J]. J Rare Earths,2019,37(9):970−977. doi: 10.1016/j.jre.2018.11.011 [20] ZHENG YIFAN, LIU YAN, ZHOU HUAN, HUANG WANZHEN, PU ZHIYING. Complete combustion of methane over Co3O4 catalysts: Influence of pH values[J]. J Alloys Compd,2018,734:112−120. doi: 10.1016/j.jallcom.2017.11.008 [21] FAYE J, BAYLET A, TRENTESAUX M, ROYER S, DUMEIGNIL F, DUPREZ D, VALANGE S, TATIBOUËT JM. Influence of lanthanum stoichiometry in La1−xFeO3−δ perovskites on their structure and catalytic performance in CH4 total oxidation[J]. Appl Catal B: Environ,2012,126:134−143. doi: 10.1016/j.apcatb.2012.07.001 [22] ZHANG G, LI C, LIU J, ZHOU LEI, LIU RUIHUA, HAN XIAO, HUANG HUI, HU HAILIANG, LIU YANG, KANG ZHENHUI. One-step conversion from metal–organic frameworks to Co3O4@N-doped carbon nanocomposites towards highly efficient oxygen reduction catalysts[J]. J Mater A,2014,2(22):8184−8189. [23] CHEN H, WEI G, LIANG X, LIU P, XI Y, ZHU J. Facile surface improvement of LaCoO3 perovskite with high activity and water resistance towards toluene oxidation: Ca substitution and citric acid etching[J]. Catal Sci Technol,2020,10(17):5829−5839. doi: 10.1039/D0CY01150A [24] PU Z, ZHOU H, ZHENG Y, HUANG W, LI X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time[J]. Appl Surf Sci,2017,410:14−21. doi: 10.1016/j.apsusc.2017.02.186 [25] ZHENG Y, FENG X, LIN D, WU E, LUO Y, YOU Y, HUANG B, QIAN Q, CHEN Q. Insights into the low-temperature synthesis of LaCoO3 derived from Co(CH3COO)2 via electrospinning for catalytic propane oxidation[J]. Chin J Chem,2019,38(2):144−150. [26] FENG Z, DU C, CHEN Y, LANG Y, SHAN B. Improved durability of Co3O4 particles supported on SmMn2O5 for methane combustion[J]. Catal Sci Technol,2018,8(15):3785−3794. doi: 10.1039/C8CY00897C [27] 刘敬伟. Pd/Co3O4/载体催化剂的制备及甲烷催化燃烧性能研究[D]. 上海: 上海大学, 2017.LIU Jing-wei. The research on the synthesis and catalytic properties of Pd/Co3O4/supporting for methane combustion[D]. Shanghai: Shanghai University, 2017. [28] YANG Q, WANG D, WANG C, LI X, LI K, YUE P, LI J. Facile surface improvement method for LaCoO3 for toluene oxidation[J]. Catal Sci Technol,2018,8(12):3166−3173. doi: 10.1039/C8CY00765A [29] 王婷. Co3O4催化剂的制备及低浓度甲烷催化燃烧的性能研究[D]. 太原: 太原理工大学, 2017.WANG Ting. Preparation and properties of Co3O4 catalyst for low concentration methane catalytic[D]. Taiyuan: Taiyuan University of Technology, 2017. [30] ROYER S, ALAMDARI H, DUPREZ D, KALIAGUINE S. Oxygen storage capacity of La1−xA′xBO3 perovskites (with A′=Sr, Ce; B=Co, Mn) relation with catalytic activity in the CH4 oxidation reaction[J]. Appl Catal B: Environ,2005,58(3/4):273−288. doi: 10.1016/j.apcatb.2004.12.010 [31] WANG Y, REN J, WANG Y, ZHANG F, LU G. Nanocasted synthesis of mesoporous LaCoO3 perovskite with extremely high surface area[J]. J Phys Chem C,2008,112:15293−15298. doi: 10.1021/jp8048394 [32] WU Y, NI X, BEAURAIN A, DUJARDIN C, GRANGER P. Stoichiometric and non-stoichiometric perovskite-based catalysts: Consequences on surface properties and on catalytic performances in the decomposition of N2O from nitric acid plants[J]. Appl Catal B: Environ,2012,125:149−157. doi: 10.1016/j.apcatb.2012.05.033 [33] LI L, YANG Q, WANG B, WANG D, CRITTENDEN J. Sacrificial carbon strategy for facile fabrication of highly-dispersed cobalt-silicon nanocomposites: Insight into its performance on the CO and CH4 oxidation[J]. J Clean Prod,2021,278:1−9. [34] QIAN Z, LIU Q L, ZHENG Y F, HAN R, SONG C F, JI N, MA D G. Enhanced catalytic performance for volatile organic compound oxidation over in-situ growth of MnOx on Co3O4 nanowire[J]. Chemosphere,2020,244:125532−125541. doi: 10.1016/j.chemosphere.2019.125532 [35] ZHANG Y, WANG M, KANG S, PAN T, DENG H, SHAN W, HE H. Investigation of suitable precursors for manganese oxide catalysts in ethyl acetate oxidation[J]. J Environ Sci,2021,104:17−26. doi: 10.1016/j.jes.2020.11.025 [36] HONG E, JEON S A, LEE S S, SHIN C H. Methane combustion over Pd/Ni-Al oxide catalysts: Effect of Ni/Al ratio in the Ni-Al oxide support[J]. Korean J Chem Eng,2018,35(9):1815−1822. doi: 10.1007/s11814-018-0090-0 [37] LAASSIRI S, BION N, DUPREZ D, ALAMDARI H S, ROYER S. Role of Mn + cations in the redox and oxygen transfer properties of BaMxAl12−xO19−δ (M = Mn, Fe, Co) nanomaterials for high temperature methane oxidation[J]. Catal Sci Technol,2013,3(9):2259−2269. doi: 10.1039/c3cy00192j [38] ZHEN H, ZHANG H, BING D, NI Y, KONG A, SHAN Y. High efficient mesoporous Co3O4 nanocatalysts for methane combustion at low temperature[J]. Chem Select,2016,1(5):979−983. [39] CHEN H, LI J, CUI W, FEI Z, QIAO X. Precise fabrication of surface-reconstructed LaMnO3 perovskite with enhanced catalytic performance in CH4 oxidation[J]. Appl Surf Sci,2020,505:1−9. [40] YANG J, HU S, SHI L, HOANG S, GUO Y. Oxygen vacancies and lewis acid sites synergistically promoted catalytic methane combustion over perovskite oxides[J]. Environ Sci Technol,2021,55(13):9243−9254. doi: 10.1021/acs.est.1c00511 -




 下载:
下载: