Hydrogen production from wood vinegar reforming over cobalt modified nickel-based catalyst
-
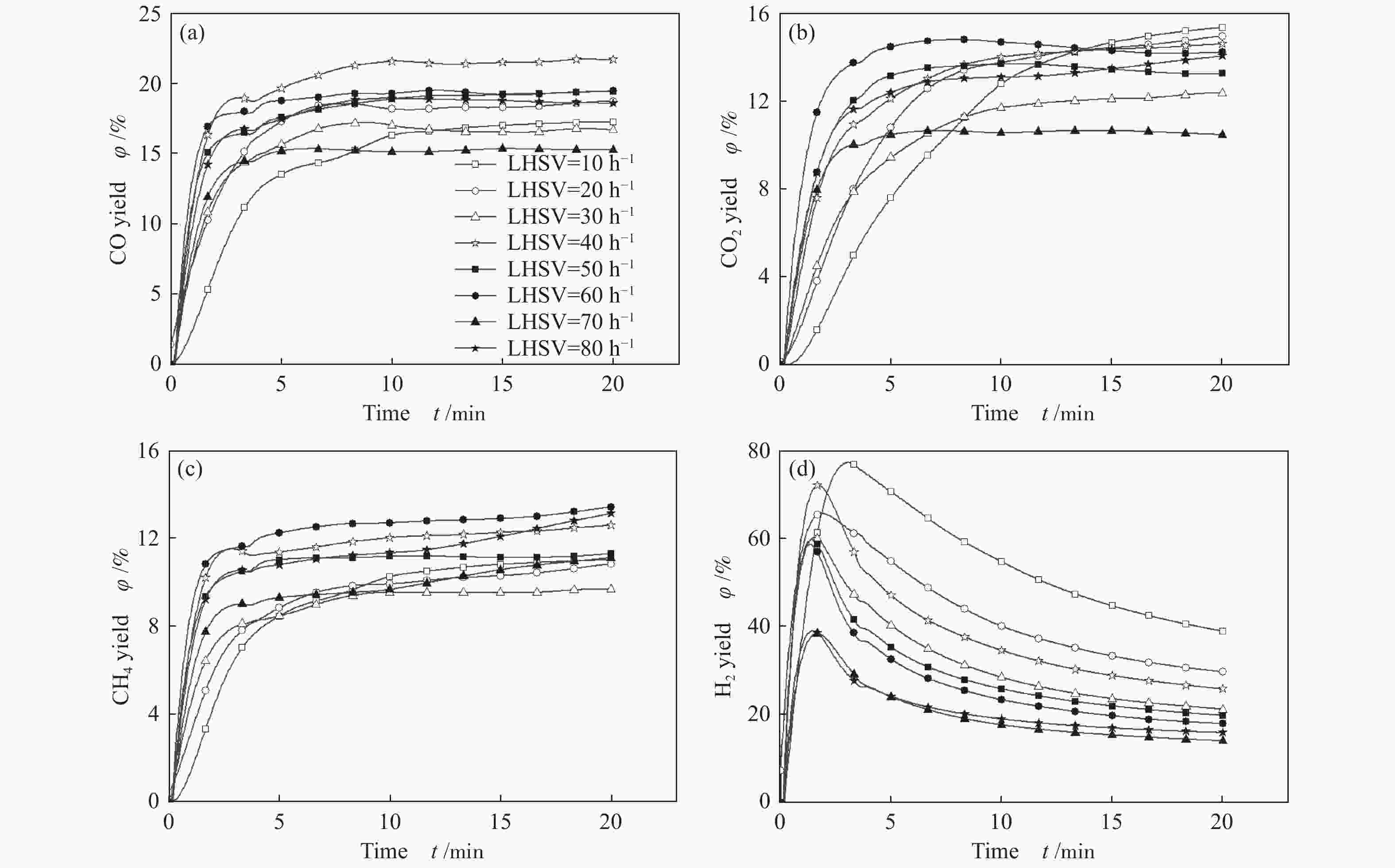
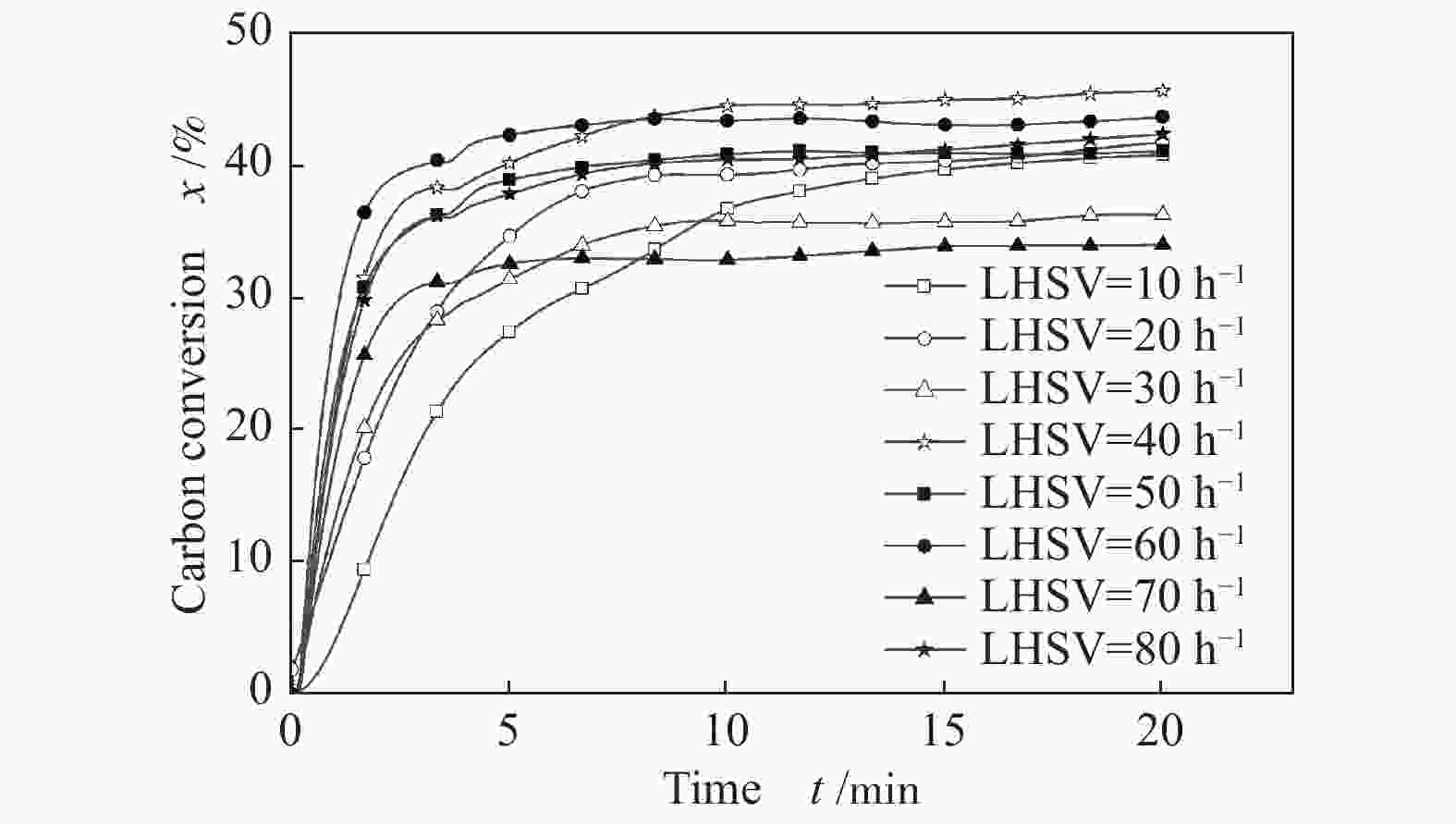
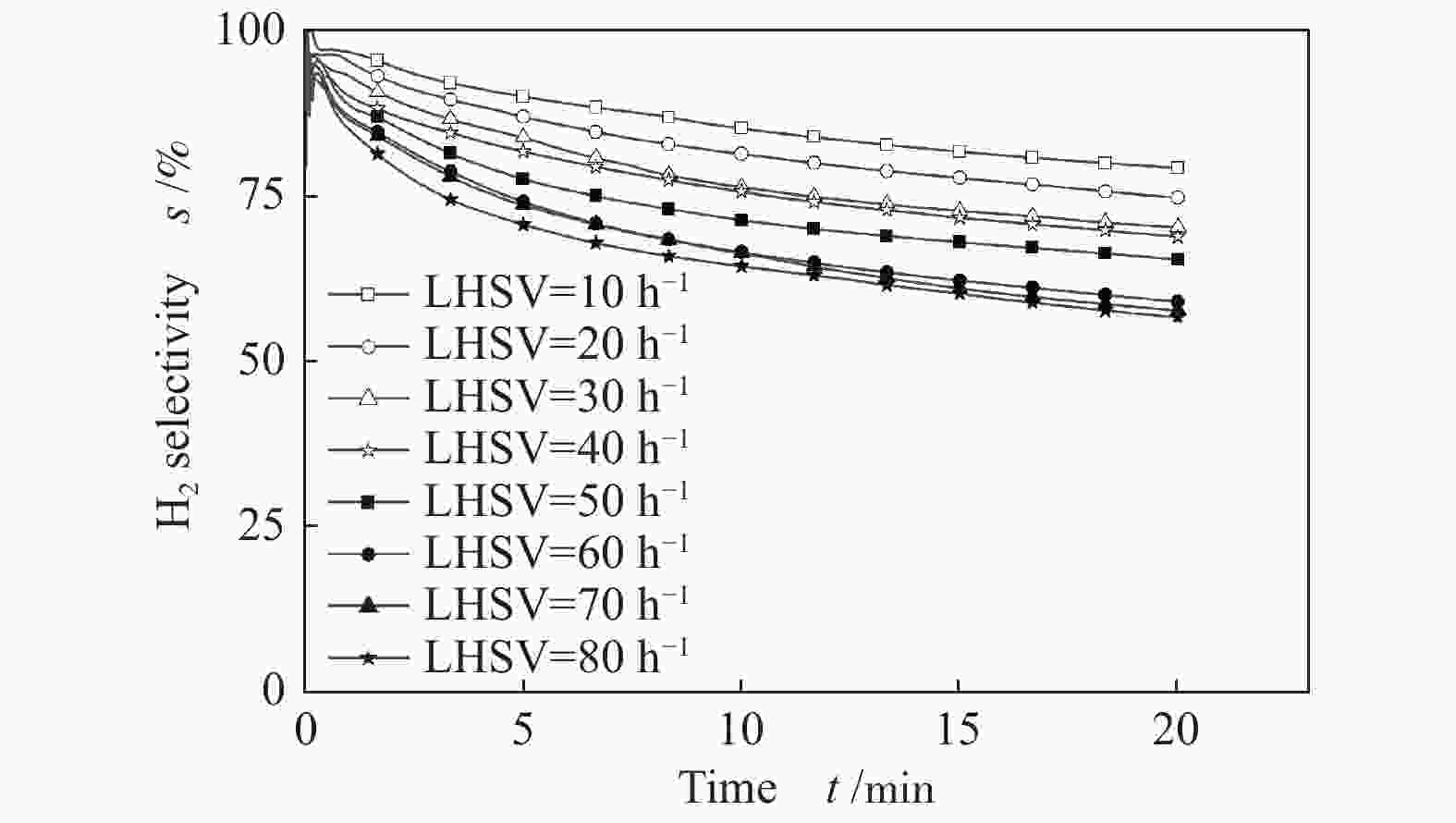
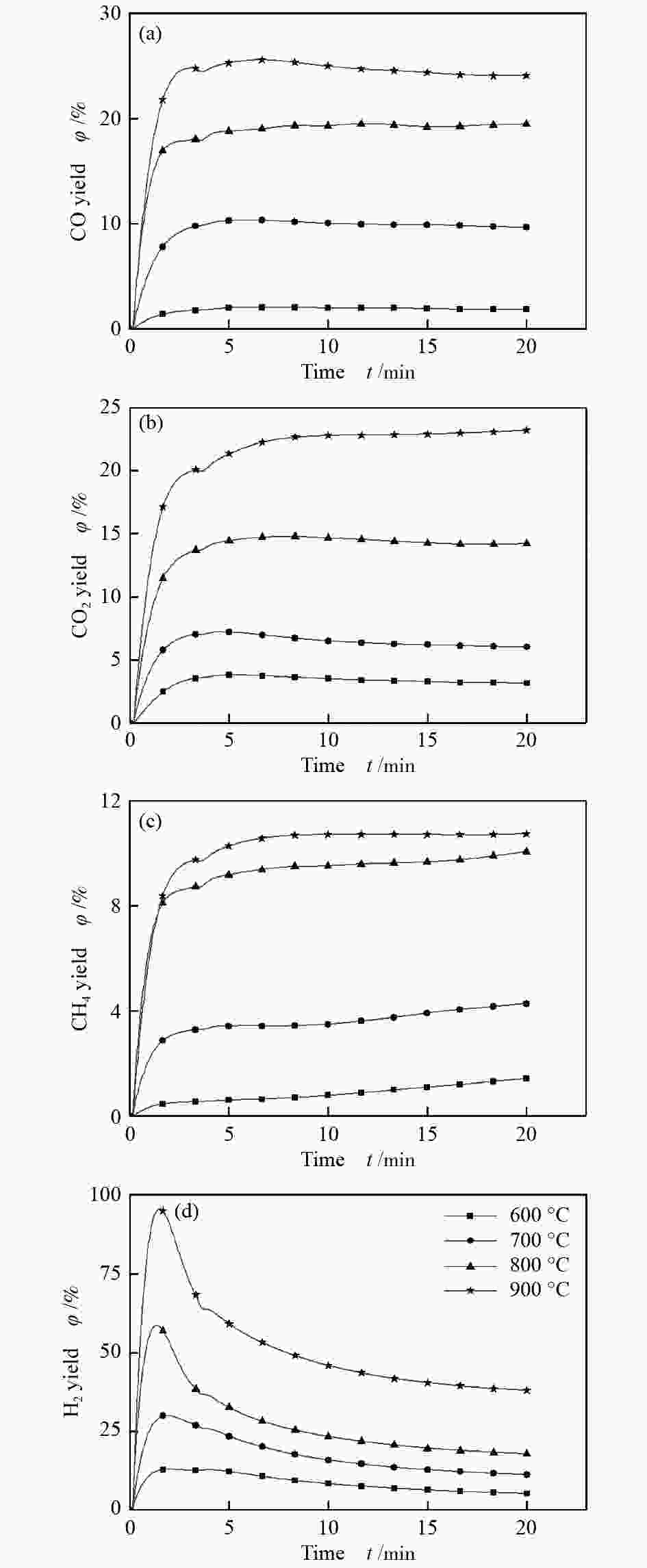
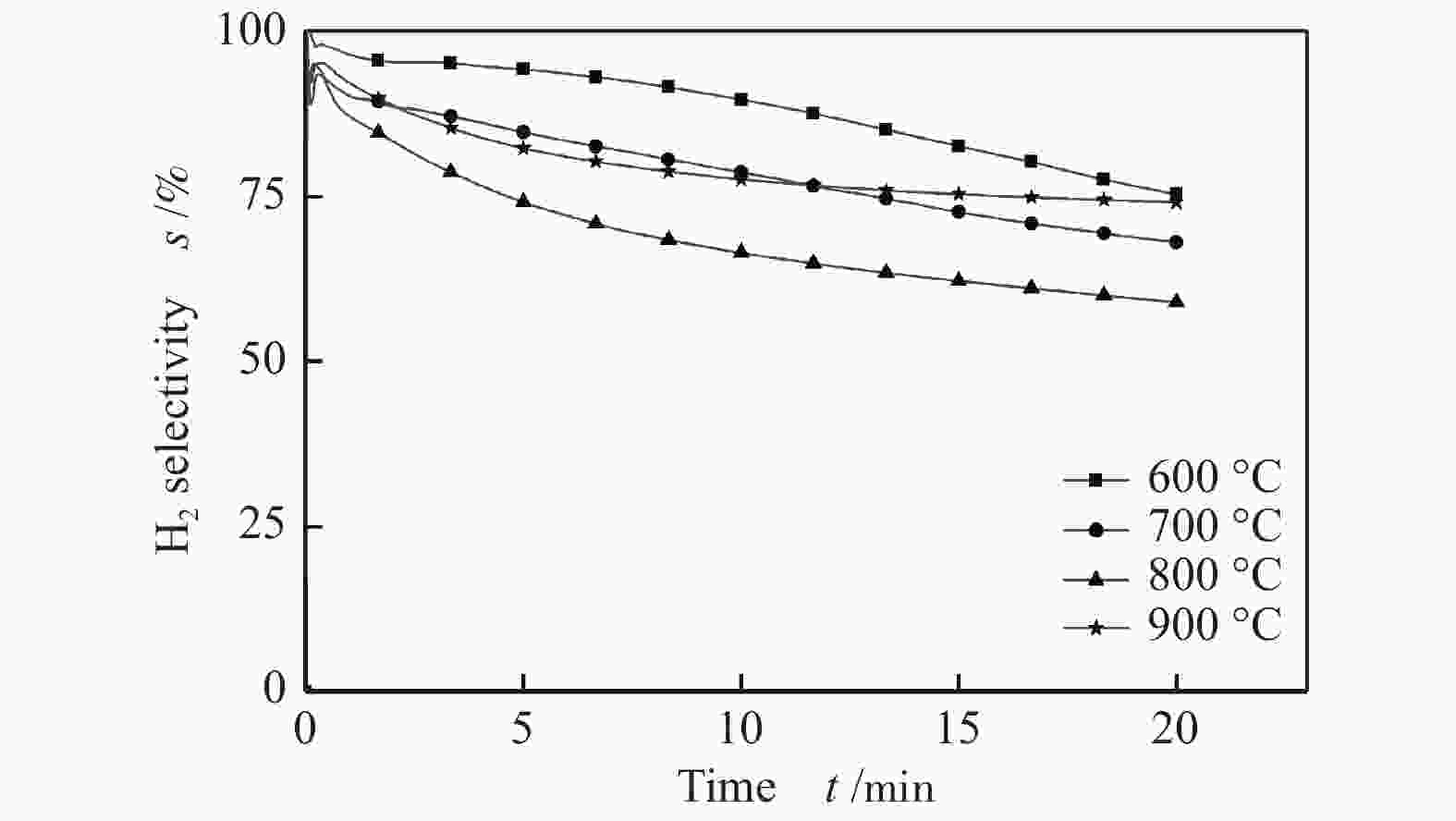
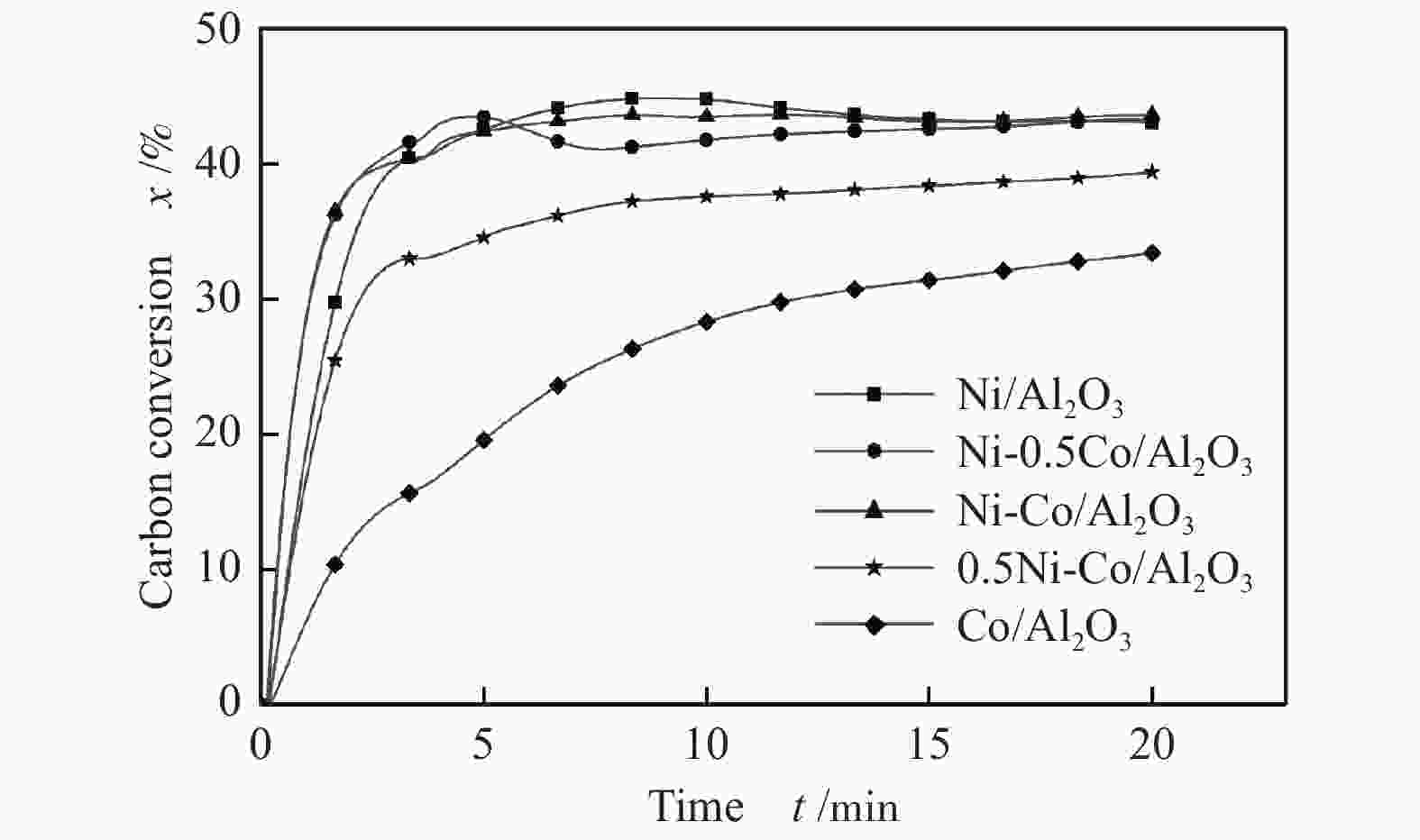
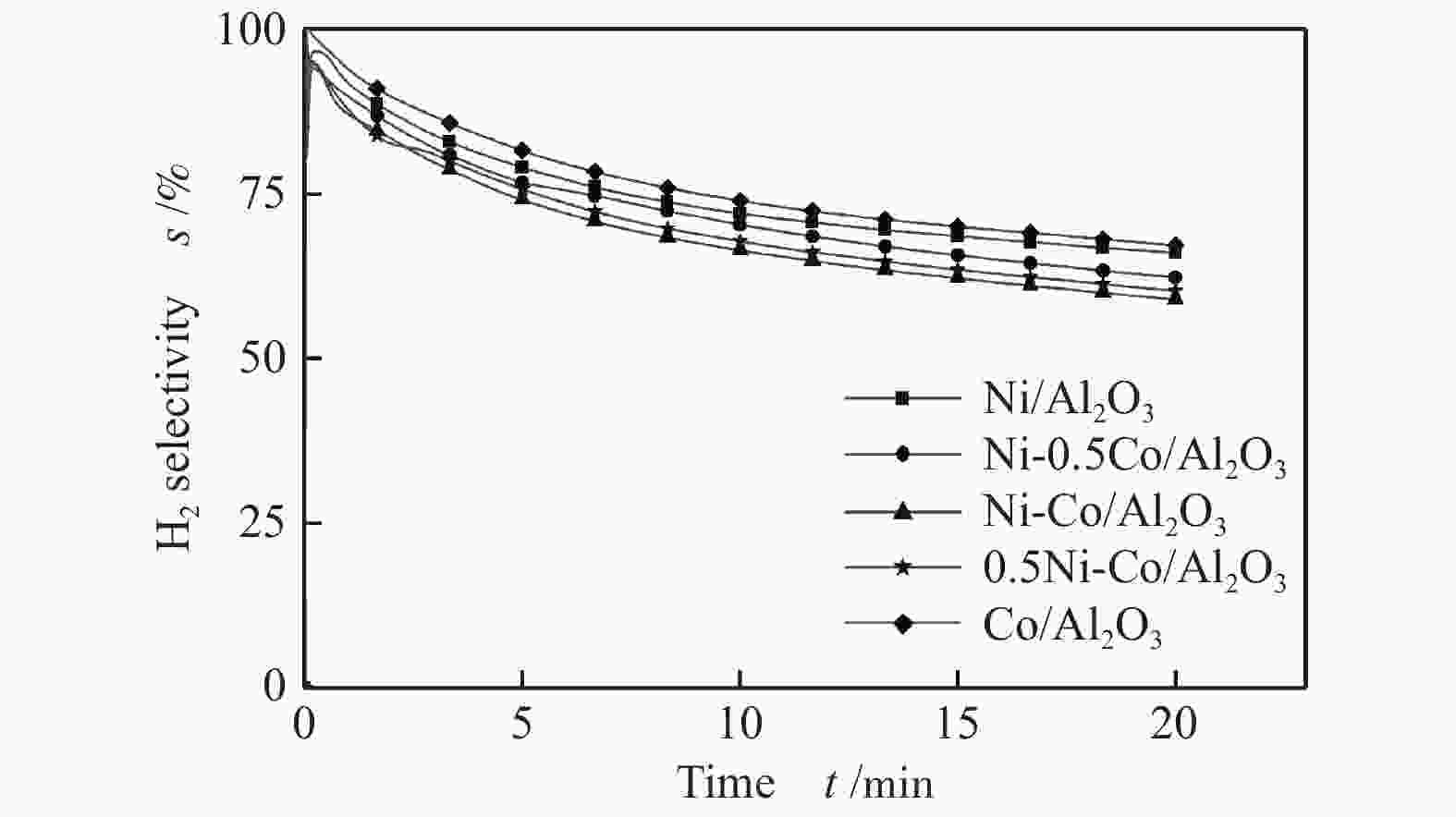
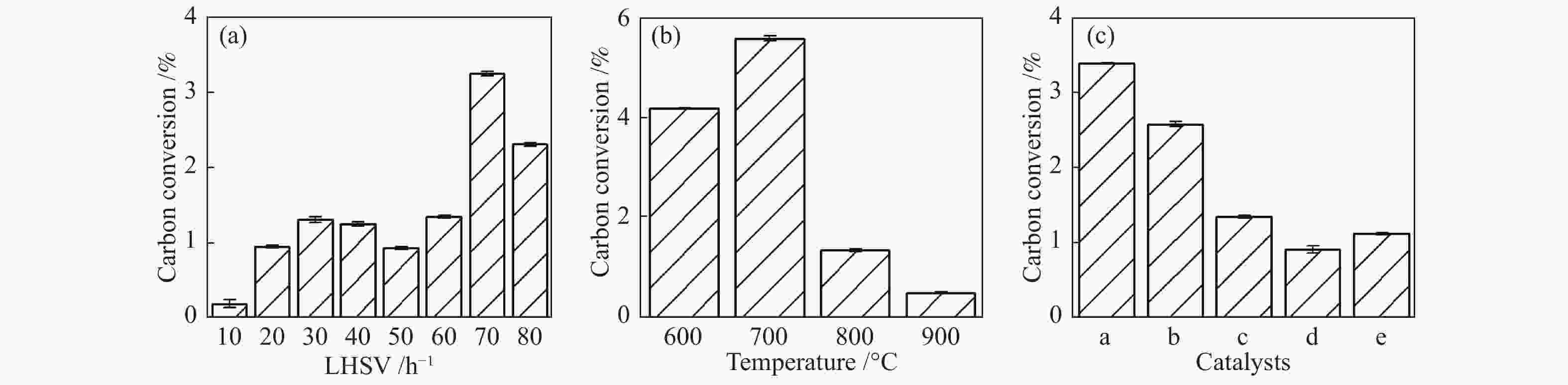
摘要: 为了实现木醋液的高值化利用,在固定床反应器中,进行木醋液催化重整制氢实验,采用浸渍法制备一系列不同Co添加量的Ni基催化剂,以产氢率、碳转化率、H2选择性和积炭量为主要评价指标,探究液时空速、反应温度、镍钴比等工况对木醋液催化重整制氢的影响,同时采用 XRF、H2-TPR、SEM及元素分析等方法对催化剂进行了表征。结果表明,液时空速增加,产气量增大,但液时空速过高会加速催化剂失活。高温有利于木醋液的催化重整制氢反应,温度到达900 ℃时,氢气产率最高。随着钴添加量的增加,反应产生的积炭降低,但氢气产率也会有所下降。因此,当液时空速为60 h−1,温度为800 ℃时,采用Ni-0.5Co/Al2O3催化剂,最适于木醋液的催化重整制氢实验。Abstract: In order to realize high value utilization of wood vinegar, a series of Ni based catalysts with different Co contents prepared by impregnation method were tested in a fixed bed reactor. The effects of liquid space-time velocity, reaction temperature and Ni/Co ratio on hydrogen production, carbon conversion, H2 selectivity and carbon deposition were investigated. The catalysts were characterized by XRF, H2-TPR, SEM and elemental analysis. The results show that the gas production increases with the increase of space-time velocity of liquid, but the catalyst deactivation is accelerated when the space-time velocity of liquid is too high. High temperature is conducive to the catalytic reforming of wood vinegar to produce hydrogen. When the temperature reaches 900 °C, the hydrogen yield is the highest. With the increase of cobalt content, the carbon deposition and hydrogen yield decrease. Therefore, when the liquid space velocity is 60 h−1 and the temperature is 800 °C, the Ni-0.5Co/Al2O3 catalyst is most conducive to the hydrogen production experiment of wood vinegar.
-
Key words:
- wood vinegar /
- hydrogen from reforming /
- nickel-based catalyst /
- carbon deposition
-
表 1 木醋液的主要成分统计
Table 1 Statistical result on the main components of wood vinegar
No RT Name Mol.formula Area/% 1 4.297 water H2O 23.95 2 4.958 acetic acid, methyl ester C3H6O2 1.73 3 7.474 acetic acid C2H4O2 16.63 4 8.496 2-propanone, 1-hydroxy- C3H6O2 6.71 5 10.026 propanoic acid C3H6O2 1.81 6 11.152 1-hydroxy-2-butanone C4H8O2 1.41 7 12.591 butanoic acid C4H8O2 0.54 8 13.05 3-cyclopentene-1-acetaldehyde, 2-oxo- C7H8O2 1.17 9 14.788 2-cyclopenten-1-one, 2-methyl- C6H8O 0.68 10 17.849 2-cyclopenten-1-one, 3-methyl- C6H8O 0.68 11 17.989 butyrolactone C4H6O2 0.98 12 19.532 2-cyclopenten-1-one, 2-hydroxy-3-methyl- C6H8O2 2.49 13 20.377 phenol C6H6O 4.99 14 20.983 phenol, 2-methoxy- C7H8O2 1.54 15 21.797 phenol, 2-methyl- C7H8O 0.94 16 22.28 nonane, 5-butyl- C13H28 0.95 17 22.819 phenol, 4-methyl- C7H8O 0.58 18 22.874 phenol, 3-methyl- C7H8O 0.84 19 23.143 propanoic acid, 2-methyl-, anhydride C8H14O3 2.23 20 23.939 phenol, 2-methoxy-4-methyl- C8H10O2 0.84 21 24.196 cyclopropyl carbinol C4H8O 5.77 22 26.393 3(2H)-furanone, dihydro-5-isopropyl- C7H12O2 0.95 23 27.214 1,4:3,6-dianhydro-α-D-glucopyranose C6H8O4 1.16 24 29.142 phenol, 2,6-dimethoxy- C8H10O3 2.07 25 31.419 phenol, 4-methoxy-3-(methoxymethyl)- C9H12O3 0.65 26 31.713 hydroquinone C6H6O2 0.71 27 37.087 1,6-anhydro-α-D-glucopyranose (levoglucosan) C6H10O5 4.04 note: only the components with relative peak area percentage greater than 0.5% were counted in the table 表 2 催化剂的组成
Table 2 Component content of catalysts
Components a b c d e Ni/% 3.95 2.78 3.12 1.87 0.00 Co/ % 0.00 1.75 2.47 3.24 3.31 表 3 木醋液催化重整制氢反应的实验方案
Table 3 Experimental scheme of hydrogen production by catalytic reforming of wood vinegar
Factors Levels LHSV/h−1 10 20 30 40 50 60 70 80 Temperature/°C 500 600 700 800 900 Ni/Co ratio 1:0 1:0.5 1:1 0.5:1 0:1 -
[1] SHARMA S, GHOSHAL S K. Hydrogen the future transportation fuel: From production to applications[J]. Renewable Sustainable Energy Rev,2015,43:1151−1158. doi: 10.1016/j.rser.2014.11.093 [2] WU Q, ZHANG S, HOU B. Study on the preparation of wood vinegar from biomass residues by carbonization process[J]. Bioresour Technol,2015,179:98−103. doi: 10.1016/j.biortech.2014.12.026 [3] 王海英, 杨国亭, 周丹. 木醋液研究现状及其综合利用[J]. 东北林业大学学报,2004,9(5):55−57. doi: 10.3969/j.issn.1000-5382.2004.05.020WANG Hai-ying, YANG Guo-ting, ZHOU Dan. Research situation and comprehensive utilization of wood vinegar[J]. J Northeast For Univ,2004,9(5):55−57. doi: 10.3969/j.issn.1000-5382.2004.05.020 [4] ORAMAHI H A, YOSHIMURA T. Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl[J]. J Wood Sci,2013,59(4):344−350. doi: 10.1007/s10086-013-1340-8 [5] MUNGKUNKAMCHAO T, KESMALA T, PIMRATCH S, TOOMSAN B, JOTHITYANGKOON D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.)[J]. Sci Hortic,2013,154:66−72. doi: 10.1016/j.scienta.2013.02.020 [6] 卢辛成, 蒋剑春, 孙康, 孙云娟. 木醋液的精制与应用研究进展[J]. 林产化学与工业,2017,37(3):1−9. doi: 10.3969/j.issn.0253-2417.2017.03.001LU Xin-cheng, JIANG Jian-chun, SUN Kang, SUN Yun-juan. Review on preparation and application of wood vinegar[J]. Chem Ind Forest Prod,2017,37(3):1−9. doi: 10.3969/j.issn.0253-2417.2017.03.001 [7] 蒋恩臣, 赵晨希, 秦丽元, 陈爱慧. 松子壳连续热解制备木醋液试验[J]. 农业工程学报,2014,30(5):262−269. doi: 10.3969/j.issn.1002-6819.2014.05.033JIANG En-chen, ZHAO Chen-xi, QIN Li-yuan, CHEN Ai-hui. Experiment of wood vinegar produced from pine nut shell continuous pyrolysis[J]. Trans CSAE,2014,30(5):262−269. doi: 10.3969/j.issn.1002-6819.2014.05.033 [8] XU X, JIANG E, LI B, WANG M. Hydrogen production from wood vinegar of camellia oleifera shell by Ni/M/γ-A12O3 catalyst[J]. Catal Commun,2013,39:106−114. doi: 10.1016/j.catcom.2013.04.024 [9] MEDRANO J A, OLIVA M, RUIZ J. Hydrogen from aqueous fraction of biomass pyrolysis liquids by catalytic steam reforming in fluidized bed[J]. Energy,2011,36:2215−2224. doi: 10.1016/j.energy.2010.03.059 [10] XU X, JIANG E, SUN Y, LI Z. Influence of mixed supports on the steam catalytic reforming of wood vinegar[J]. Energy Fuels,2017,31(2):1678−1688. doi: 10.1021/acs.energyfuels.6b03000 [11] FU P, ZHANG A, LUO S, YI W, HU S, ZHANG Y. Catalytic steam reforming of biomass-derived acetic acid over two supported Ni catalysts for hydrogen-rich syngas production[J]. ACS Omega,2019,4(8):13585−13593. doi: 10.1021/acsomega.9b01985 [12] ZHANG A, LI Z, YI W, FU P, WANG L, LIANG C, LUO S. Study the reaction mechanism of catalytic reforming of acetic acid through the instantaneous gas production[J]. Int J Hydrogen Energy,2019,44(39):21279−21289. doi: 10.1016/j.ijhydene.2019.06.060 [13] LI L, JIANG B, TANG D, ZHANG Q, ZHEG Z. Hydrogen generation by acetic acid steam reforming over Ni-based catalysts derived from La1−x CexNiO3 perovskite[J]. Int J Hydrogen Energy,2018,43(14):6795−6803. doi: 10.1016/j.ijhydene.2018.02.128 [14] SIANG J, LEE C, WANG C. Hydrogen production from steam reforming of ethanol using a ceria-supported iridium catalyst: effect of different ceria supports[J]. Int J Hydrogen Energy,2010,35(8):3456−3462. doi: 10.1016/j.ijhydene.2010.01.067 [15] ZHANG B, TANG X, LI Y. Steam reforming of bio-ethanol for the production of hydrogen over ceria-supported Co, Ir and Ni catalysts[J]. Catal Commun,2006,7(6):367−372. doi: 10.1016/j.catcom.2005.12.014 [16] HE S, MEI Z, LIU N. Ni/SBA-15 catalysts for hydrogen production by ethanol steam reforming: Effect of nickel precursor[J]. Int J Hydrogen Energy,2017,42(21):14429−14438. doi: 10.1016/j.ijhydene.2017.02.115 [17] XIE H Q, YU Q B, YAO X. Hydrogen production via steam reforming of bio-oil model compounds over supported nickel catalysts[J]. J Energy Chem,2015,24(3):299−308. doi: 10.1016/S2095-4956(15)60315-1 [18] CONSTANTINOU D A, EFSTATHIOU A M. Low-temperature purification of gas streams from phenol by steam reforming over novel supported-Rh catalysts[J]. Appl Catal B: Environ,2010,96(3/4):276−289. doi: 10.1016/j.apcatb.2010.02.007 [19] ARTETXE M, NAHIL M A, OLAZAR M, WILLIAMSP T. Steam reforming of phenol as biomass tar model compound over Ni/Al2O3 catalyst[J]. Fuel,2016,184:629−636. doi: 10.1016/j.fuel.2016.07.036 [20] 王一双, 陈明强, 刘少敏, 杨忠连, 沈朝萍, 刘珂. 负载NiO-Fe2O3的凹凸棒石对生物油模型物催化重整制氢性能的影响[J]. 燃料化学学报,2015,43(12):1470−1475. doi: 10.3969/j.issn.0253-2409.2015.12.010WANG Yi-shuang, CHEN Ming-qiang, LlU Shao-min, YANG Zhong-lian, SHEN Chao-ping, LIU Ke. Hydrogen production via cataylic steam reforming of bio-oil model compounds over NiO-Fe2O3-loaded palygouskite[J]. J Fuel Chem Technol,2015,43(12):1470−1475. doi: 10.3969/j.issn.0253-2409.2015.12.010 [21] NOGUEIRA F, ASSAF P, CARVALHO H, ASSAF E. Catalytic steam reforming of acetic acid as a model compound of bio-oil[J]. Appl Catal B: Environ,2014,160(7):188−199. [22] HE Z, YANG M, WANG X, ZHAO Z, DUAN A. Effect of the transition metal oxide supports on hydrogen production from bio-ethanol reforming[J]. Catal Today,2012,194(1):2−8. doi: 10.1016/j.cattod.2012.05.004 [23] XU X, ZHANG C, LIU Y, ZHAI Y, ZHANG R, TANG X. Catalytic reforming of acetic acid as a model compound of bio-oil for hydrogen production over Ni-CeO2-MgO/olivine catalysts[J]. Environ Prog Sustainable Energy,2015,34(3):915−922. doi: 10.1002/ep.12062 [24] NABGAN W, ABDULLAH T A T, MAT R. Production of hydrogen via steam reforming of acetic acid over Ni and Co supported on La2O3 catalyst[J]. Int J Hydrogen Energy,2017,42(14):8975−8985. doi: 10.1016/j.ijhydene.2016.04.176 [25] ZHANG F, WANG M, ZHU L. A comparative research on the catalytic activity of La2O3 and γ-Al2O3 supported catalysts for acetic acid steam reforming[J]. Int J Hydrogen Energy,2017,42(6):3667−3675. doi: 10.1016/j.ijhydene.2016.06.264 [26] WURZLER G T, RABELO-ETO R C, MATTOS L V. Steam reforming of ethanol for hydrogen production over MgO-supported Ni-based catalysts[J]. Appl Catal A: Gen,2016,518:115−128. doi: 10.1016/j.apcata.2015.11.020 [27] 吕衍安, 赵星岭, 索掌怀, 廖卫平, 金明善. 负载Ni催化剂上低温甘油蒸汽重整制氢[J]. 燃料化学学报,2015,43(6):684−691. doi: 10.3969/j.issn.0253-2409.2015.06.007LV Yan-an, ZHAO Xing-ling, SUO Zhang-huai, LIAO Wei-ping, JIN Ming-shan. Low-temperature steam reforming of glycerol for hydrogen production over supported nickel catalysts[J]. J Fuel Chem Technol,2015,43(6):684−691. doi: 10.3969/j.issn.0253-2409.2015.06.007 [28] MORAES T S, NETO R C R, RIBEIRO M C, MATTOS L V, KOURTELESIS M, LADAS S, VERYKIOS X, BELLOT NORONHA F. The study of the performance of PtNi/CeO2-nanocube catalysts for low temperature steam reforming of ethanol[J]. Catal Today,2015,242:35−49. doi: 10.1016/j.cattod.2014.05.045 [29] ZHANG Z M, WANG Y R, SUN K. Steam reforming of acetic acid over Ni-Ba/Al2O3 catalysts: Impacts of barium addition on coking behaviors and formation of reaction intermediates[J]. J Energy Chem,2020,43:208−219. doi: 10.1016/j.jechem.2019.08.023 [30] VICENTE J, MONTERO C, EREÑA J, AZKOITI M J, BILBAO J, GAYUBO A G. Coke deactivation of Ni and Co catalysts in ethanol steam reforming at mild temperatures in a fluidized bed reactor[J]. Int J Hydrogen Energy,2014,39(24):12586−12596. doi: 10.1016/j.ijhydene.2014.06.093 [31] ZHANG A, LIANG C, WANG L, LI Z, YI W, FU P, WANG S. Study on the trigger mechanism and carbon behavior of catalytic reforming of wood vinegar[J]. Int J Hydrogen Energy,2020,45(29):14669−14678. doi: 10.1016/j.ijhydene.2020.03.196 -





 下载:
下载: