Variance in the catalytic performance of nano-ZSM-5 zeolites during the reaction process of methanol to aromatics and its relation to the structural properties
-
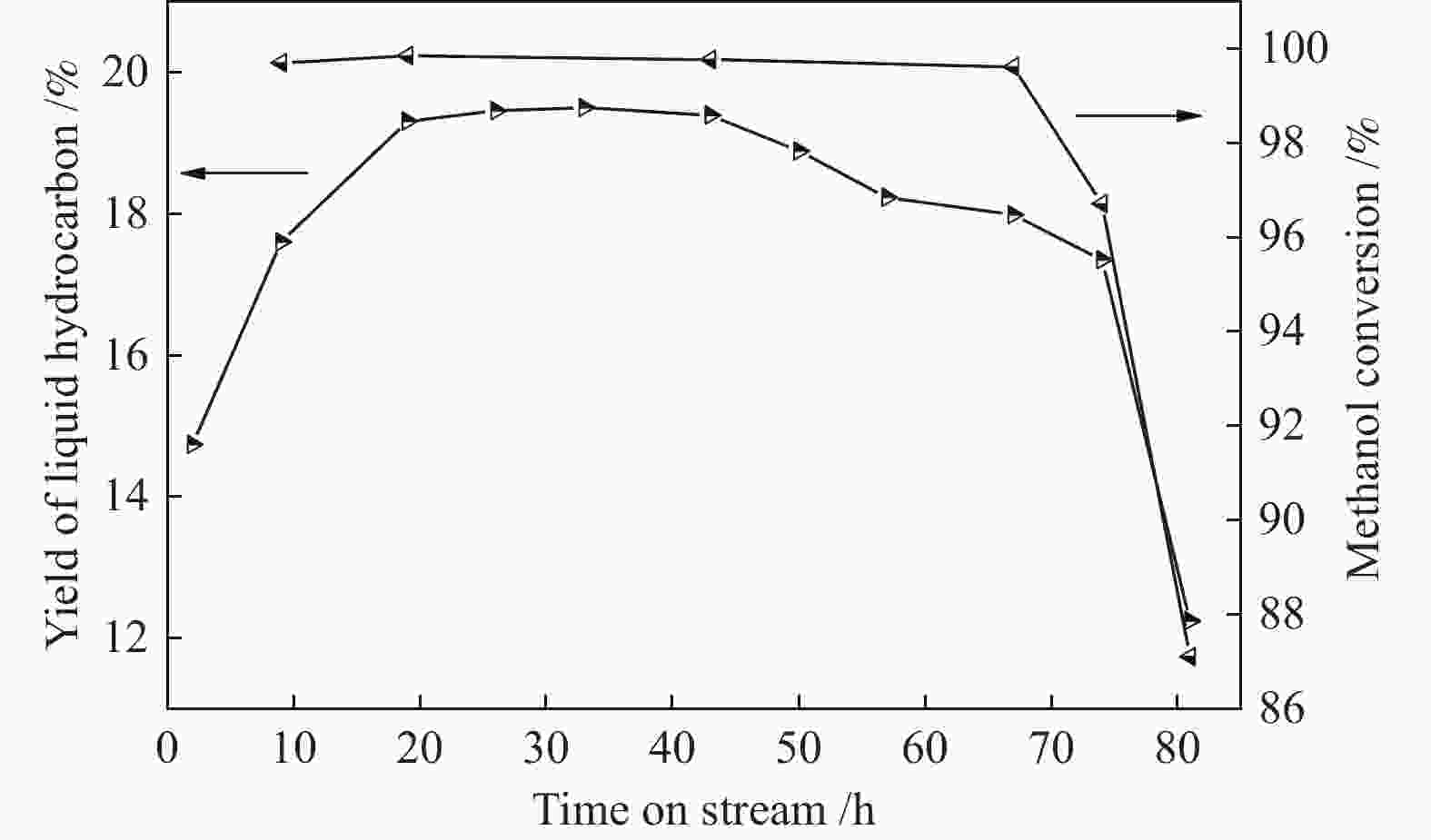
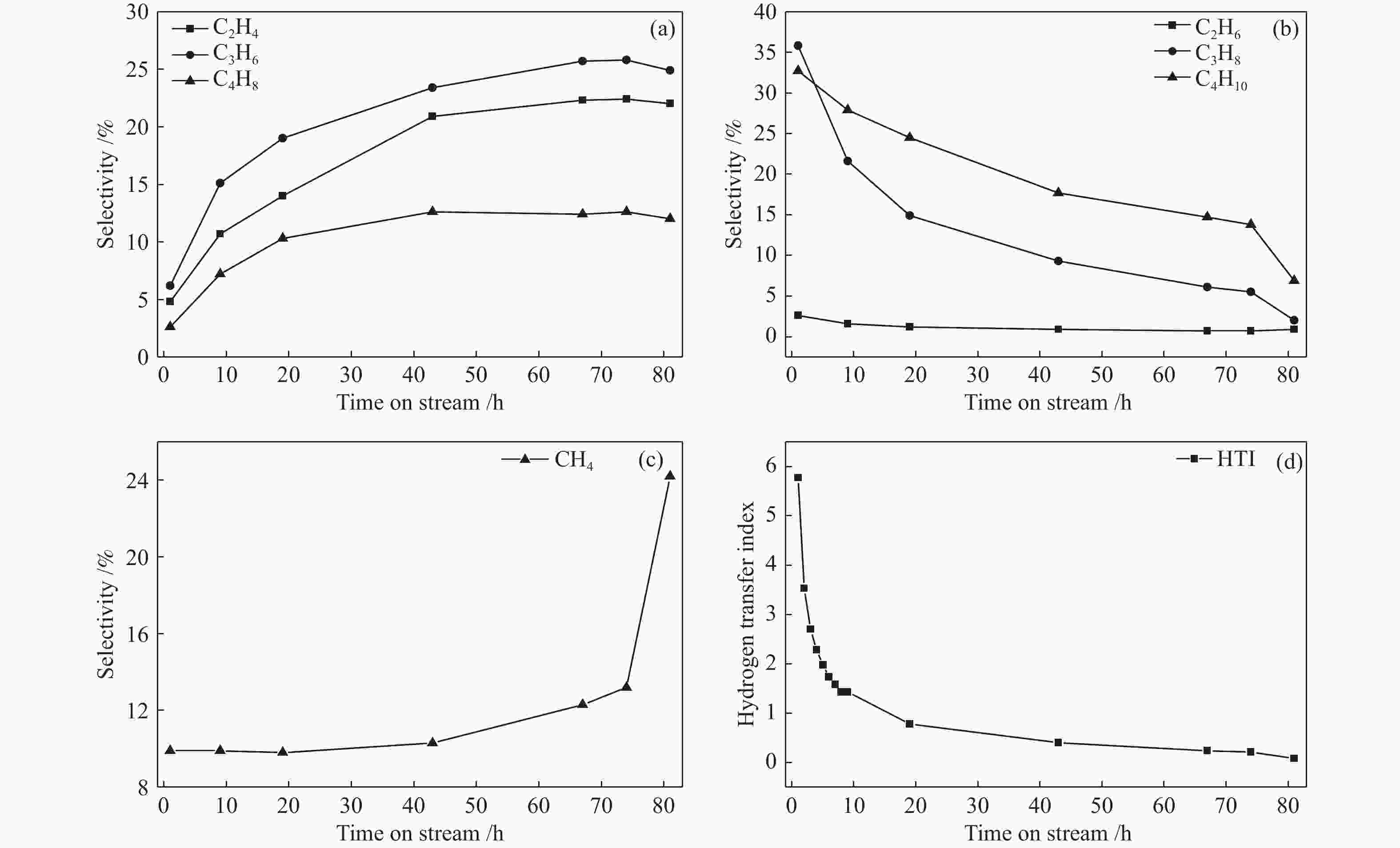
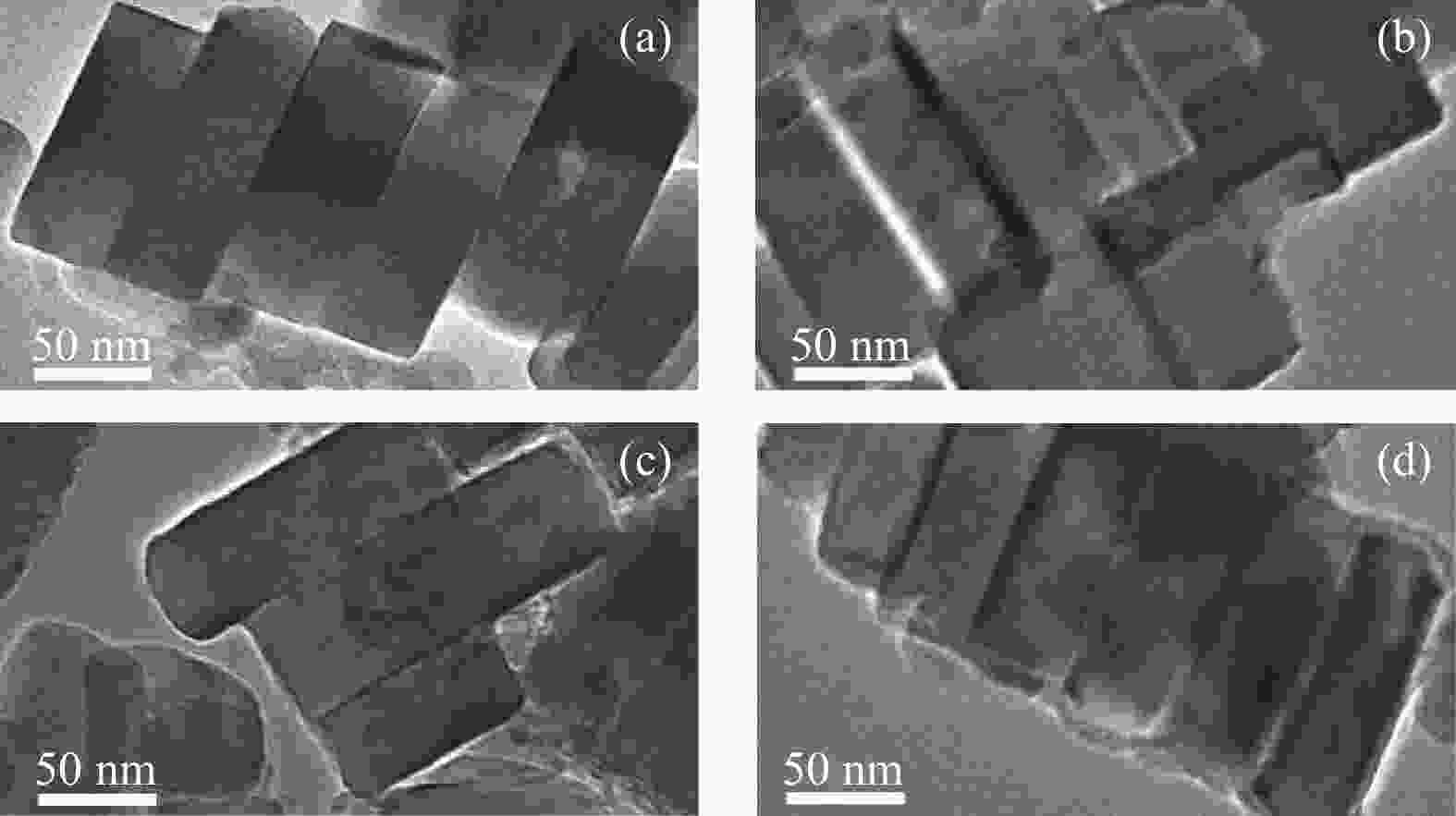
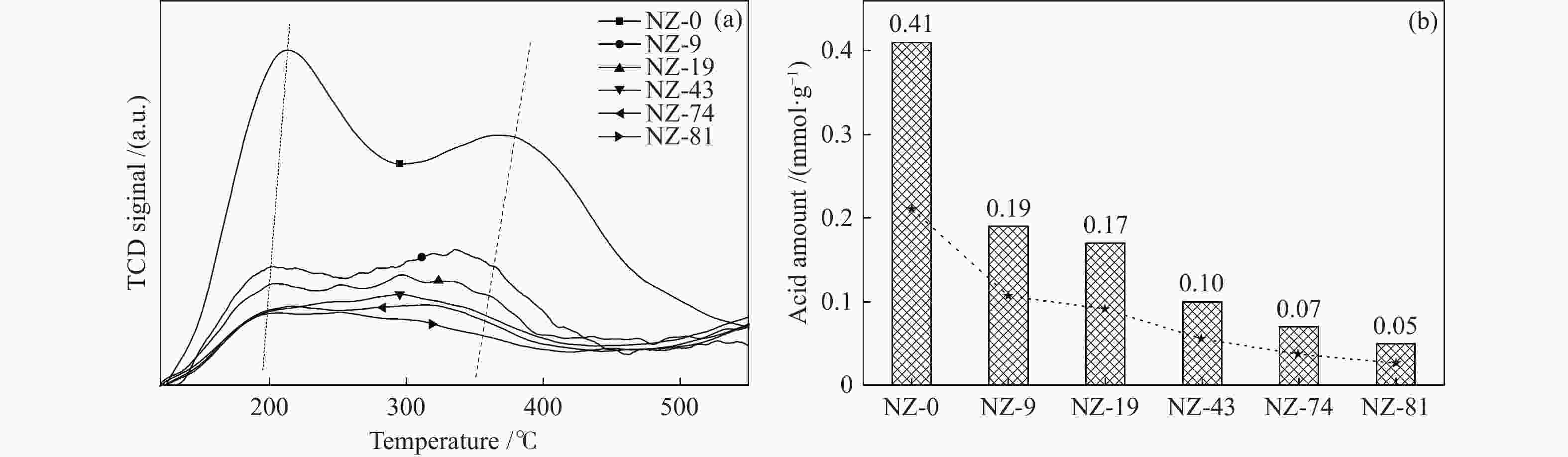
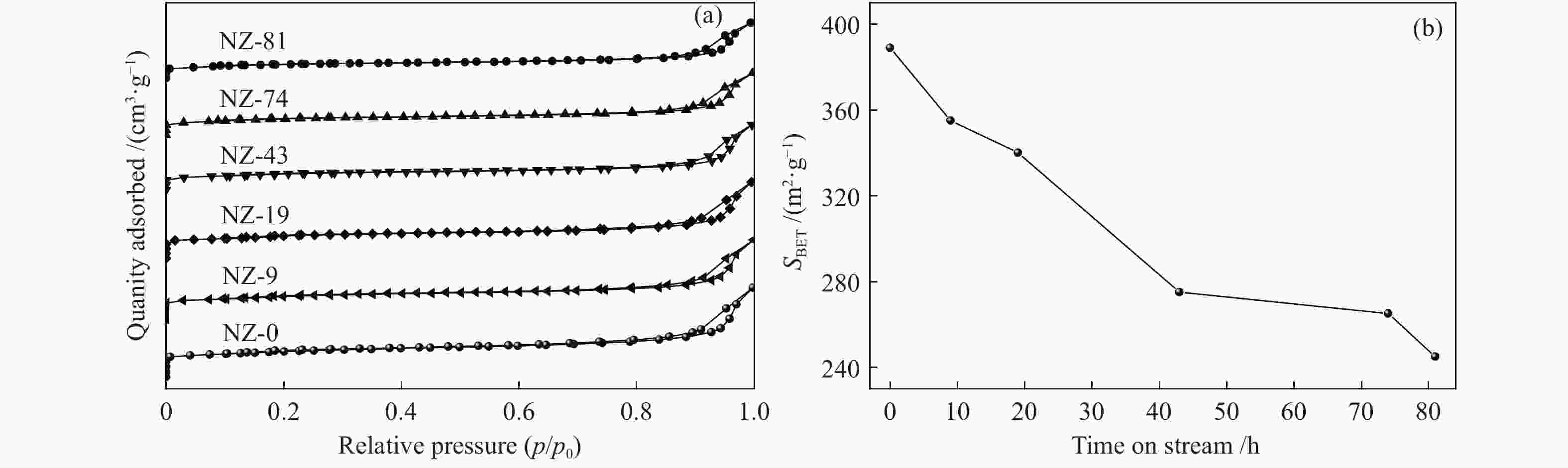
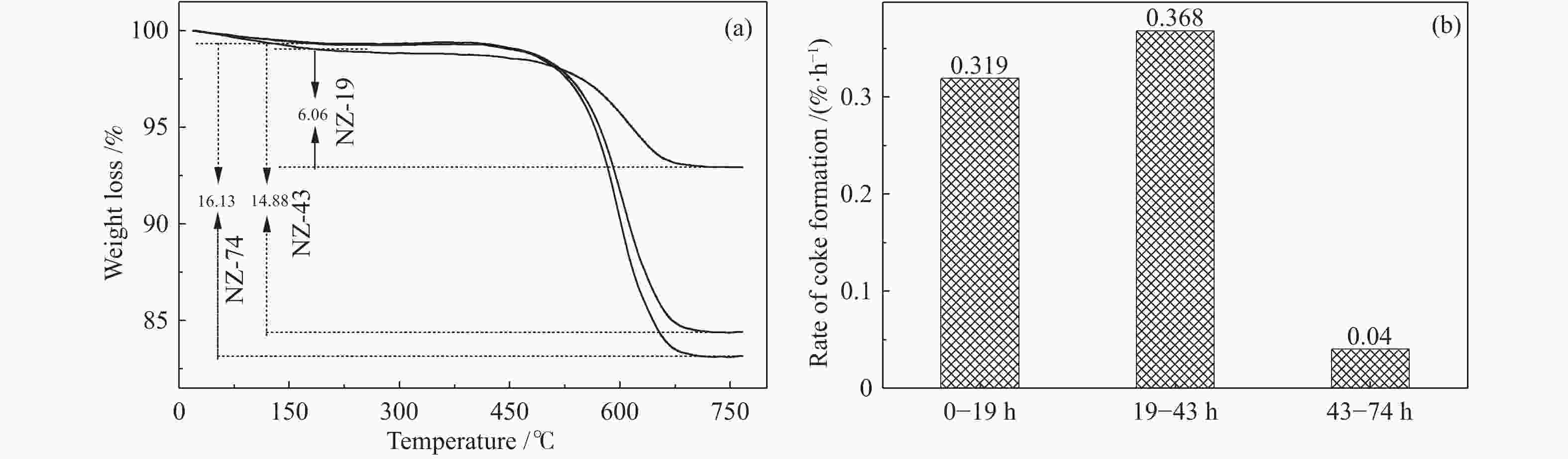
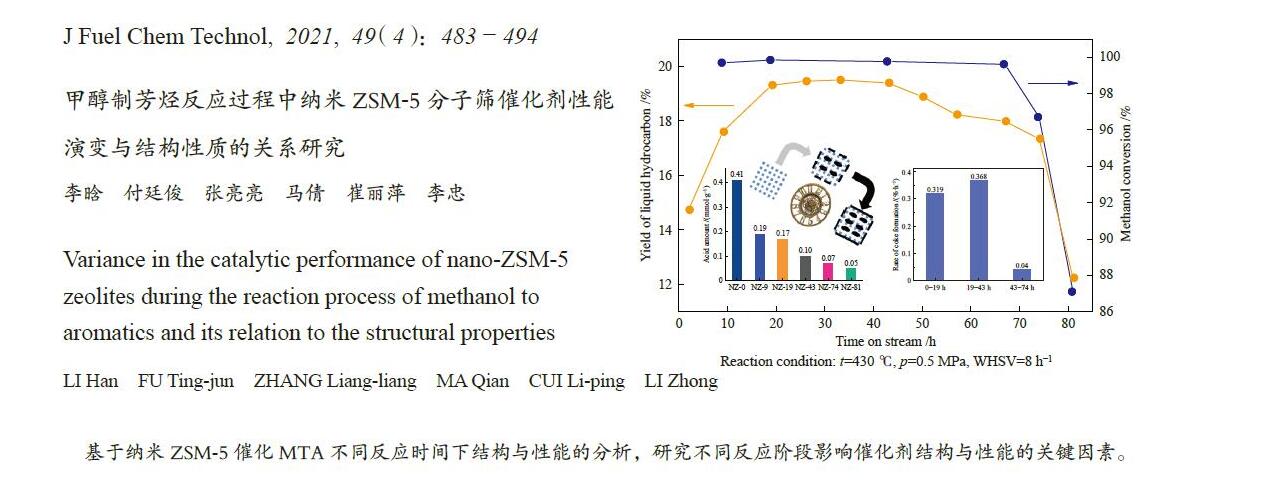
摘要: ZSM-5分子筛催化甲醇制芳烃反应中,存在产品选择性低和催化稳定性差等问题。本研究通过XRD、物理吸附、NH3-TPD、TEM、TG及27Al MAS NMR等手段表征分析反应过程中ZSM-5的结构变化,结合催化性能演变,探讨不同反应阶段影响其结构与性能的关键因素。结果表明,反应初期19 h内高温水热对铝结构的破坏使酸量由0.41 mmol/g明显降至0.17 mmol/g,低碳烯烃选择性显著增加,液烃收率由14.7%快速增至19.3%。稍后的24 h稳定反应阶段中,积炭速率增加,催化剂比表面积由340 m2/g显著降至275 m2/g,酸量继续下降至0.10 mmol/g;液烃收率仍维持在19.5%,说明该反应仅需少量酸即可维持。之后31 h内积炭不断累积,虽然积炭速率明显降低,但催化剂逐渐失活;外表面积炭显著增加,比表面积和酸量继续缓慢减小,液烃收率降至17.3%,芳烃选择性也随之降低。反应末期的7 h里,积炭严重覆盖了ZSM-5酸位并堵塞孔道,液烃收率突降至12.2%,CH4选择性由13.2%骤增至24.2%;积炭对外表面酸的覆盖减少了表面异构化,对二甲苯在二甲苯中选择性由24.4%增至33.1%。考虑到积炭对失活后团聚晶粒的整体包裹,应设法减少晶粒外表面间的接触,以提升分子对反应活性位的可接近性和催化剂的容碳能力。该研究为芳构化催化剂制备时酸性和形貌的控制提供了依据。Abstract: Low selectivity to target products and poor catalytic stability remain two crucial issues for the conversion of methanol to aromatics (MTA) catalyzed by ZSM-5 zeolites. In this work, the variance in catalytic performance of ZSM-5 zeolites with the time on stream during a long term MTA test was monitored and related to the structural changes which were characterized by XRD, physisorption, NH3-TPD, TEM, TG and 27Al MAS NMR; the key structural factors affecting the catalytic performance and structure were then investigated. The results illustrate that within the early 19 h after starting the reaction, the amount of acid sites decreases significantly from 0.41 to 0.17 mmol/g due to the damage to the structure of aluminum species under high temperature hydrothermal conditions; the selectivity to light alkenes increases significantly, accompanying with a rapid increase of the liquid hydrocarbons yield from 14.7% to 19.3%. In the next stable reaction stage of 24 h, the rate of coke formation increases and the surface area decreases significantly from 340 to 275 m2/g, whilst the amount of acid sites decreases continuously to 0.10 mmol/g; the liquid hydrocarbon yield keeps above 19.5%, suggesting that a small amount of acid sites will be sufficient to stably bolster the MTA reaction. In contrast, in the next 31 h, although the rate of coke formation decreases obviously, the catalyst is deactivated gradually, dominantly by the coke deposition on the external surface; in this period, the surface area and the amount of acid sites decreases continuously and slowly and the liquid hydrocarbon yield drops to 17.3%, accompanying with a decrease in the selectivity to aromatics. At the end of the reaction of 7 h, the liquid hydrocarbon yield drops to 12.2%, due to the complete coverage of the acid sites and serious blockage of the pore by coke deposition; meanwhile, the selectivity to CH4 increases significantly from 13.2% to 24.2%, whereas the fraction of p-xylene in xylenes increases from 24.4% to 33.1%, owing to the suppression of isomerization on the external surface acid sites which are covered by coke deposition. As the agglomerated particles are covered by coke in the deactivated catalyst, it is then proposed that a diminution of the contact between the external surfaces of particles could improve the accessibility of the reactant molecules to the active sites and enhance the coke capacity. The results may provide some relevant suggestions for the control of acidity and morphology in the preparation of MTA catalysts.
-
Key words:
- methanol /
- aromatics /
- ZSM-5 zeolite /
- coke /
- reaction process /
- structure change
-
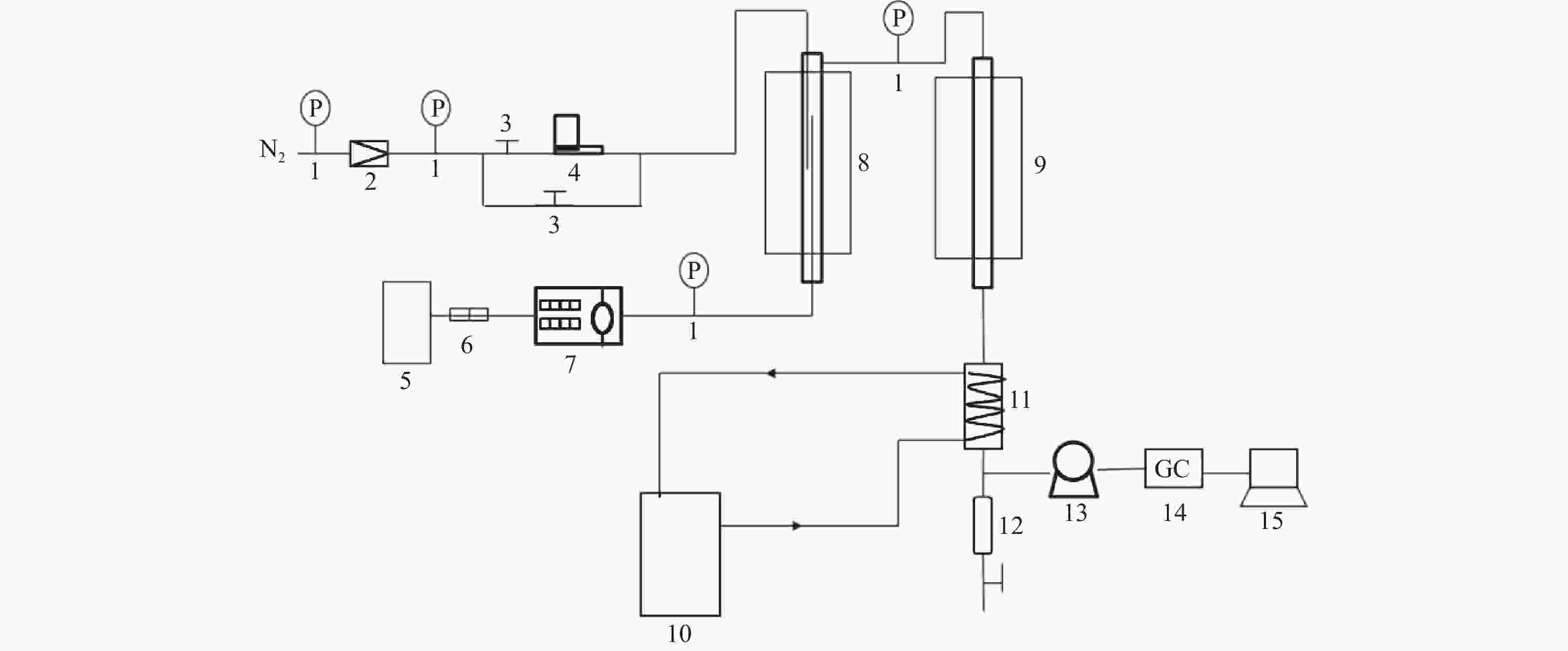
图 1 MTA反应固定床评价装置示意图
Figure 1 Diagram of fixed bed reactor of MTA reaction
1-pressure gauge;2-pressure reducing value;3-globe value;4-gas flowmeter;5-stock tank;6-filter;7-micro tube pump;8-preheater;9-reactor;10-condensate recirculating tank;11-condensator;12-liquid storage tank;13-wet gas flowmeter;14-gas chromatograph;15-computer
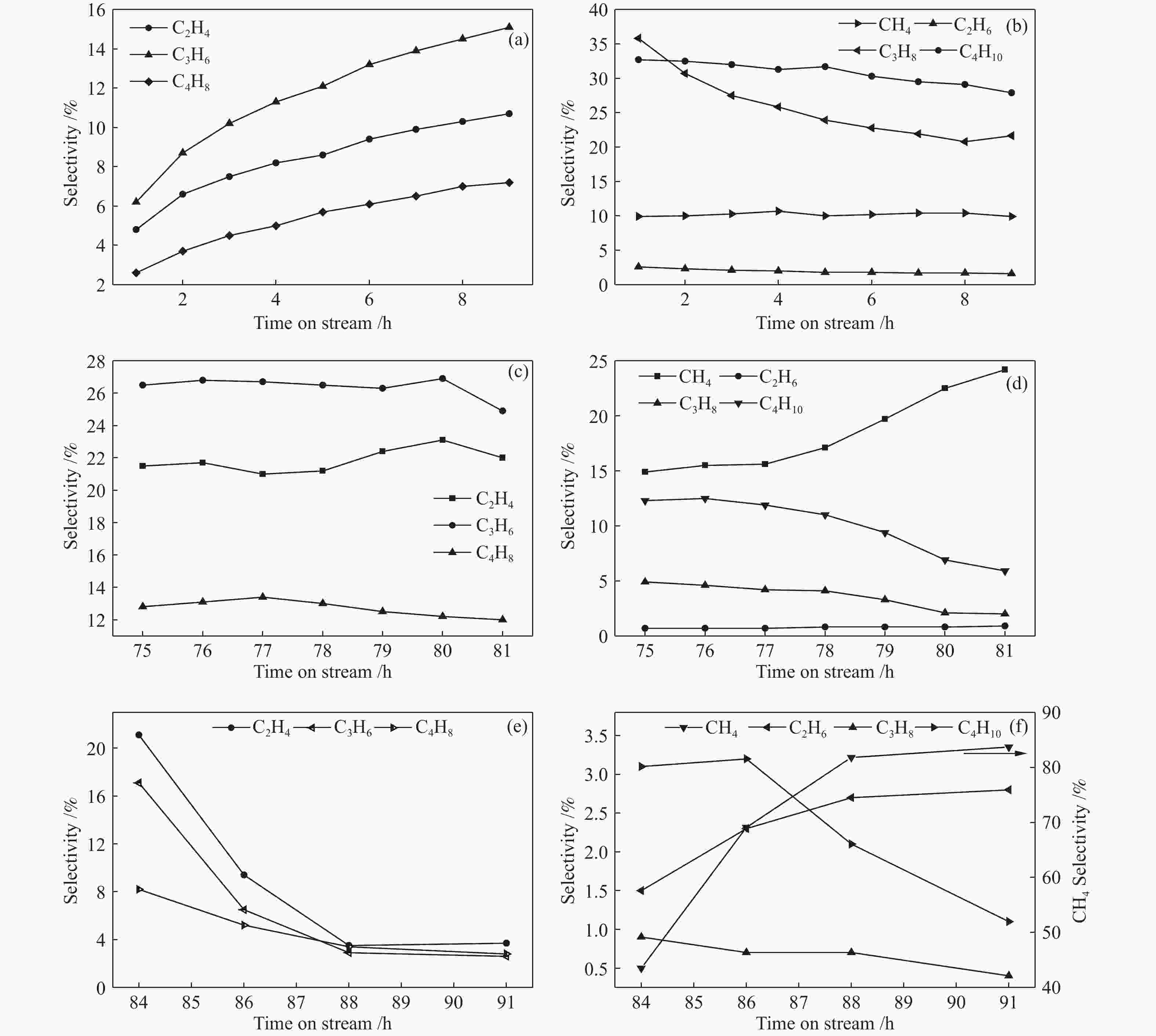
图 5 反应前9 h (a) 烯烃和 (b) 烷烃选择性变化;失活前7 h (c) 烯烃和 (d) 烷烃选择性;失活后 (e) 烯烃和 (f) 烷烃选择性
Figure 5 Changes in (a) alkene selectivity and (b) alkane selectivity within first 9 h; the changes in (c) alkene selectivity and (d) alkane selectivity within 7 h before the catalyst deactivation; the change in (e) alkene selectivity and (f) alkane selectivity after catalyst deactivation
表 1 不同反应时间ZSM-5分子筛的织构性质
Table 1 Textural properties of the ZSM-5 after different reaction times
Sample SBET
(m2·g−1)SMicro/
(m2·g−1)SExter/
(m2·g−1)vTotal/
(cm3·g−1)vMicro/
(cm3·g−1)vMeso/
(cm3·g−1)NZ-0 389 294 95 0.54 0.138 0.402 NZ-9 355 288 67 0.48 0.137 0.343 NZ-19 340 277 63 0.46 0.132 0.329 NZ-43 275 218 57 0.40 0.102 0.298 NZ-74 265 212 53 0.38 0.095 0.285 NZ-81 245 197 48 0.34 0.086 0.254 SBET=BET surface area, calculated by the BET model
Smicro=micropore area, SExter=external surface surface area, determined by the t-plot method
vtotal=total pore volume, determined from the absorbed amount at p/p0=0.99
vmicro=micropore volume, calculate by the t-plot method -
[1] GAO P, XU J, QI G D, WANG C, WANG Q, ZHAO Y X, ZHANG Y H, FENG N D, ZHAO X L, LI J L, DENG F. A Mechanistic study of methanol-to-aromatics reaction over Ga-modified ZSM-5 zeolites: Understanding the dehydrogenation process[J]. ACS Catal,2018,8(10):9809−9820. doi: 10.1021/acscatal.8b03076 [2] PAN D H, SONG X H, YANG X H, GAO L J, WEI R P, ZHANG J, XIAO G M. Efficient and selective conversion of methanol to para-xylene over stable H[Zn, Al]ZSM-5/SiO2 composite catalyst[J]. Appl Catal A: Gen,2018,557:15−24. doi: 10.1016/j.apcata.2018.03.006 [3] QIAO J, WANG J Q, FRENKEL A I, TENG J W, CHEN X Q, XIAO J X, ZHANG T Z, WANG Z D, YUAN Z Q, YANG, W M. Methanol to aromatics: Isolated zinc phosphate groups on HZSM-5 zeolite enhance BTX selectivity and catalytic stability[J]. RSC Adv,2020,10:5961−5971. doi: 10.1039/C9RA09657D [4] 郭淑佳, 王森, 罗耀亚, 罗莉, 董梅, 秦张峰, 樊卫斌, 王建国. H-ZSM-5分子筛形貌对ZnCr2O4/H-ZSM-5双功能催化剂合成气制芳烃催化性能的影响[J]. 燃料化学学报,2020,48(8):970−979. doi: 10.3969/j.issn.0253-2409.2020.08.009GUO Shu-jia, WANG Sen, LUO Yao-ya, LUO Li, DONG Mei, QIN Zhang-feng, FAN Wei-bin, WANG Jian-guo. Effect of H-ZSM-5 zeolite morphology on the performance of bifuctional ZnCr2O4/H-ZSM-5 catalysts in the direct conversion of syngas into aromatics[J]. J Fuel Chem Technol,2020,48(8):970−979. doi: 10.3969/j.issn.0253-2409.2020.08.009 [5] INUI T, MAKINO Y, OKAZUMI F, NAGANO S, MIYAMOTO A. Selective aromatization of light paraffins on platinum-ion-exchanged gallium-silicate bifunctional catalysts[J]. Chem Inform,1987,26(4):647−652. [6] ZHAO J J, WANG Y Q, SUN C, ZHAO A J, WANG C, ZHANG X, WANG Z Y, ZHAO T T, LIU W R, LU J X. Synthesis of hierarchical ZSM-5 aggregates by an alkali-treated seeds method with cetyltrimethylammonium bromide for the methanol to gasoline reaction[J]. React Kinet Mech Catal,2019,128:1079−1096. doi: 10.1007/s11144-019-01671-0 [7] JAVDANI A, AHMADPOUR J, YARIPOUR F. Nano-sized ZSM-5 zeolite synthesized via seeding technique for methanol conversions: A review[J]. Microporous Mesoporous Mater,2019,284:443−458. doi: 10.1016/j.micromeso.2019.04.063 [8] NI Y M, ZHU W L. Formaldehyde intermediate participating in the conversion of methanol to aromatics over zinc modified H-ZSM-5[J]. J Energy Chem,2021,54:174−178. doi: 10.1016/j.jechem.2020.05.063 [9] TREPS L, GOMEZ A, BRUIN T D, CHIZALLET C. Environment, stability and acidity of external surface sites of silicalite-1 and ZSM-5 micro and nano slabs, sheets, and crystals[J]. ACS Catal,2020,10(5):3297−3312. doi: 10.1021/acscatal.9b05103 [10] 邵娟, 付廷俊, 常江伟, 万威利, 齐瑞岳, 李忠. ZSM-5分子筛催化甲醇制汽油反应中的晶粒粒径效应研究[J]. 燃料化学学报,2017,45(1):75−83. doi: 10.3969/j.issn.0253-2409.2017.01.011SHAO Juan, FU Ting-jun, CHANG Jiang-wei, WAN Wei-li, QI Rui-yue, LI Zhong. Effect of ZSM-5 crystal size on its catalytic properties for conversion of methanol to gasoline[J]. J Fuel Chem Technol,2017,45(1):75−83. doi: 10.3969/j.issn.0253-2409.2017.01.011 [11] XU Y F, WANG J, MA G Y, LIN J H, DING M Y. Designing of hollow ZSM-5 with controlled mesopore sizes to boost gasoline production from syngas[J]. ACS Sustainable Chem Eng,2019,7(21):18125−18132. [12] BARBERA K, BONINO F, BORDIGA S, JANSSENS T V W, BEATO P. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects[J]. J Catal,2011,280(2):196−205. doi: 10.1016/j.jcat.2011.03.016 [13] FU T J, MA Z, WANG Y J, SHAO J, MA Q, ZHANG C M, CUI L P, LI Z. Si/Al ratio induced structure evolution during desilication-recrystallization of silicalite-1 to synthesize nano-ZSM-5 catalyst for MTH reaction[J]. Fuel Process Technol,2019,194:106122. doi: 10.1016/j.fuproc.2019.106122 [14] YANG C G, QIU M H, HU S W, CHEN X Q, ZENG G F, LIU Z Y, SUN Y H. Stable and efficient aromatic yield from methanol over alkali treated hierarchical Zn-containing HZSM-5 zeolites[J]. Microporous Mesoporous Mater,2016,231:110−116. doi: 10.1016/j.micromeso.2016.05.021 [15] ROWNAGHI A A, REZAEI F, HEDLUND J. Selective formation of light olefin by n-hexane cracking over HZSM-5: Influence of crystal size and acid sites of nano- and micrometer-sized crystals[J]. Chem Eng J,2012,191:528−533. doi: 10.1016/j.cej.2012.03.023 [16] SHEN K, WANG N, CHEN X D, CHEN Z H, LI Y W, CHEN J Y, QIAN W Z. Seed-induced and additive-free synthesis of oriented nanorod-assembled meso/macroporous zeolites: Toward efficient and cost-effective catalysts for the MTA reaction[J]. Catal Sci Technol,2017,7(21):5143−5153. doi: 10.1039/C7CY01647F [17] CHOI M, NA K, KIM J, SAKAMOTO Y, TERASAKI O, RYOO R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts[J]. Chem Inform,2009,461(828):246−249. [18] SHEN K, QIAN W Z, WANG N, SU C, WEI F. Fabrication of c-axis oriented ZSM-5 hollow fibers based on an in situ solid-solid transformation mechanism[J]. J Am Chem Soc,2013,135(41):15322−15325. doi: 10.1021/ja408624x [19] YANG L Z, LIU Z Y, LIU Z, PENG W Y, LIU Y Q, LIU C Q, LIU C G. Correlation between H-ZSM-5 crystal size and catalytic performance in the methanol-to-aromatics reaction[J]. Chin J Catal,2017,38(4):683−690. doi: 10.1016/S1872-2067(17)62791-8 [20] WAN Z J, LI G K, WANG C F, TANG H, ZHANG D K. Relating coke formation and characteristics to deactivation of ZSM-5 zeolite in methanol to gasoline conversion[J]. Appl Catal A: Gen,2018,519:141−151. [21] SUN L Y, WANG Y Q, CHEN H B, SUN C, MENG F J, GAO F, WANG X. Direct synthesis of hierarchical ZnZSM-5 with addition of CTAB in a seeding method and improved catalytic performance in methanol to aromatics reaction[J]. Catal Today,2018,316:91−98. doi: 10.1016/j.cattod.2018.01.015 [22] FENG W, GAO X F, DING C M, JIA Y M, LIU P. Effect of weak base modification on ZSM-5 catalyst for methanol to aromatics[J]. Appl Organomet Chem,2016,31(6):1−7. [23] WAN Z J, WU W, LI G, WANG C F, YANG H, ZHANG D K. Effect of SiO2/Al2O3 Ratio on the Performance of Nanocrystal ZSM-5 Zeolite Catalysts in Methanol to Gasoline Conversion[J]. Appl Catal A: Gen,2016,523:312−320. doi: 10.1016/j.apcata.2016.05.032 [24] GAO Y, ZHENG B H, WU G, MA F W, LIU C T. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization[J]. RSC Adv,2016,6:83581−83588. doi: 10.1039/C6RA17084F [25] PINILLA-HERRERO I, BORFECCHIA E, HOLZINGER J, MENTZEL U V,. JOENSEN F, LOMACHENKO K A, BORDIGA S, LAMBERTI C, BERLIER G, OLSBYE U, SVELLE S, SKIBSTED J, BEATO P. High Zn/Al ratios enhance dehydrogenation vs hydrogen transfer reactions of Zn-ZSM-5 catalytic systems in methanol conversion to aromatics[J]. J Catal,2018,362:146−163. doi: 10.1016/j.jcat.2018.03.032 [26] ZHANG G Q, BAI T, CHEN T F, FAN W T, ZHANG X. Conversion of methanol to light aromatics on Zn-modified nano-HZSM-5 zeolite catalysts[J]. Ind Eng Chem Res,2014,53:14932−14940. doi: 10.1021/ie5021156 [27] ZHANG Y P, LI M G, XING E H, LUO Y B, SHU X T. Coke evolution on mesoporous ZSM-5 during methanol to propylene reaction[J]. Catal Commun,2019,119:67−70. doi: 10.1016/j.catcom.2018.10.009 [28] DAI C Y, DU K, CHEN Z S, CHEN H Y, GUO X W, MA X X. Synergistic catalysis of multi-stage pore-Rich H-BZSM-5 and Zn-ZSM-5 for the production of aromatic hydrocarbons from methanol via lower olefins[J]. Ind Eng Chem Res,2020,59(47):20693−20700. doi: 10.1021/acs.iecr.0c05225 [29] LIU B, SLOCOMBE D, ALKINANY M, WANG J, ARDEN J, VAI A, GONZALEZ-CORTES S, XIAO T, KUZNETSOV V, EDWARDS P P. Advances in the study of coke formation over zeolite catalysts in the methanol-to-hydrocarbon process[J]. Appl Petrochem Res,2016,6(3):1−7. [30] WANG N, HOU Y L, SUN W J, CAI D L, CHEN Z H, LIU L M, GE B, HU L, QIAN W Z, WEI F. Modulation of b-axis thickness within MFI zeolite: Correlation with variation of product diffusion and coke distribution in the methanol-to-hydrocarbons conversion[J]. Appl Catal B: Environ,2019,243:721−733. doi: 10.1016/j.apcatb.2018.11.023 [31] CHOUDHARY V R, BANERJEE S, PANJALA D. Product distribution in the aromatization of dilute ethene over H-GaAlMFI zeolite: effect of space velocity[J]. Microporous Mesoporous Mater,2002,51(3):203−210. doi: 10.1016/S1387-1811(01)00483-8 [32] CHEN H Y, YANG M F, SHANG W J, TONG Y, LIU B Y, HAN X L, ZHANG J B, HAO Q Q, SUN M, MA X X. Organosilane Surfactant-directed Synthesis of Hierarchical ZSM-5 Zeolites with Improved Catalytic Performance in MTP Reaction[J]. Ind Eng Chem Res,2018,57:10956−10966. doi: 10.1021/acs.iecr.8b00849 [33] IBANEZ M, GAMERO M, RUIZ-MARTINE J, WECKHUYSEN B M, AGUAYO A T, BILBAO J, CASTANO P. Simultaneous coking and dealumination of zeolite H-ZSM-5 during the transformation of chloromethane into olefins[J]. Catal Sci Technol,2016,6:296−306. doi: 10.1039/C5CY00784D [34] GAYUBO A G, AGUAYO A T, OLAZAR M, VIVANCO R, BLBAO J. Kinetics of the irreversible deactivation of the HZSM-5 catalyst in the MTO process.[J]. Chem Eng Sci,2003,58(23/24):5239−5249. doi: 10.1016/j.ces.2003.08.020 [35] YU Z W, LI S H, WANG Q, ZHENG A M, JUN X, CHEN L, DENG F. Brønsted/lewis acid synergy in H-ZSM-5 and H-MOR zeolites studied by 1H and 27Al DQ-MAS solid-state NMR spectroscopy[J]. J Phys Chem C,2011,115:22320−22327. doi: 10.1021/jp203923z [36] MA Q, FU T J, WANG Y J, LI H, CUI L P, LI Z. Development of mesoporous ZSM-5 zeolite with microporosity preservation through induced desilication[J]. J Mater Sci,2020,55:11870−11890. doi: 10.1007/s10853-020-04855-5 [37] LI M R, ZHOU Y P, JU C, FANG Y M. Remarkable increasing of ZSM-5 lifetime in methanol to hydrocarbon reaction by post engineering in fluoride media[J]. Appl Catal A: Gen,2016,512:1−8. doi: 10.1016/j.apcata.2015.12.001 [38] KIM J, CHOI M, RYOOR. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process[J]. J Catal,2010,269(1):219−228. doi: 10.1016/j.jcat.2009.11.009 -




 下载:
下载: