Catalytic hydrodeoxygenation of lignite-derived model compounds to monomeric hydrocarbons over Co/Al2O3
-
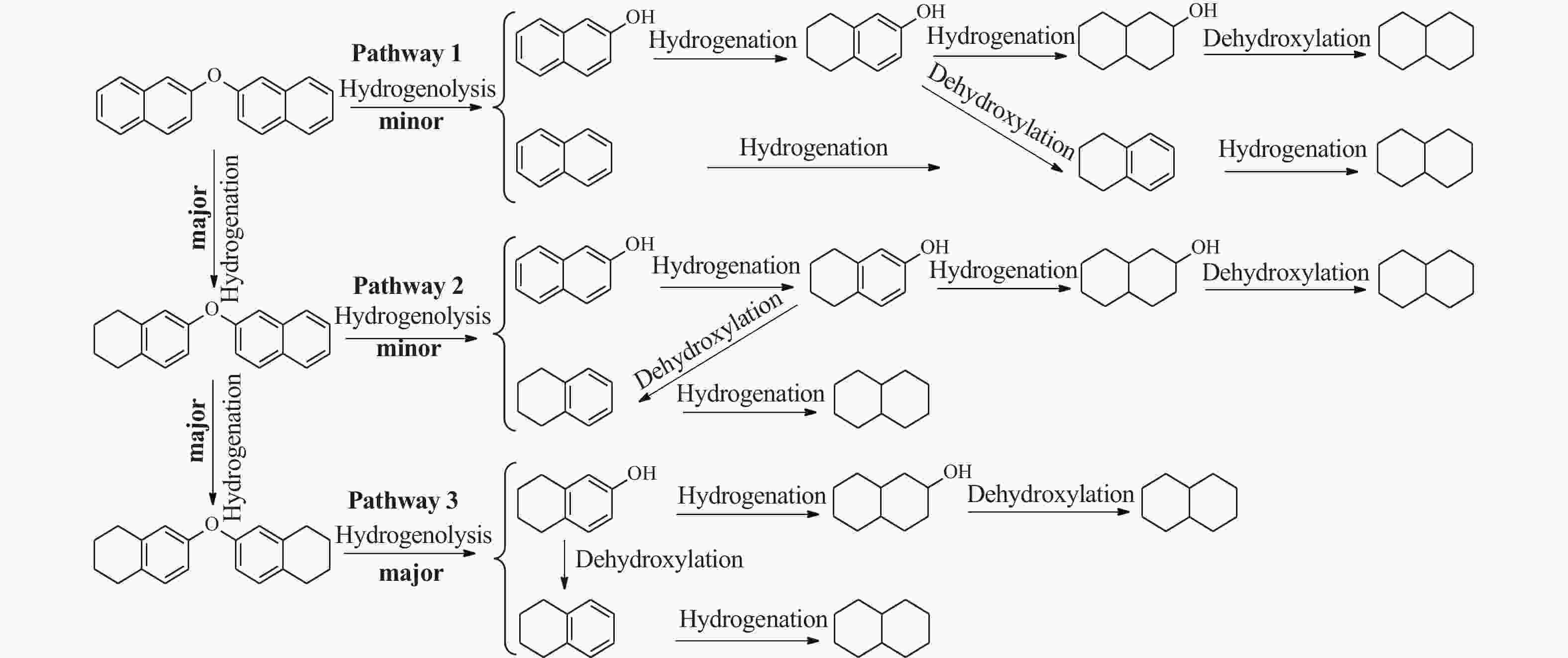
摘要: 以钴铝类水滑石(CoAl-LDH)为前驱体,经焙烧和氢气还原制备了Co/Al2O3催化剂。采用X 射线衍射(XRD)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)和X射线光电子能谱(XPS)等表征手段研究了前驱体及催化剂的理化性质。以2-萘基醚为褐煤模型化合物,考察了Co/Al2O3催化其加氢脱氧制单体烃的性能。结果表明,Co/Al2O3-700催化剂具有最高的加氢脱氧活性,在温度250 ℃和氢气压力2 MPa反应条件下,反应90 min时2-萘基醚完全转化为单体烃(十氢化萘和四氢化萘)。2-萘基醚先加氢生成6,6′-氧代二(1,2,3,4-四氢萘),然后断裂C−O键生成四氢化萘和5,6,7,8-四氢-2-萘酚是主要反应路径。此外,Co/Al2O3-700对褐煤衍生苄醚和苯醚模型化合物加氢脱氧同样具有很高的催化活性。
-
关键词:
- Co/Al2O3催化剂 /
- 加氢脱氧 /
- 褐煤衍生模型化合物 /
- 单体烃类化合物
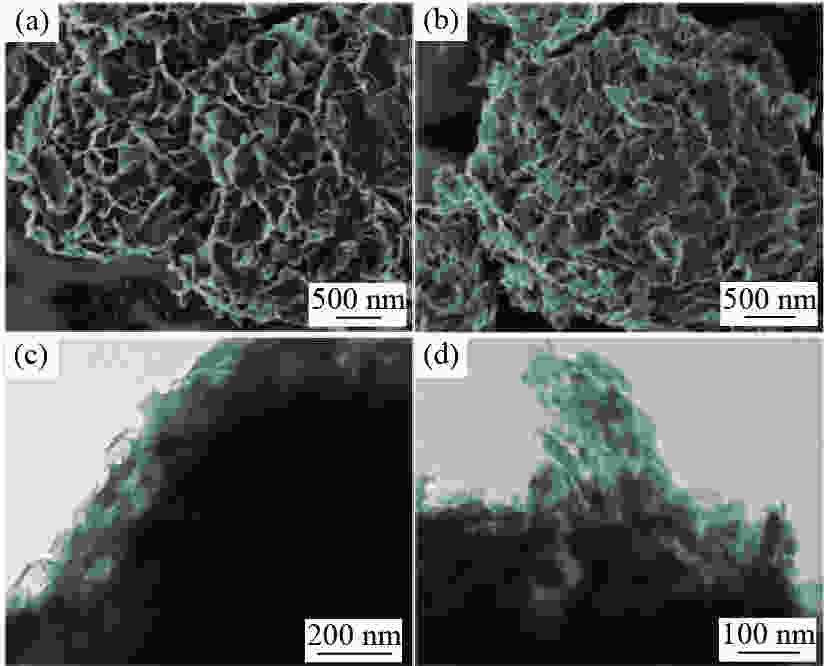
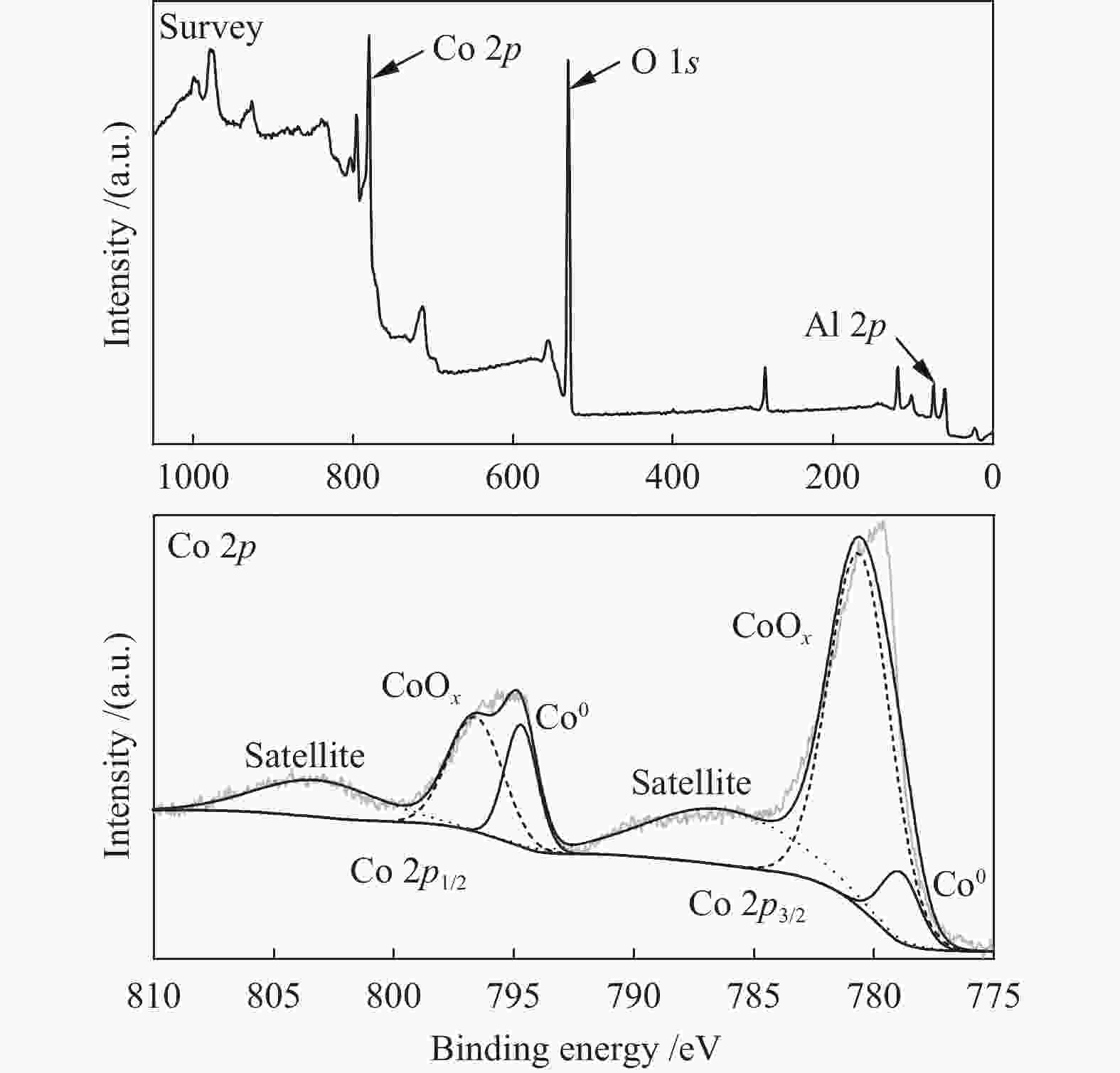
Abstract: A Co/Al2O3 catalyst was synthesized by facile calcination and hydrogen reduction of a cobalt-aluminum hydrotalcite CoAl-LDH, and the X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray photon spectroscopy (XPS) were used to characterize the physical and chemical properties of the precursor and catalysts. Using 2-naphthyl ether as the lignite derived model compound, the catalytic performance of Co/Al2O3 on the hydrodeoxygenation of 2-naphthyl ether to monomeric hydrocarbons was investigated. The results show that Co/Al2O3-700 has the highest hydrodeoxygenation activity. Under the conditions of 250 ℃, 2 MPa of initial H2 pressure and 90 min of holding time, the 2-naphthyl ether is completely converted to monomeric hydrocarbons (decalin and tetralin), in which the 2-naphthyl ether is first converted to 6,6'-oxybis (1,2,3,4-tetrahydronaphthalene) by hydrogenation and then the tetralin and 5,6,7,8-tetrahydronaphthalene-2-naphthol are formed by the cleavage of C−O bond. In addition, Co/Al2O3-700 also shows high activity for the hydrodeoxygenation of lignite-derived benzyl ether and phenyl ether model compounds. -
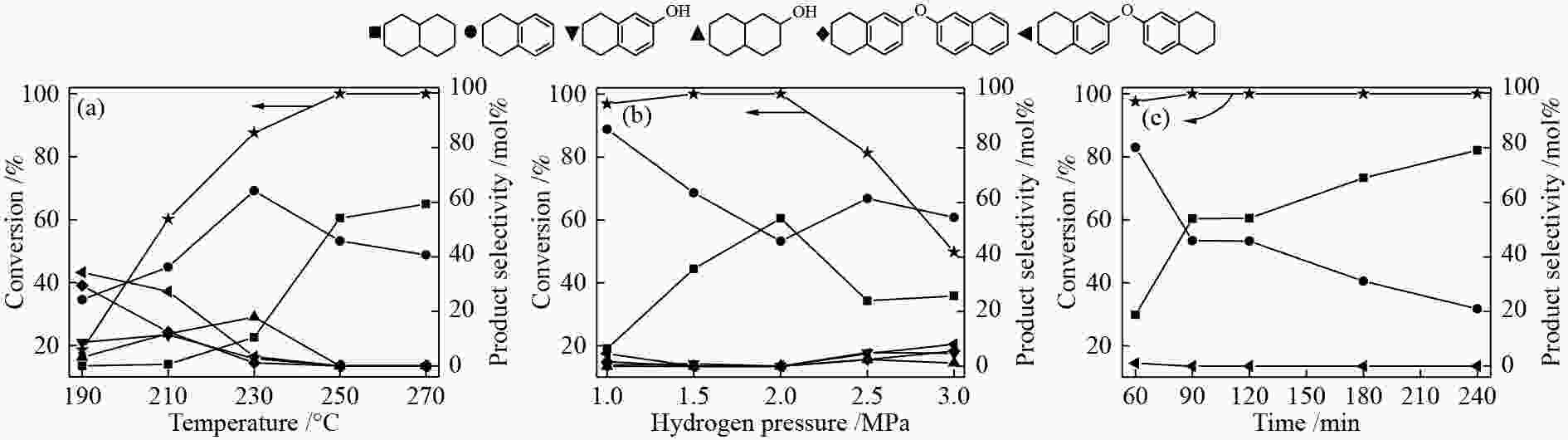
图 7 反应温度(a)氢气压力(b)和反应时间(c)对于2-萘醚转化的影响
Figure 7 Effects of temperature (a), hydrogen pressure (b) and time (c) on 2-naphthyl ether conversion reaction conditions: 50 mg 2-naphthyl ether, 25 mg Co/Al2O3-700, 2 MPa H2, 2 h (a); 50 mg 2-naphthyl ether, 25 mg Co/Al2O3-700, 250 ℃, 2 h (b); 50 mg 2-naphthyl ether, 25 mg Co/Al2O3-700, 250 ℃, 2 MPa H2 (c)
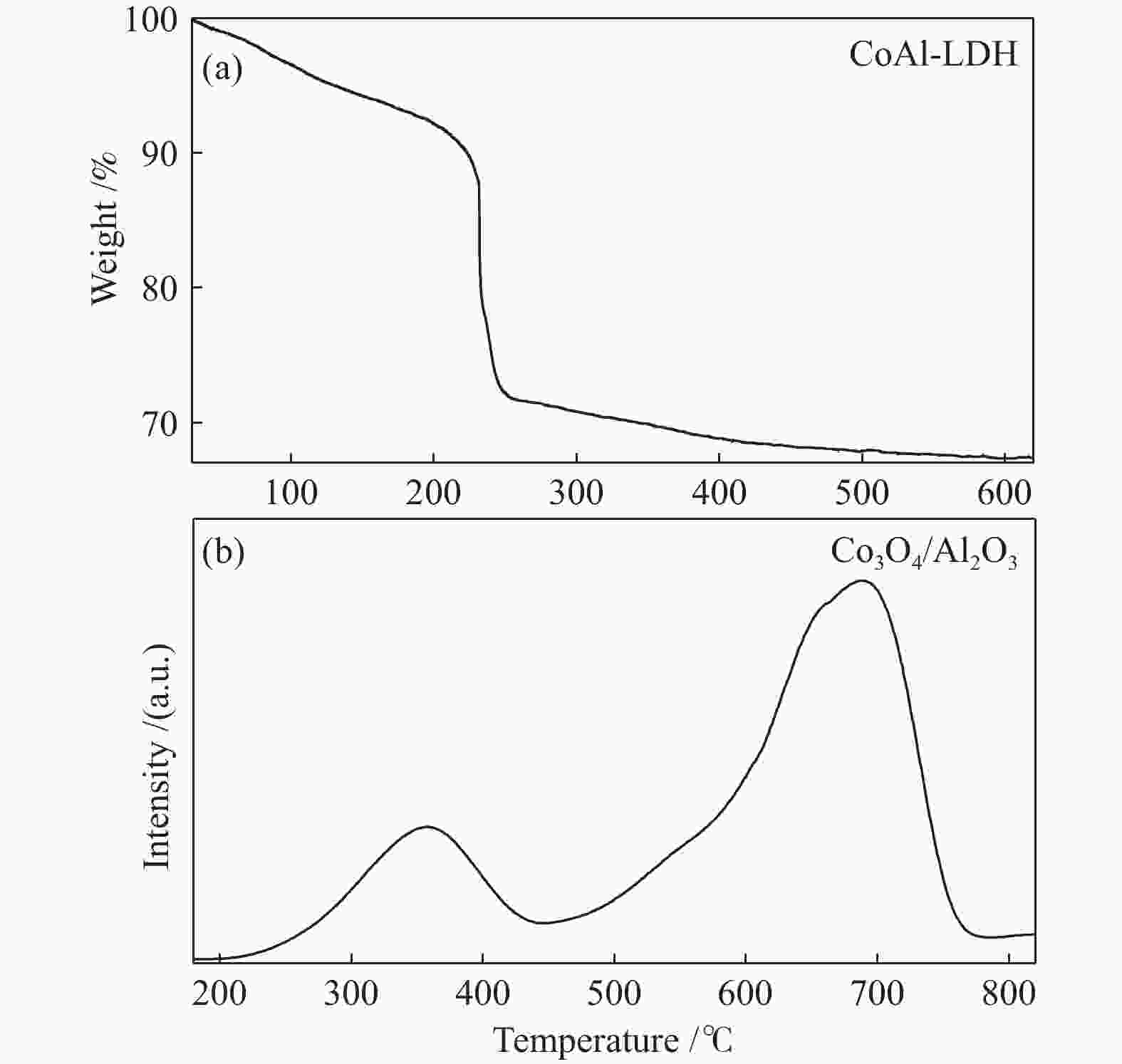
表 1 CoAl-LDH、Co3O4/Al2O3和Co/Al2O3-700的物理性质
Table 1 Textural property of CoAl-LDH, Co3O4/Al2O3 and Co/Al2O3-700
Sample Surface
area A/
(m2·g−1)aPore
volume v/
(cm3·g−1)bPore
diameter
d/nmbCobalt
content
w/%cCoAl-LDH 67.46 0.39 3.83 32.5 Co3O4/Al2O3 132.16 0.24 3.41 49.8 Co/Al2O3-700 71.97 0.48 3.82 57.5 a calculated by the BET method; b calculated by the BJH method; c measured with LA-ICP-MS 表 2 还原温度对催化剂催化2-萘基醚加氢脱氧的影响
Table 2 Effect of catalyst reduction temperature (RT) on the hydrodeoxygenation of 2-naphthyl ether
Entry RT/°C Conv./% Product selectivity /% 





1 600 68.3 10.2 62.1 11.4 7.8 2.7 5.8 2 700 87.6 10.5 64.2 17.9 2.7 1.2 3.5 3 800 84.5 7.8 60.7 19.5 5.0 1.9 5.1 reaction conditions: 50 mg 2-naphthyl ether, 25 mg Co/Al2O3-t, 15 mL n-hexane, 2 MPa H2, 230°C, 120 min 表 3 2-萘基醚及其加氢产物中C-O键的解离能
Table 3 The BDE of C-O bonds in 2-naphthyl ether and its hydrogenation products
Compound 
BDE/ (kJ·mol−1) 344.7 344.3 347.4 333.7 -
[1] 水恒福, 刘健龙, 王知彩, 张德祥. 小龙潭褐煤不同气氛下液化性能的研究[J]. 燃料化学学报,2009,37(3):257−261. doi: 10.3969/j.issn.0253-2409.2009.03.001SHUI Heng-fu, LIU Jian-long, WANG Zhi-cai, ZHANG De-xiang. Preliminary study on liquefaction properties of Xiaolongtan lignite under different atmospheres[J]. J Fuel Chem Technol,2009,37(3):257−261. doi: 10.3969/j.issn.0253-2409.2009.03.001 [2] 刘鹏, 周扬, 鲁锡兰, 王岚岚, 潘铁英, 张德祥. 先锋褐煤在水热处理过程中的结构演绎[J]. 燃料化学学报,2016,44(2):129−137. doi: 10.3969/j.issn.0253-2409.2016.02.001LIU Peng, ZHOU Yang, LU Xi-lan, WANG Lan-lan, PAN Tie-ying, ZHANG De-xiang. Structural evolution of Xianfeng lignite during hydrothermal treatment[J]. J Fuel Chem Technol,2016,44(2):129−137. doi: 10.3969/j.issn.0253-2409.2016.02.001 [3] 李文英, 李旺, 冯杰. 褐煤直接液化过程中存在的问题与思考[J]. 煤炭学报,2020,45(1):414−423.LI Wen-ying, LI Wang, FENG Jie. An overview on issues for lignite direct liquefaction[J]. J China Coal Soc,2020,45(1):414−423. [4] 胡发亭, 王学云, 毛学锋, 李军芳, 赵鹏. 煤直接液化制油技术研究现状及展望[J]. 洁净煤技术,2020,26(1):99−109.HU Fa-ting, WANG Xue-yun, MAO Xue-feng, LI Jun-fang, ZHAO Peng. Research progress and prospect of direct liquefaction technology from coal to oil[J]. Clean Coal Technol,2020,26(1):99−109. [5] WANG J P, LI G Y, GUO R, LI A Q, LIANG Y H. Theoretical and Experimental Insight into Coal Structure: Establishing a Chemical Model for Yuzhou Lignite[J]. Energy Fuels,2017,31:124−132. doi: 10.1021/acs.energyfuels.6b01854 [6] MATHEWS J P, CHAFFEE A L. The molecular representations of coal-A review[J]. Fuel,2012,96:1−14. doi: 10.1016/j.fuel.2011.11.025 [7] ZHANG J W, LU G P, CAI C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100(Fe) supported Pd-Ni BMNPs[J]. Green Chem,2017,19:4538−4543. doi: 10.1039/C7GC02087B [8] KIM J K, LEE J K, KANG K H, SONG J C, SONF I K. Selective cleavage of C-O bond in benzyl phenyl ether to aromatics over Pd-Fe bimetallic catalyst supported on ordered mesoporous carbon[J]. Appl Catal A: Gen,2015,498:142−149. doi: 10.1016/j.apcata.2015.03.034 [9] XIE T, CAO J P, ZHU C, ZHAO X Y, ZHAO M, ZHAO Y P, WEI X Y. Selective cleavage of C-O bond in benzyl phenyl ether over Pd/AC at room temperature[J]. Fuel Process Technol,2019,188:190−196. doi: 10.1016/j.fuproc.2019.02.022 [10] SONG Q L, ZHAO Y P, WU F P, LI G S, FAN X, WANG R Y, CAO J P, WEI X Y. Selective hydrogenolysis of lignin-derived aryl ethers over Co/C@N catalysts[J]. Renewable Energy,2020,148:729−738. doi: 10.1016/j.renene.2019.10.160 [11] 张因, 郭健健, 王杰, 李海涛, 赵永祥. 以NiAl-NO3-LDH为前驱体制备Ni-Al2O3催化剂及其催化乙酰丙酸加氢性能[J]. 高等学校化学学报,2019,40(8):1686−1696. doi: 10.7503/cjcu20190068ZHANG Yin, GUO Jian-jian, WANG Jie, LI Hai-tao, ZHAO Yong-xiang. Preparation of Ni-Al2O3 Catalysts Derived from Hydrotalcite and Its Catalytic Performance for Hydrogenation of Levulinic Acid[J]. Chem J Chin Univ,2019,40(8):1686−1696. doi: 10.7503/cjcu20190068 [12] LONG X D, SUN P, LI Z L, LANG R, XIA C G, LI F W. Magnetic Co/Al2O3 catalyst derived from hydrotalcite for hydrogenation of levulinic acid to γ-valerolactone[J]. Chin J Catal.,2015,36:1512−1518. doi: 10.1016/S1872-2067(15)60934-2 [13] 蔡中顺, 朱子慧, 潘菁, 孙妍妍, 习玲玲, 侯昭胤. Co-Al催化剂在环氧丙醇加氢制备1, 3-丙二醇中的应用[J]. 高等学校化学学报,2020,41(8):1818−1825. doi: 10.7503/cjcu20200236CAI Zhong-shun, ZHU Zi-hui, PAN Jing, SUN Yan-yan, XI Ling-ling, HOU Zhao-yin. Application of Co-Al Catalysts in Hydrogenation of Glycidol to 1, 3-Propanediol[J]. Chem J Chin Univ,2020,41(8):1818−1825. doi: 10.7503/cjcu20200236 [14] LIANG S S, HOU Y C, WU W Z, LI L, REN S H. New insights into the primary reaction products of Naomaohu coal via breaking weak bonds with supercritical ethanolysis[J]. Energy Fuels,2019,33:6294−6301. doi: 10.1021/acs.energyfuels.9b01154 [15] LI Z K, WEI X Y, YAN H L, ZONG Z M. Insight into the structural features of Zhaotong lignite using multiple techniques[J]. Fuel, 2015, 153: 176-182. [16] SANATI S, REZVANI Z. 3-g-C3N4 nanosheet@CoAl-layered double hydroxide composites for electrochemical energy storage in supercapacitors[J]. Chem Eng J,2019,(362):743−757. [17] JIANG L, GUO H W, LI C Z, ZHOU P, ZHANG Z H. Selective cleavage of lignin and lignin model compounds without external hydrogen catalyzed by heterogeneous nickel catalysts[J]. Chem Sci,2019,10:4458−4468. doi: 10.1039/C9SC00691E [18] SHIMURA K, MIYAZAWA T, HANAOKA T, HIRATA S. Fischer-Tropsch synthesis over alumina supported cobalt catalyst: Effect of promoter addition[J]. Appl Catal A: Gen,2015,494:1−11. doi: 10.1016/j.apcata.2015.01.017 [19] SONG T, REN P, DUAN Y N, WANG Z Z, CHEN X F, YANG Y. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes[J]. Green Chem,2018,20:4629−4637. doi: 10.1039/C8GC01374H [20] ZHAO H Y, HAO J X, BAN Y P, SHA Y F, H. ZHOU H C, LIU Q S. Novel and efficient cobalt catalysts synthesized by one-step solution phase reduction for the conversion of biomass derived ethyl levulinate[J]. Catal Today,2019,319:145−154. doi: 10.1016/j.cattod.2018.08.011 [21] YAO Z W, ZHANG X H, PENG F, YU H, WANG H J, YANG J. Novel highly efficient alumina-supported cobalt nitridecatalyst for preferential CO oxidation at high temperatures[J]. Int J Hydrogen Energy,2011,36:1955−1959. doi: 10.1016/j.ijhydene.2010.11.082 [22] WANG H L, RUAN H, FENG M Q, QIN Y L, JOB H, LUO L L, WANG C M, ENGELHARD M H, KUHN E, CHEN X W, TUCKER M P, YANG B. One-pot process for hydrodeoxygenation of lignin to alkanes using Ru-based bimetallic and bifunctional catalysts supported on zeolite Y[J]. ChemSusChem,2017,10:1846−1856. doi: 10.1002/cssc.201700160 [23] HE J Y, ZHAO C, LERCHER J A. Ni-catalyzed cleavage of aryl ethers in the aqueous phase[J]. J Am Chem Soc,2012,134:20768−20775. doi: 10.1021/ja309915e [24] ZHANG J G, TEO J, CHEN X, ASAKURA H, TANAKA T, TERAMURA K, YAN N. A Series of NiM (M = Ru, Rh, and Pd) Bimetallic Catalysts for Effective Lignin Hydrogenolysis in Water[J]. ACS Catal,2014,4:1574−1583. doi: 10.1021/cs401199f [25] SI X G, ZHAO Y P, SONG Q L, CAO J P, WANG R Y, WEI X Y. Hydrogenolysis of lignin-derived aryl ethers to monomers over a MOF-derived Ni/N-C catalyst[J]. React Chem Eng,2020,5:886−895. doi: 10.1039/D0RE00040J [26] LIU G H, ZONG Z M, LIU F J, MENG X L, ZHANG Y Y, WANG S K, LI S, ZHU C, WEI X Y, MA F Y, LIU J M. Deep hydroconversion of ethanol-soluble portion from the ethanolysis of Dahuangshan lignite to clean liquid fuel over a mordenite supported nickel catalyst[J]. J Anal Appl Pyrolysis,2019,39:13−21. [27] LUO Z C, ZHENG Z X, LI L, CUI Y T, ZHAO C. Bimetallic Ru-Ni catalyzed aqueous-phase guaiacol hydrogenolysis at low H2 pressures[J]. ACS Catal,2017,7(12):8304−8313. doi: 10.1021/acscatal.7b02317 [28] WU H R, SONG J L, XIE C, WU C Y, CHEN C J, HAN B X. Efficient and mild transfer hydrogenolytic cleavage of aromatic ether bonds in lignin-derived compounds over Ru/C[J]. ACS Sustainable Chem Eng,2018,6:2872−2877. doi: 10.1021/acssuschemeng.7b02993 [29] TU C Y, CHEN J W, LI W L, WANG H Y, DENG K X, VINOKUROV V A, HUANG W. Hydrodeoxygenation of bio-derived anisole to cyclohexane over bi-functional IM-5 zeolite supported Ni catalysts[J]. Sustainable Energy Fuels,2019,3:3462−3472. doi: 10.1039/C9SE00554D -





 下载:
下载: