Preparation of N-doped Mo/HZSM-5 catalyst and its effect on performance of non-oxidative methane aromatization reaction
-
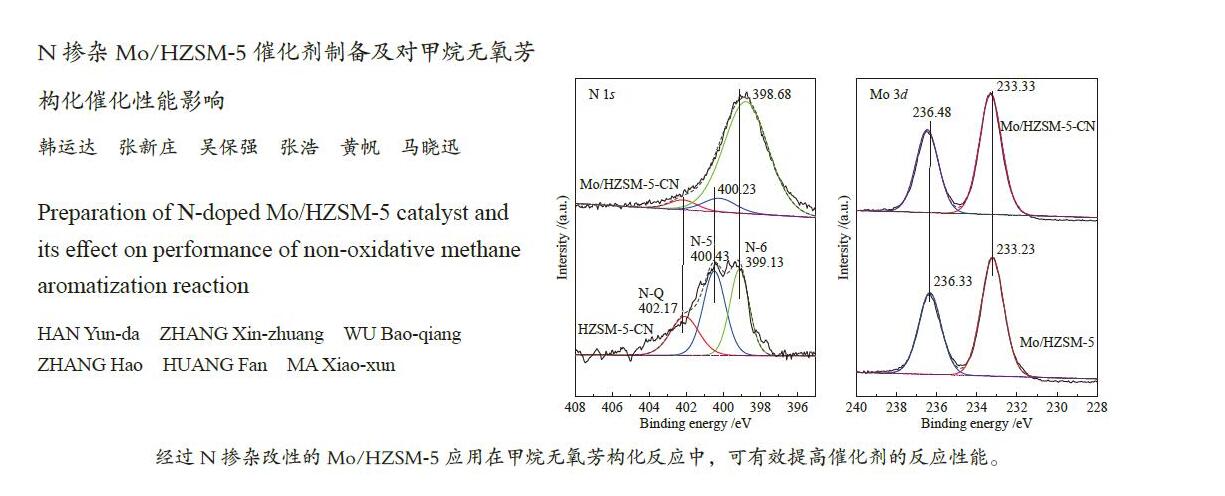
摘要: 采用三聚氰胺作为N源,N掺杂改性HZSM-5沸石分子筛后负载Mo活性金属组分,制备了一种用于甲烷无氧芳构化反应(MDA)的催化剂。采用XPS、N2吸附-脱附、XRD、H2-TPR、TEM和NH3-TPD对催化剂性质和Mo金属组分状态进行了分析表征,并考察了催化剂的甲烷无氧芳构化反应催化性能。结果表明,HZSM-5经过N掺杂改性后,会在分子筛表面生成一层含氮基团,有序调控了分子筛的酸性位点;同时会诱导Mo金属组分在催化剂表面更好的锚定落位。此方法制备的Mo/HZSM-5-CN催化剂能有效提高MDA反应的甲烷转化率和芳烃选择性,减缓了积炭的生成,展现出更优良的催化性能。Abstract: Catalysts for methane dehydroaromatization (MDA) were prepared using melamine as nitrogen source, doped with modified HZSM-5 zeolites and loaded with active metal component Mo. Using XPS, N2-isothermal adsorption and desorption, XRD, H2-TPR, TEM and NH3-TPD, properties of the catalyst and state of active metal components were analyzed. Its catalytic performance for MDA reaction was investigated. The results showed that HZSM-5 after modified with melamine not only formed a layer of carbon and nitrogen groups on surface of the zeolites, but also controlled the medium and strong acid sites of the zeolites in orderly. At the same time it induced better anchor of Mo metal components on surface of the catalyst. The Mo/HZSM-5-CN catalyst prepared by this method could effectively increase methane conversion rate and aromatic selectivity of the MDA reaction, slow down generation of carbon deposits, and exhibit better catalytic performance.
-
Key words:
- melamine /
- N-doped /
- non-oxidative methane aromatization /
- HZSM-5
-
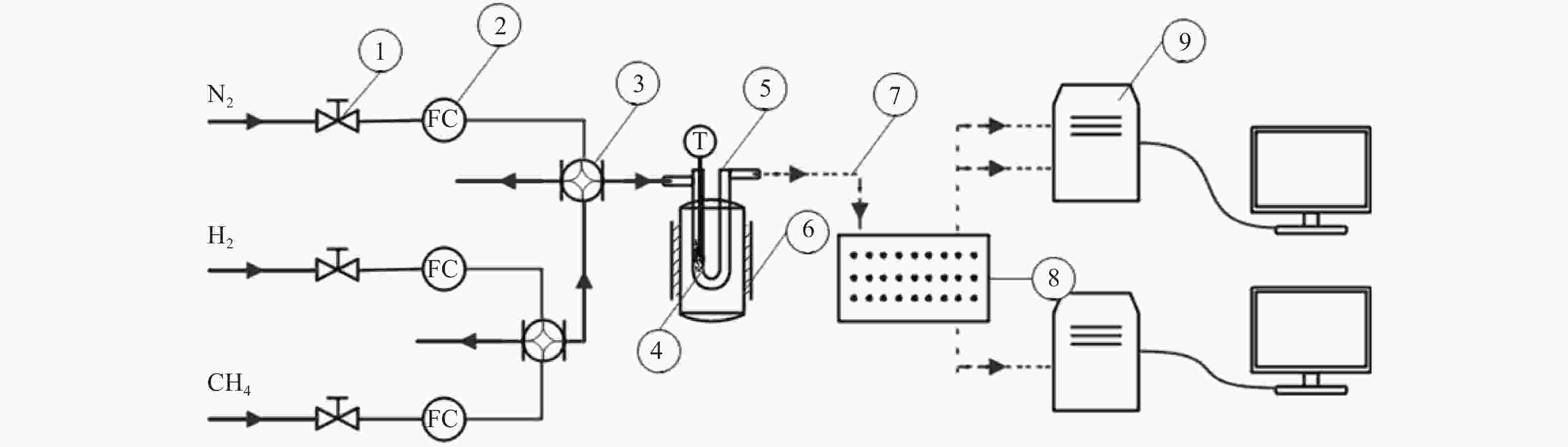
图 1 催化剂MDA催化性能评价装置
Figure 1 Catalytic performance evaluation device of MDA
1: single phase valve; 2: mass flow controller; 3: four-way valve; 4: distribution board; 5: U shaped quartz tube reactor; 6: reaction heating furnace; 7: pipeline with temperature control; 8: ten way valve; 9: online chromatography
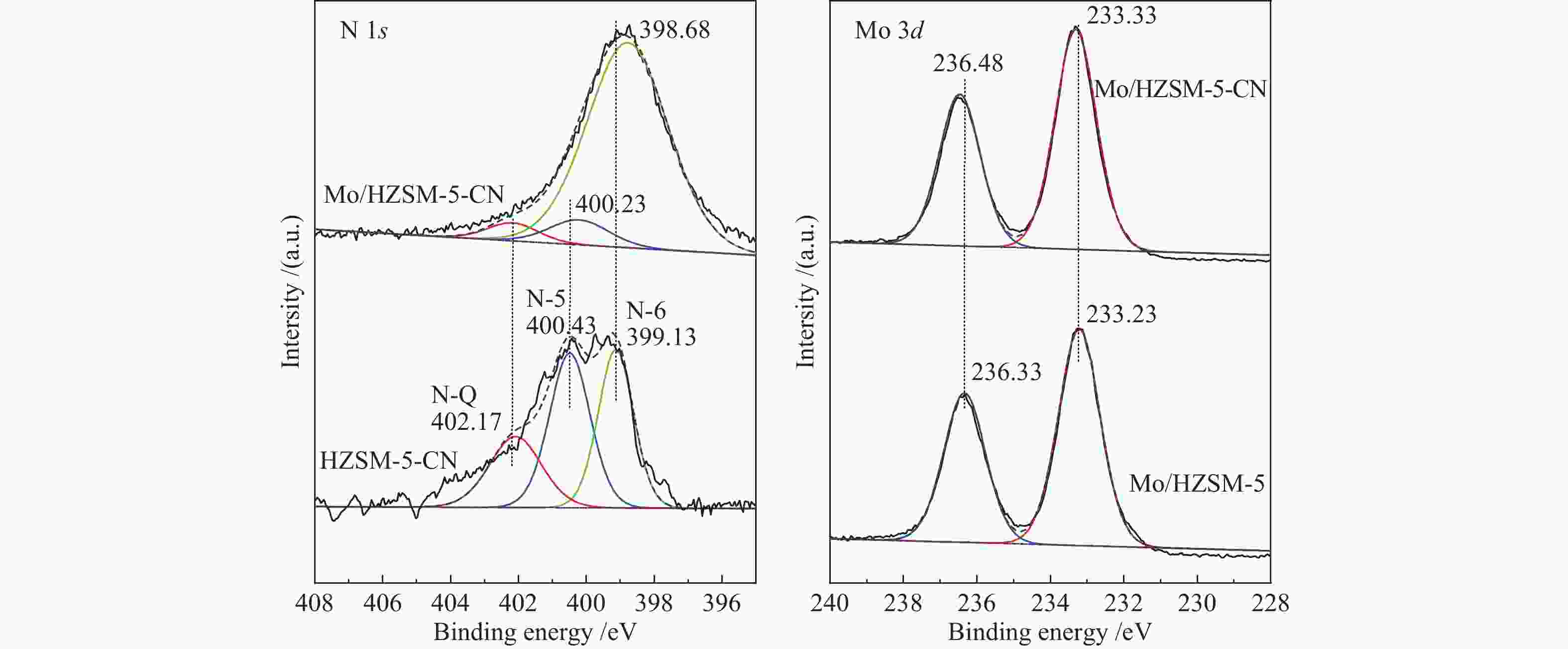
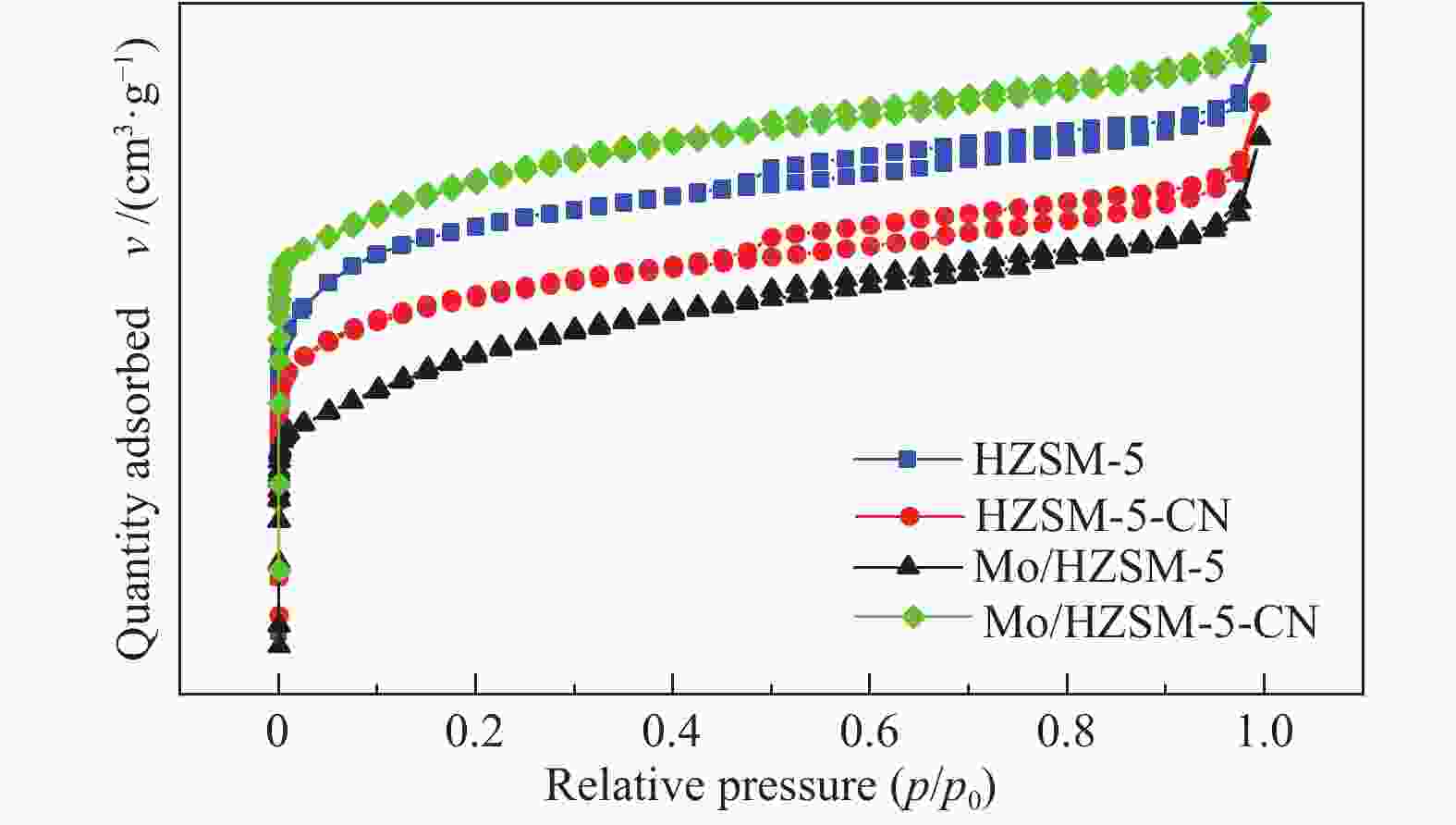
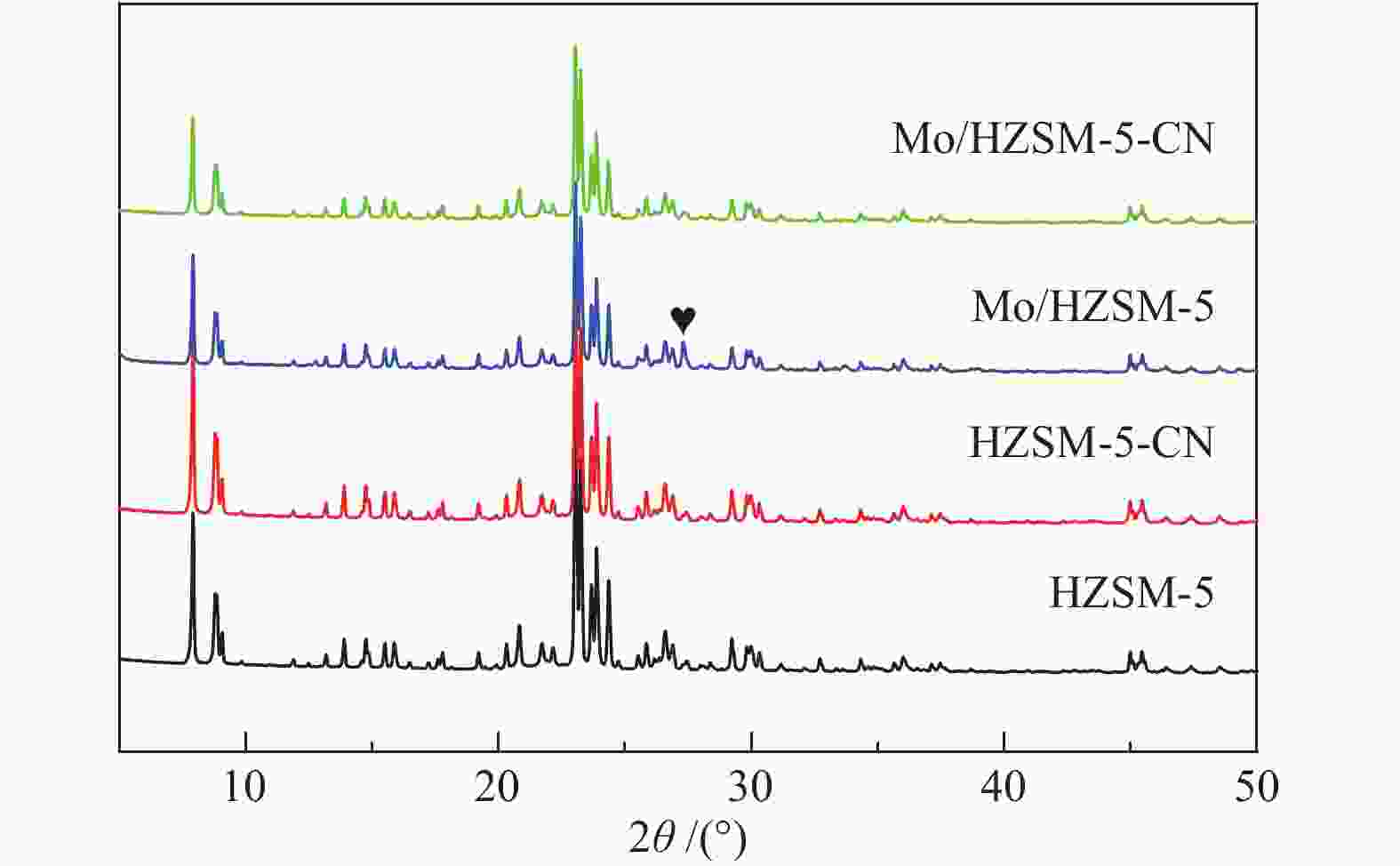
表 1 改性前后催化剂的比表面积和孔道结构分布
Table 1 Surface area and pore structure distribution of the catalysts before and after modification
Catalyst Surface area
A/(m2·g−1)Pore volume
v/(cm3·g−1)total micropore external total micropore HZSM-5 367 328 39 0.198 0.139 HZSM-5-CN 318 279 38 0.180 0.118 Mo/HZSM-5 293 246 47 0.179 0.112 Mo/HZSM-5-CN 300 255 45 0.167 0.114 表 2 改性前后催化剂的酸量分布
Table 2 Amounts of acid sites of the catalysts before and after modification
Catalyst Weak
acid/
(mmol·g−1)Medium
acid/
(mmol·g−1)Strong
acid/
(mmol·g−1)Total/
(mmol·g−1)HZSM-5 0.534 0.109 0.388 1.031 HZSM-5-CN 0.465 0.105 0.215 0.785 Mo/HZSM-5 0.391 0.122 0.116 0.629 Mo/HZSM-5-CN 0.362 0.192 0.147 0.701 表 3 催化剂MDA反应中不同时间点活性与积炭
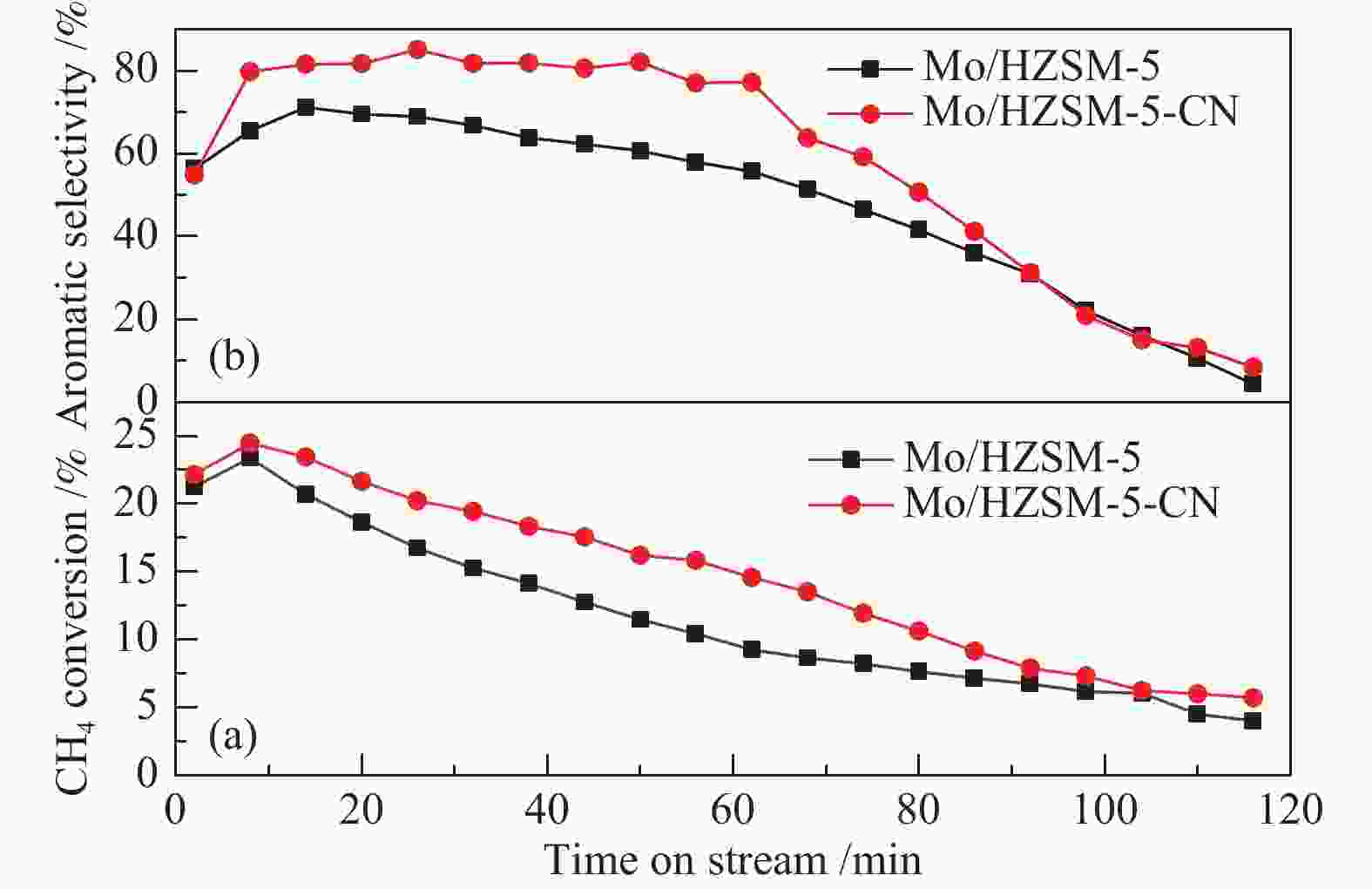
Table 3 Analysis of catalyst activity and carbon deposition in MDA reaction
Catalyst Time/
minConversion/
%Selectivity/
%Carbon
depostion/
%Mo/HZSM-5 20 18.65 69.54 12.8 40 13.54 63.88 60 9.79 56.81 80 7.63 41.60 Mo/HZSM-5-CN 20 21.67 81.75 10.9 40 18.03 82.10 60 15.10 77.50 80 10.63 50.64 -
[1] 韩凤山, 林克芝. 世界芳烃生产技术的发展趋势[J]. 当代石油石化,2006,14(5):30−35.HAN Feng-shan, LIN Ke-zhi. The development trend of world aromatics production technology[J]. Contemp Pet Petrochem,2006,14(5):30−35. [2] WANG L, TAO L, XIE M, XU G, XU Y. Dehydrogenation and aromatization of methane under non-oxidizing conditions[J]. Catal Lett,1993,21(1):35−41. [3] 贺黎明, 沈贺军. 甲烷的转化和利用[M]. 北京: 化学工业出版社, 2005.HE Li-ming, SHEN He-jun. Conversion and Utilization of Methane[M]. Beijing: Chem Ind Press, 2005. [4] 赵群, 杨慎, 王红岩. 中国页岩气开发现状及前景预判[J]. 环境影响评价,2019,41(1):14−18.ZHAO Qun, YANG Shen, WANG Hong-yan. China's shale gas development status and prospect prediction[J]. Environ Impact Assess,2019,41(1):14−18. [5] ARGAUER R J, LANDOLTG R. Crystalline zeolite ZSM-5 and method of preparing the same: US, 3702886[P], 1972. [6] ZHANG C L, LI S, YUAN Y, ZHANG W X, LIN L W. Aromatization of methane in the absence of oxygen over Mo-based catalysts supported on different types of zeolites[J]. Catal Lett,1998,56(4):207−213. doi: 10.1023/A:1019046104593 [7] WECKHUYSEN, B M, WANG D, ROSYNEK, M P, LUNSFORD, J H. Conversion of methane to benzene over transition metal ion ZSM-5 zeolites: I. catalytic characterization[J]. J Catal,1998,175:338−346. doi: 10.1006/jcat.1998.2010 [8] YANG J, DENG F, ZHANG M, LUO Q, YE C. W/HZSM-5 catalyst for methane dehydroaromatization: a multinuclear MAS NMR study[J]. J Mol Catal A: Chem,2003,202:239−246. doi: 10.1016/S1381-1169(03)00187-0 [9] WANG L, OHNISHI R, ICHIKAWA M. Novel rhenium-based catalysts for dehydrocondensation of methane with CO/CO2 towards ethylene and benzene[J]. Catal Lett, 1999, 62: 29–33. [10] 苏玲玲, 马丁, 刘秀梅, 徐奕德, 包信和. Re/HZSM-5体系上的甲烷无氧芳构化反应[J]. 催化学报,2002,23(1):41−45. doi: 10.3321/j.issn:0253-9837.2002.01.010SU Ling-ling, MA Ding, LIU Xiu-mei, XU Yi-de, BAO Xin-he. Oxygen-free aromatization of methane on Re/HZSM-5 system[J]. Chin J Catal,2002,23(1):41−45. doi: 10.3321/j.issn:0253-9837.2002.01.010 [11] LIU B S, ZHANG Y, LIU J F, TIAN M, ZHANG F M, AU C T. Characteristic and mechanism of methane dehydroaromatization over Zn-based/HZSM-5 catalysts under conditions of atmospheric pressure and supersonic jet expansion[J]. J Phys Chem C,2011,115:16954−16962. doi: 10.1021/jp2027065 [12] TAN P L, AU C T, LAI S Y. Methane dehydrogenation and aromatization over 4 wt% Mn/HZSM-5 in the absence of an oxidant[J]. Catal Lett,2006,112:239−245. doi: 10.1007/s10562-006-0209-5 [13] LI S, ZHANG C, KAN Q, WANG D, WU T, LIN L. The function of Cu(II) ions in the Mo/CuH-ZSM-5 catalyst for methane conversion under non-oxidative condition[J]. Appl Catal A: Gen,1999,187:199−206. doi: 10.1016/S0926-860X(99)00231-8 [14] MA D, SHU Y, ZHANG C, ZHANG W, BAO X. Synthesis and characterization of galloaluminosilicate (MFI) and their evaluation in methane dehydro-aromatization[J]. J Mol Catal A: Chem,2001,168:139−146. doi: 10.1016/S1381-1169(00)00513-6 [15] DING W, MEITZNER G D, IGLESIA E. The Effects of Silanation of External Acid Sites on the Structure and Catalytic Behavior of Mo/H–ZSM5[J]. J Catal,2002,206(1):14−22. doi: 10.1006/jcat.2001.3457 [16] KIKUCHI A, KOJIMA R, MA H, BAI J, ICHIKAWA M. Study on Mo/HZSM-5 catalysts modified by bulky aminoalkyl-substituted silyl compounds for the selective methane-to-benzene (MTB) reaction[J]. J Catal,2006,242(2):349−356. [17] WU P, KAN Q, WANG X, WANG D, XING H, YANG P. Acidity and catalytic properties for methane conversion of Mo/HZSM-5 catalyst modified by reacting with organometallic complex[J]. Appl Catal A: Gen,2005,282(1/2):39−44. doi: 10.1016/j.apcata.2004.11.042 [18] MARTÍNEZ A, PERIS E, SASTRE G. Dehydroaromatization of methane under non-oxidative conditions over bifunctional Mo/ITQ-2 catalysts[J]. Cataly Today,2005,107:676−684. [19] 张根, 高佳良, 张新庄, 程序, 代成义, 马晓迅. 氨水改性纳米HZSM-5对其甲烷无氧芳构化性能影响[J]. 西北大学学报(自然科学版), 2018, 48(4): 63−69.ZHANG Gen, GAO Jia-liang, ZHANG Xin-zhuang, CHENG-Xu, DAI Cheng-yi, MA Xiao-xun. The effect of ammonia modified nano-HZSM-5 on the performance of dehydroaromatization[J]. J Northwest Univ, Nat Sci Ed, 2018, 48(4): 63−69. [20] TAN P L, AU C T, LAI S Y. Effects of acidification and basification of impregnating solution on the performance of Mo/HZSM-5 in methane aromatization[J]. Appl Catal A: Gen,2007,324:36−41. [21] SONG Y, SUN C, SHEN W, LIN L. Hydrothermal post-synthesis of HZSM-5 zeolite to enhance the coke-resistance of Mo/HZSM-5 catalyst for methane dehydroaromatization reaction: Reconstruction of pore structure and modification of acidity[J]. Appl Catal A: Gen,2007,317(2):266−274. doi: 10.1016/j.apcata.2006.10.037 [22] CHU N, YANG J, WANG J, YU S, LU J, ZHANG Y. A feasible way to enhance effectively the catalytic performance of methane dehydroaromatization[J]. Catal Commun,2010,11(6):513−517. doi: 10.1016/j.catcom.2009.12.004 [23] ANDERSON S, JHONNY V R, KARIM, S, DÍAZ URBANO, AVELINO C, SIBELE P. Dandelion-like micro-spherical MCM-22 zeolite using BP2000 as a hard template[J]. Acs Omega,2018,3(6):6217−6223. doi: 10.1021/acsomega.8b00647 [24] NA J D, LIU G Z, JI M L. Synthesis and properties of ZSM-5/MCM-41 composite zeolite with a core-shell structure for cracking of supercritical n-dodecane[J]. Adv Mater Res,2012,528:267−271. doi: 10.4028/www.scientific.net/AMR.528.267 [25] 吴保强, 马晓迅, 梁斌, 韩运达. 甘油辅助HZSM-5分子筛的制备及其甲烷无氧芳构化催化性能研究[J]. 燃料化学学报,2020,48(7):821−832.WU Bao-qiang, MA Xiao-xun, LIANG Bin, HAN Yun-da. Preparation of glycerol-assisted HZSM-5 molecular sieve and its catalytic performance for dehydroaromatization[J]. J Fuel Chem Technol,2020,48(7):821−832. [26] Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers[J]. Angew Chem, 2006, 118(19): 3162−3165. [27] LEE W, MAITI U, LEE J, LIM J, HAN T, KIM, S. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications[J]. Chem Commun,2014,50(52):6818−6830. [28] FUJITA S I, WATANABE H, KATAGIRI A. Nitrogen and oxygen-doped metal-free carbon catalysts for chemo selective transfer hydrogenation of nitrobenzene, styrene, and 3-nitrostyrene with hydrazine[J]. J Mol Catal A: Chem,2014,393:257−262. doi: 10.1016/j.molcata.2014.06.021 [29] LIM J W, JEONG E, JUNG, M J, LEE S I, LEE Y S. Effect of simultaneous etching and N-doping on the surface and electrochemical properties of AC[J]. J Ind Eng Chem,2012,18(1):116−122. doi: 10.1016/j.jiec.2011.11.074 [30] ZHANG B, WEN Z, CI S, MAO S, CHEN J, HE Z. Synthesizing nitrogen-doped activated carbon and probing its active sites for oxygen reduction reaction in microbial fuel cells[J]. ACS Appl Mater Interfaces,2014,6(10):7464−7470. doi: 10.1021/am5008547 [31] WANG K, WANG R, LI H, WANG H, MAO X, LINKOV V. N-doped carbon encapsulated Co3O4 nanoparticles as a synergistic catalyst for oxygen reduction reaction in acidic media[J]. Int J Hydrogen Energy,2015,40(10):3875−3882. doi: 10.1016/j.ijhydene.2015.01.100 [32] ZHAO A, MASA J, XIA W. Very low amount of TiO2 on N-doped carbon nanotubes significantly improves oxygen reduction activity and stability of supported Pt nano-particles[J]. Phys Chem Chem Phys,2015,17(16):10767−10773. [33] ZHANG G J, SUN Y H, XU Y. Catalytic performance of N-doped activated carbon supported cobalt catalyst for carbon dioxide reforming of methane to synthesis gas[J]. J Taiwan Inst Chem Eng,2018,93:234−244. [34] LI M, XU F, LI H, WANG Y. Nitrogen-doped porous carbon materials: promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation[J]. Catal Sci Technol,2016,6(11):3670−3693. [35] SOARES O S G P, ROCHA R P, GONALVES A G, FIGUEIREDO J L, ÓRFO J J, PEREIRA M F. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes[J]. Appl Catal B: Environ, 2016, 195: 296−303 [36] RESTIVO J, ROCHA R P, SILVA A M T, ÓRFÃO J J M, PEREIRA M F R, FIGUEIREDO J L. Catalytic performance of heteroatom-modified carbon nanotubes in advanced oxidation processes[J]. Chin J Catal,2014,35(6):896−905. doi: 10.1016/S1872-2067(14)60103-0 [37] ROCHA R P, GONÇALVES A G, PASTRANA-MARTÍNEZ L M. Nitrogen-doped graphene-based materials for advanced oxidation processes[J]. Catal Today,2015,249:192−198. doi: 10.1016/j.cattod.2014.10.046 [38] SUN Y, ZHANG G, XU Y, ZHANG Y, L V Y, ZHANG R. Comparative study on dry reforming of methane over Co-M (M=Ce, Fe, Zr) catalysts supported on N-doped activated carbon[J]. Fuel Process Technol,2019,192:1−12. doi: 10.1016/j.fuproc.2019.04.017 [39] RAHMAN M, INFANTES-MOLINA A, HOFFMAN A S, BARE S R, KHATIB S J. Effect of Si/Al ratio of ZSM-5 support on structure and activity of Mo species in methane dehydroaromatization[J]. Fuel, 2020, 278: 118290. [40] CHENG X, YAN P, ZHANG X Z. Enhanced methane dehydroaromatization in the presence of CO2 over Fe- and Mg-modified Mo/ZSM-5[J]. Mol Catal,2017,437:114−120. -




 下载:
下载: