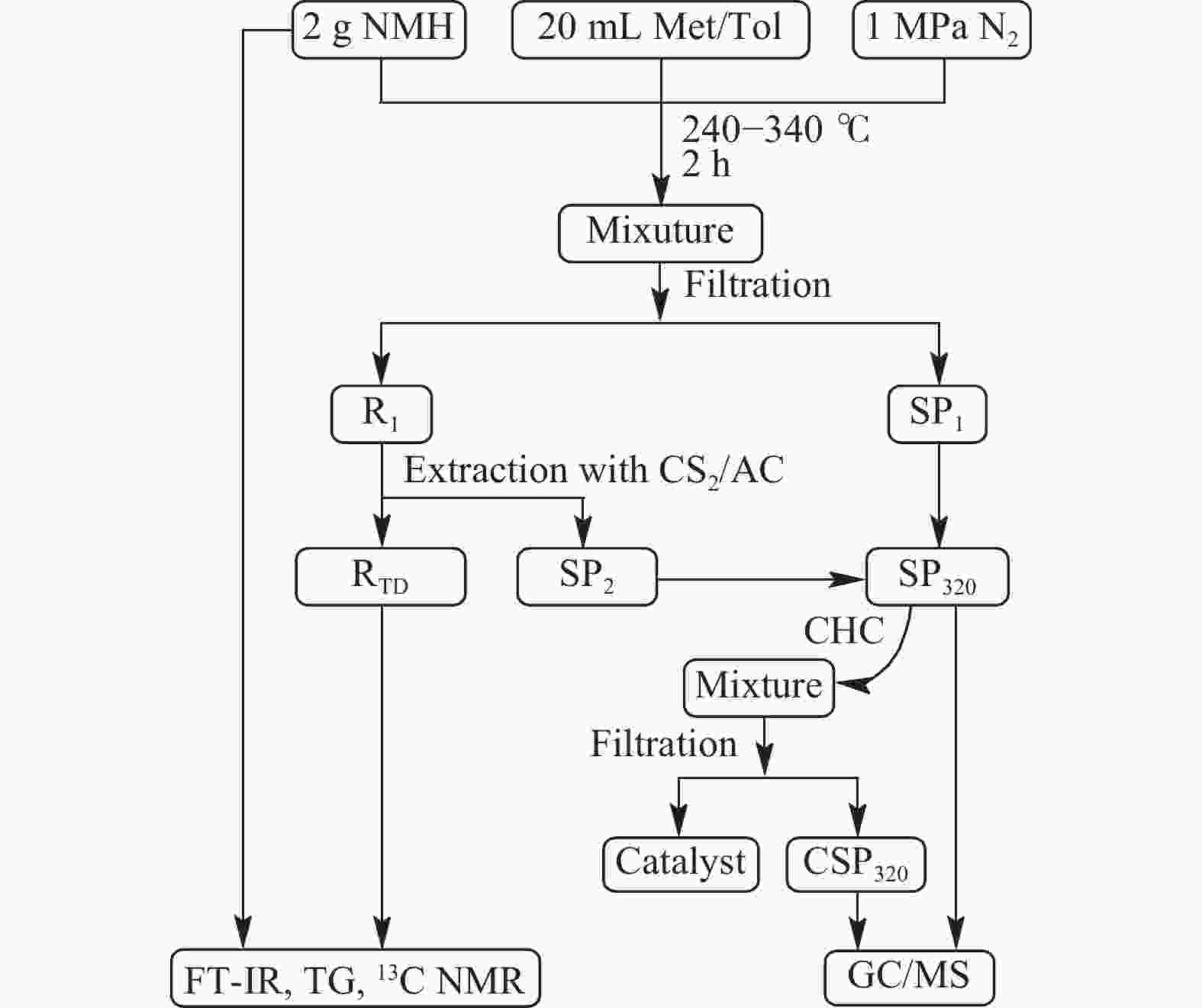
Thermal dissolution of Naomaohu sub-bituminous coal and catalytic hydroconversion of its soluble portions
-
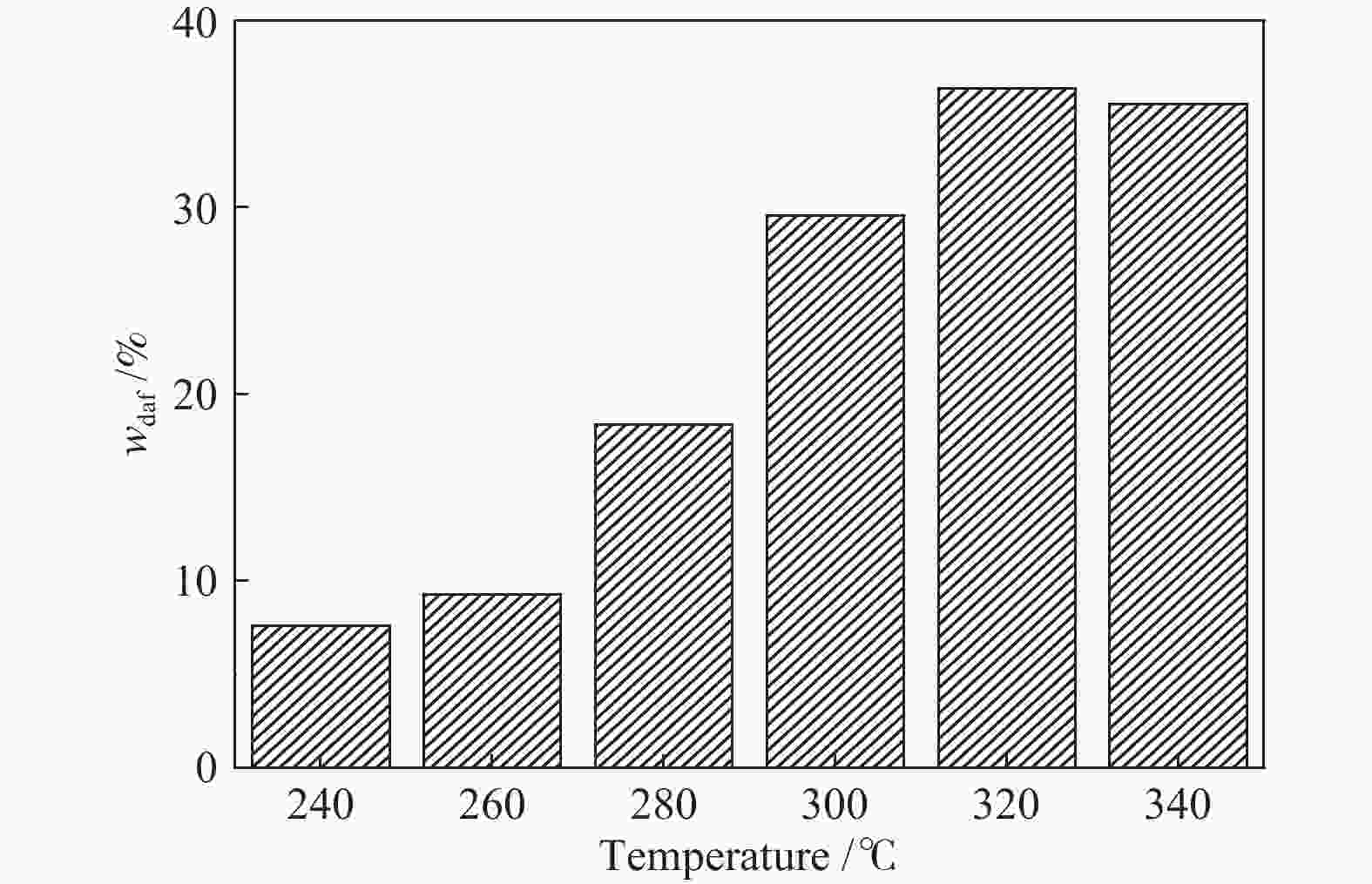
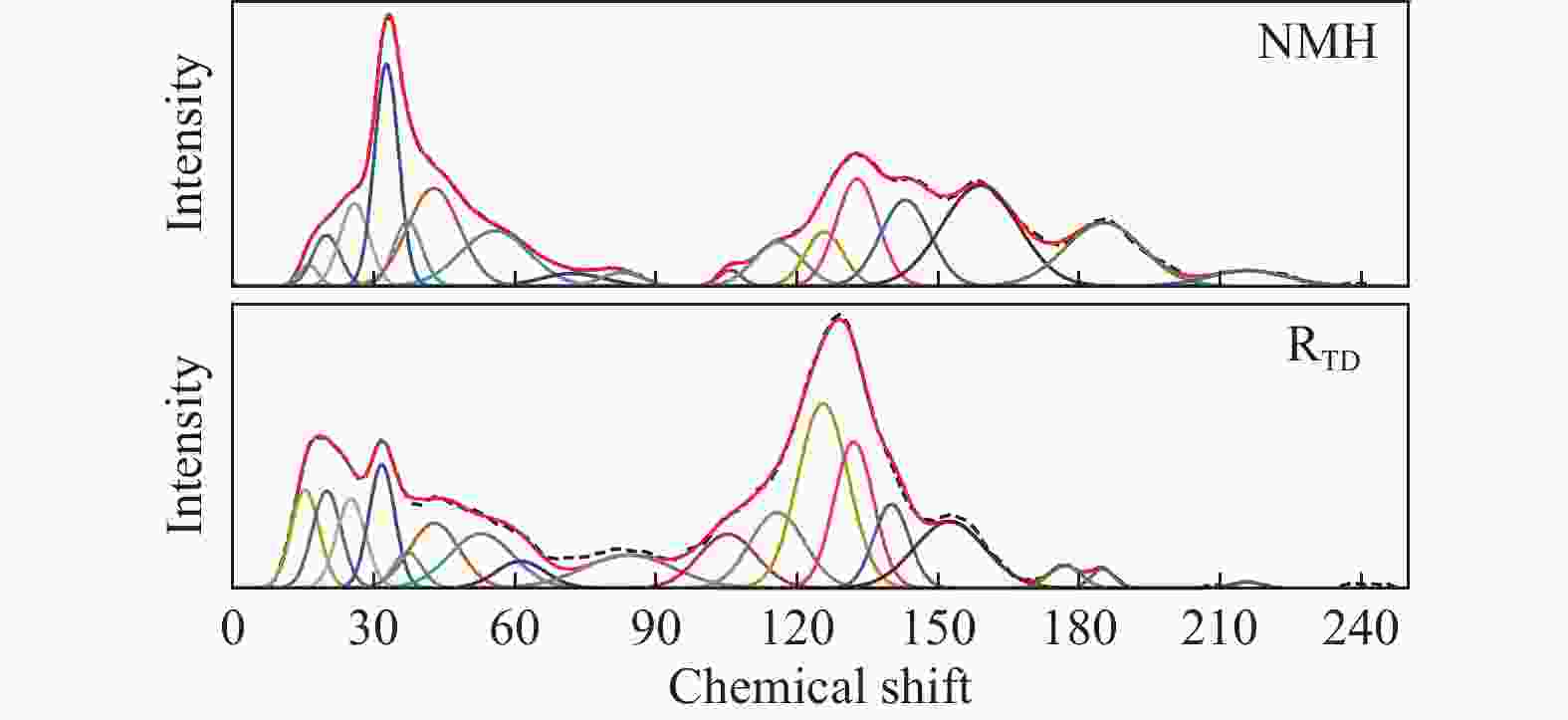
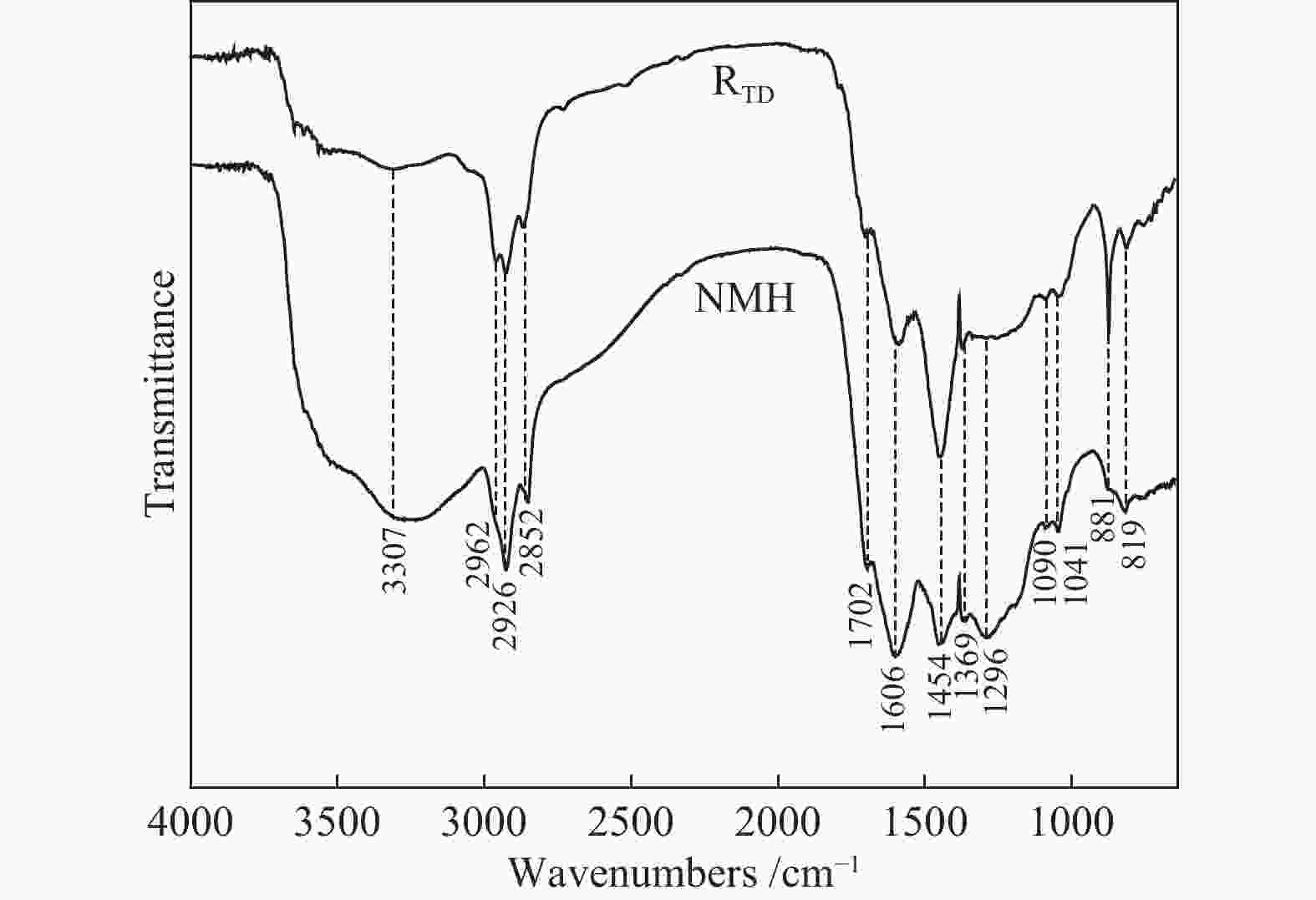
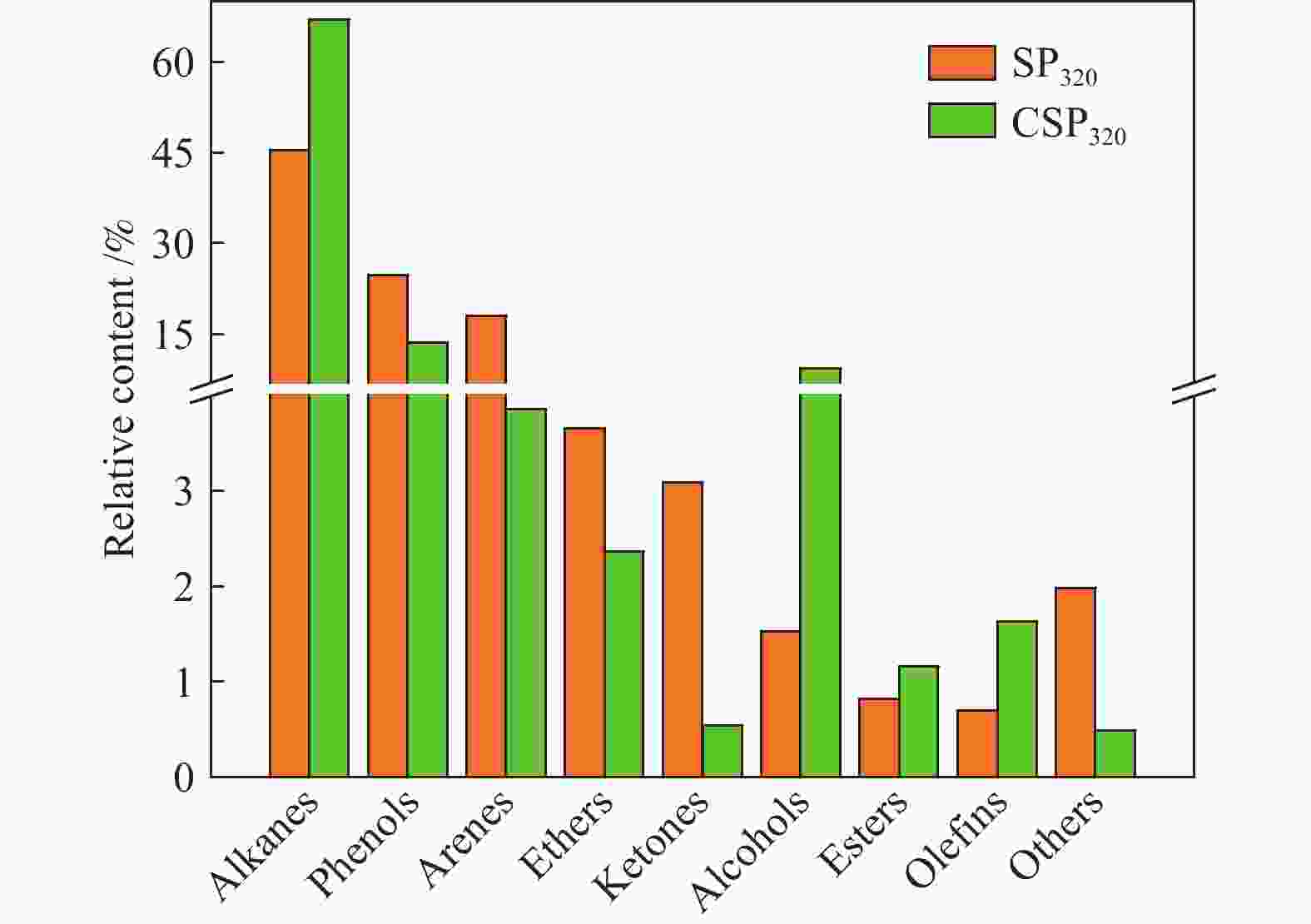
摘要: 采用等体积甲醇/甲苯混合溶剂对淖毛湖次烟煤(NMH)进行热溶得到热溶物和热溶残渣(RTD),利用Co/C@N-700催化剂催化320 ℃热溶物(SP320)加氢转化得到CSP320。利用气相色谱质谱联用仪(GC/MS)分析了SP320催化加氢前后的组成和结构特征,利用傅里叶变换红外光谱(FT-IR)、热重以及固体 13C核磁共振(13C NMR)分析了NMH和RTD的热解反应性和结构特征。热溶物收率随温度升高而增加,320 ℃达到最大值(36.46%)。GC/MS分析表明,烷烃、芳烃和酚类是SP320中的主要族组分,相对含量分别为45.45%、18.03%和24.75%;经催化加氢后芳烃和酚类相对含量分别降低至3.86%和13.6%,烷烃和醇类含量分别提高至66.99%和9.36%,其中,环烷烃由8种增加至24种,这说明Co/C@N-700对SP320中芳烃和酚类加氢转化具有较高活性。与NMH相比,RTD具有较高的热稳定性,骨架结构中芳香碳含量较高,而羰基碳含量较低;RTD的FT-IR谱图中O−H、−CH2−、C=O以及C−O−C对应的吸收峰强度显著减弱,芳香C=C的吸收峰强度明显增强。
-
关键词:
- 次烟煤 /
- 热溶 /
- 催化加氢转化 /
- 固体 13C核磁共振
Abstract: Naomaohu sub-bituminous coal (NMH) was thermally dissolved in isometric methanol/toluene mixed solvent affording soluble portions (SPs) and thermal dissolution residues (RTD), then hydroconversion of SP320 was catalyzed over Co/C@N-700 catalyst affording CSP320. The composition and structural characteristics of SP320 before and after catalytic hydroconversion were analyzed with a gas chromatograph/mass spectrometer (GC/MS), and pyrolysis reactivity and structural characteristics of NMH and RTD were characterized with Fourier transform-infrared spectroscopy (FT-IR), thermogravimetry, as well as solid state 13C nuclear magnetic resonance (13C NMR). The SPs yields increase with increasing temperature, and reach a maximum (36.46%) at 320 oC. GC/MS analysis shows that SP320 are mainly composed of alkanes, phenols and arenes, and their relative contents are 45.45%, 18.03% and 24.75%, respectively. After catalytic hydroconversion, relative contents of arenes and phenols in CSP320 decrease to 3.86% and 13.6%, respectively, while those of alkanes and alcohols increase to 66.99% and 9.36%, respectively, and kinds of cycloalkanes increase from 8 to 24. These results indicate that arenes and phenols in SP320 could be hydrogenated into alkanes and alcohols catalyzed by Co/C@N-700. Compared to NMH, RTD possesses higher thermal stability, more aromatic carbons, and less carbonyl carbons in its skeleton structure. In addition, intensity of adsorption peaks attributed to O−H, −CH2−, C=O and C−O−C in the FT-IR spectrum of RTD are weaker, while adsorption peaks assigned to aromatic C=C are stronger than those of NMH. -
表 1 NMH的工业分析和元素分析
Table 1 Proximate and ultimate analyses of NMH
Sample Proxiamte analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf C H N S Odiff NMH 7.65 5.68 50.80 68.55 5.34 0.91 0.26 24.94 diff: by difference 表 2 NMH和RTD的TG/DTG曲线特征参数
Table 2 Characteristic parameters of NMH and RTD from TG/DTG curves
Sample W110−900 /% W110−330 /% W330−600 /% W600−900 /% tp /℃ NMH 46.49 7.14 29.19 10.16 450 RTD 34.29 4.44 19.72 10.13 463 W110−900:total weight loss; W110−330: weight loss assigned to release of bonded water and decarboxylation; W330−600: weight loss ascribed to cleavage of covalent bonds; W600−900: weight loss attributed to decomposition of carbonates and condensation of aromatic rings; tp: peak temperature of maximum weight loss rate 表 3 NMH和RTD中不同类型碳的相对含量
Table 3 Relative contents of different carbon types in NMH and RTD
Carbon type Symbol Chemical shift Molar content/% NMH RTD RCH3 ${f}_{{\rm{al}}}^{1}$ 16.1/15.3 0.66 5.06 ArCH3 ${f}_{ {\rm{al}} }^{a}$ 19.8/19.9 2.78 5.33 RCH2CH3 ${f}_{ {\rm{al}} }^{2}$ 25.8/25.1 5.88 4.25 RCH2R ${f}_{ {\rm{al}} }^{3}$ 32.6/31.6 10.53 6.10 R3CH ${f}_{{\rm{al}}}^{4}$ 37.1/37.1 3.63 1.39 CR4 ${f}_{{\rm{al}}}^{5}$ 42.7/42.8 10.45 6.85 RCH2OR ${f}_{{\rm{al}}}^{{\rm{O}}1}$ 55.7/52.8−61.2 8.32 4.49 R2CHOR ${f}_{{\rm{al}}}^{{\rm{O}}2}$ 72.1−82.5/84.1 1.10 2.42 R3COR ${f}_{{\rm{al}}}^{{\rm{O}}3}$ 0.95 8.02 
${f}_{{\rm{a}}}^{{\rm{O}}1}$ 105.7/105.2 0.62 4.35 
${f}_{{\rm{a}}}^{{\rm{O}}2}$ 115.9/115.8 4.51 7.35 
${f}_{{\rm{a}}}^{{\rm{H}}}$ 125.7/125.5 3.99 15.95 
${f}_{{\rm{a}}}^{{\rm{b}}}$ 132.7/132.1 10.17 12.23 
${f}_{{\rm{a}}}^{{\rm{s}}}$ 143.0/140.0 8.19 4.13 
${f}_{{\rm{a}}}^{{\rm{O}}3}$ 158.9/152.4 16.10 9.66 RCOOH/R ${f}_{{\rm{a}}}^{{\rm{c}}1}$ 185.2/176.9−185.0 10.12 2.12 RCOH/R/Ar ${f}_{{\rm{a}}}^{{\rm{c}}2}$ 215.8/215.5 2.00 0.30 表 4 SP320和CSP320中GC/MS可检测芳烃
Table 4 Arenes identified by GC/MS in SP320 and CSP320
Compound RC/% Compound RC/% SP320 CSP320 SP320 CSP320 Ethylbenzene 6.73 2-ethyl-1,3-dimethylbenzene 0.28 0.14 p-xylene 4.77 1,2,3,4-tetramethylbenzene 0.21 0.17 m-xylene 1.54 0.38 naphthalene 0.11 o-xylene 0.33 (1-methyl-but-1-enyl)benzene 0.05 1-ethyl-2-methylbenzene 0.18 1,2,3,4,5-pentamethylbenzene 0.18 Propylbenzene 0.62 1,1,3-trimethyl-1H-indene 0.16 1-ethyl-3-methylbenzene 0.10 1-methylnaphthalene 0.13 Mesitylene 0.12 (1,4-dimethyl-pent-2-enyl)benzene 0.20 1,2,4-trimethylbenzene 0.55 0.32 2,3-dimethylnaphthalene ·0.24 1-ethyl-2-methylbenzene 0.27 1,4-dimethylnaphthalene 0.06 1-ethyl-4-methylbenzene 0.47 0.32 1,2,4-triethylbenzene Isobutylbenzene 0.07 1,4,6-trimethylnaphthalene 0.43 0.38 Allylbenzene 0.14 1,4-diisopropyl-2,5-dimethylbenzene 0.20 1-ethyl-3,5-dimethylbenzene 0.12 3,4-dimethylbiphenyl 1.23 0.17 1,2,3,5-tetramethylbenzene 0.20 1-ethyl-3,5-diisopropylbenzene 0.30 表 5 SP320和CSP320中GC/MS可检测酚类
Table 5 Phenols identified by GC/MS in SP320 and CSP320
Compound RC/% Compound RC/% SP320 CSP320 SP320 CSP320 Phenol 0.02 2,5-diethylphenol 0.30 o-cresol 0.38 2-isopropyl-5-methylphenol 1.13 0.67 p-cresol 0.05 5-isopropyl-2-methylphenol 0.94 2,6-dimethylphenol 1.53 1.2 2,3,4,6-tetramethylphenol 5.86 2.13 2,4-dimethylphenol 0.62 2,3,5,6-tetramethylphenol 3.89 1.76 2,5-dimethylphenol 0.37 2-(tert-butyl)-5-methylphenol 0.2 2-isopropyl-5-methylphenol 0.26 2,3,5-trimethylbenzene-1,
4-diol1.05 2,4,6-trimethylphenol 2.89 2.7 2,5-di-tert-butylphenol 0.71 2,4,5-trimethylphenol 6.23 2,6-di-tert-butyl-4-methylphenol 1.07 2,3,6-trimethylphenol 1.17 2,3,5,6-teramethyl-benzene-1,
4-diol0.11 3,4,5-trimethylphenol 0.49 5,7-dimethyl-naphthalen-1-ol 0.04 2-ethyl-4,5-dimethylphenol 0.42 2,5,8-trimethyl-naphthalen-1-ol 0.16 表 6 SP320和CSP320中GC/MS可检测醇类
Table 6 Alcohols identified by GC/MS in SP320 and CSP320
Compound RC/% Compound RC/% SP320 CSP320 SP320 CSP320 4-isopropenyl-1-methylcyclohexanol 0.32 2-isopropyl-5-methylcyclohexanol 2.59 (4-tert-butyl-phenyl)methanol 1.21 1.36 2,4,5-trimethylcyclohexanol 1.41 1,2-dimethylcyclohexanol 1.64 4-methyl-heptan-4-ol 1.73 Cyclohexanol 0.28 3-phenyl-propan-1-ol 0.35 表 7 SP320和CSP320中GC/MS可检测烷烃
Table 7 Alkanes identified by GC/MS in SP320 and CSP320
Compound RC/% Compound RC/% SP320 CSP320 SP320 CSP320 Octane 1.08 1.03 8-methylheptadecane 0.30 1,2,3-trimethylcyclohexane 0.48 2.15 1,1,3-trimethylindan 0.49 1,2-dimethylcyclohexane 0.44 4.64 tridecane 1.86 isopropylcyclopentane 0.31 1,4,7-trimethylindan 0.08 1,4-dimethylcyclohexane 0.55 4.17 2,3,6-trimethyldecane 0.35 2,4-dimethylheptane 0.38 0.32 tetradecane 0.71 0.98 1-ethyl-2-methylcyclopentane 2.14 4,5,7-trimethylindan 0.13 2-methyl-bicycloheptane 0.51 pentadecane 1.68 1.08 2,5-dimethylheptane 0.17 hexadecane 2.73 2.46 ethylcyclohexane 0.12 3-methyltridecane 0.60 propylcyclopentane 0.04 2,6,10-trimethylpentadecane 0.14 0.04 nonane 0.22 0.42 heptadecane 3.89 4.01 1-ethyl-4-methylcyclohexane 1.01 2-methylnonadecane 0.10 1,1-dimethylcyclopentane 0.58 3,6-dimethyldecane 0.31 methylcycloheptane 0.36 0.48 2,6,10-trimethyltetradecane 0.17 cycloheptane 0.06 octadecane 7.25 5.96 decane 0.17 9-methylnonadecane 0.46 2,2,3-trimethylbicycloheptane 0.08 3-methylheptadecane 2.23 4-methyldecane 0.08 nonadecane 0.60 3.84 cyclooctane 0.08 2-methylheptadecane 0.48 indan 0.10 icosane 9.36 12.85 4-ethyldecane 0.81 henicosane 2.18 1.94 1-methylindan 0.30 0.32 2-methylhexadecane 1.62 undecane 0.24 0.57 3-methylhexadecane 0.15 0.12 1,2-dimethylindan 0.15 docosane 0.41 1,3-dimethylindan 0.55 3-methylhenicosane 0.46 1,6-dimethylindan 0.35 0.43 tetracosane 0.84 5.85 5-methylindan 0.25 2-methyloctadecane 0.22 4-methylindan 0.48 0.64 pentacosane 0.30 dodecane 0.56 0.78 heptacosane 0.55 2.53 4,7-dimethylindan 0.19 2,6,11-trimethyldodecane 0.24 2,4-dimethylundecane 0.30 octacosane 0.80 -
[1] LIU F J, WEI X Y, FAN N H, ZONG Z M. Separation and structural characterization of the value-added chemicals from mild degradation of lignites: A review[J]. Appl Energy,2016,170:415−436. doi: 10.1016/j.apenergy.2016.02.131 [2] ZHANG L P, HU S, CHEN Q D, XIAO L F, Hassan S S, JIANG L, WANG Y, SU S, XIANG J. Molecular structure characterization of the tetrahydrofuran-microwave-extracted portions from three Chinese low-rank coals[J]. Fuel,2017,189:178−185. doi: 10.1016/j.fuel.2016.10.082 [3] SHUI H F, ZHOU Y, LI H P, WANG Z C, LEI Z P, REN S B, PAN C X, WANG W W. Thermal dissolution of Shenfu coal in different solvents[J]. Fuel,2013,108:385−390. doi: 10.1016/j.fuel.2012.11.005 [4] 潘春秀, 刘华龙, 祝婉婉, 李海平, 刘锦润, 魏贤勇, 水恒福, 王知彩. 神府次烟煤在不同温度下的热溶产物表征[J]. 燃料化学学报,2015,43(4):416−421.PAN Chun-xiu, LIU Hua-long, ZHU Wan-wan, LI Hai-ping, LIU Jin-run, WEI Xian-yong, SHUI Heng-fu, WANG Zhi-cai. Characterization of the thermal dissolution products of a subbituminous coal at different temperature[J]. J Fuel Chem Technol,2015,43(4):416−421. [5] HAO P, BAI Z Q, HOU R R, XU J L, BAI J, GUO Z X, KONG L X, LI W. Effect of solvent and atmosphere on product distribution, hydrogen consumption and coal structural change during preheating stage in direct coal liquefaction[J]. Fuel,2018,211:783−788. doi: 10.1016/j.fuel.2017.09.122 [6] VASIREDDY S, MORREALE B, CUGINI A, SONG C S, SPIVEY J J. Clean liquid fuels from direct coal liquefaction: chemistry, catalysis, technological status and challenges[J]. Energy Environ Sci,2011,4:311−345. doi: 10.1039/C0EE00097C [7] MOCHIDA I, OKUMA O, YOON S H. Chemicals from direct coal liquefaction[J]. Chem Rev,2014,114:1637−1672. doi: 10.1021/cr4002885 [8] LIU G H, ZONG Z M, LIU F J, MENG X L, ZHANG Y Y, WANG S K, LI S, ZHU C, WEI X Y, MA F Y, LIU J M. Deep hydroconversion of ethanol-soluble portion from the ethanolysis of Dahuangshan lignite to clean liquid fuel over a mordenite supported nickel catalyst[J]. J Anal Appl Pyrolysis,2019,139:13−21. doi: 10.1016/j.jaap.2019.01.002 [9] ZHANG W, CHEN J Z, LIU R L, WANG S P, CHEN L M, LI K G. Hydrodeoxygenation of lignin-derived phenolic monomers and dimers to alkane fuels over bifunctional zeolite-supported metal catalysts[J]. ACS Sustainable Chem Eng,2014,2(4):683−691. doi: 10.1021/sc400401n [10] YAO G, WU G J, DAI W L, GUAN N J, LI L D. Hydrodeoxygenation of lignin-derived phenolic compounds over bi-functional Ru/H-Beta under mild conditions[J]. Fuel,2015,150:175−183. doi: 10.1016/j.fuel.2015.02.035 [11] GUAN W X, CHEN X, JIN S H, LI C, TSANG C W, LIANG C H. Highly Stable Nb2O5−Al2O3 composites supported Pt catalysts for hydrodeoxygenation of diphenyl Ether[J]. Ind Eng Chem Res,2017,56(47):14034−14042. doi: 10.1021/acs.iecr.7b03736 [12] LIU Z Q, WEI X Y, WU H H, LI W T, ZHANG Y Y, ZONG Z M MA F Y, LIU J M. Difunctional nickelmicrofiber attapulgite modified with an acidic ionic liquid for catalytic hydroconversion of lignite-related model compounds[J]. Fuel,2017,204:236−242. doi: 10.1016/j.fuel.2017.05.039 [13] KIM J K, LEE J W, KANG K H, SONG J C, SONG I K. Selective cleavage of C-O bond in benzyl phenyl ether to aromatics over Pd–Fe bimetallic catalyst supported on ordered mesoporous carbon[J]. Appl Catal A: Gen,2015,498:142−149. doi: 10.1016/j.apcata.2015.03.034 [14] LIU X H, JIA W D, XU G Y, ZHANG Y, FU Y. Selective hydrodeoxygenation of lignin-derived phenols to cyclohexanols over Co-based catalysts[J]. ACS Sustainable Chem Eng,2017,5(10):8594−8601. doi: 10.1021/acssuschemeng.7b01047 [15] HU Y H, JIANG G C, XU G Q, MU X D. Hydrogenolysis of lignin model compounds into aromatics with bimetallic Ru-Ni supported onto nitrogen-doped activated carbon catalyst[J]. Mol Catal,2018,445:316−326. doi: 10.1016/j.mcat.2017.12.009 [16] 宋庆露. Co/C@N催化淖毛湖 热溶物及其模型化合物的加氢转化[D]. 徐州: 中国矿业大学, 2020.SONG Qing-lu, Catalytic Hydroconversion of Naomaohu Soluble Portion and its Model Compounds over Co/C@N[D]. Xuzhou: China university of mining and technology, 2020. [17] SONG Q L, ZHAO Y P, WU F P, LI G S, FAN X, WANG R Y, CAO J P, WEI X Y. Selective hydrogenolysis of lignin-derived aryl ethers over CoC@N catalysts[J]. Renewable Energy,2020,148:729−738. doi: 10.1016/j.renene.2019.10.160 [18] ZHAO Y P, XIAO J, DING M, EDDINGS E G, WEI X Y, FAN X, ZONG Z M. Sequential extraction and thermal dissolution of Baiyinhua lignite in isometric CS2/Acetone and toluene/methanol binary solvents[J]. Energy Fuels,2016,30(1):47−53. doi: 10.1021/acs.energyfuels.5b01775 [19] GIVEN P H, MARZEC A, BARTON W A, LYNCH L J, GERSTEIN B C. The concept of a mobile or molecular phase within the macromolecular network of coal: A debate[J]. Fuel,1986,65(2):155−163. doi: 10.1016/0016-2361(86)90001-3 [20] SHI L, LIU Q Y, GUO X J, WU W Z, LIU Z Y. Pyrolysis behavior and bonding information of coal — A TGA study[J]. Fuel Process Technol,2013,108:125−132. doi: 10.1016/j.fuproc.2012.06.023 [21] LI Z K, WEI X Y, YAN H L, ZONG Z M. Insight into the structural features of Zhaotong lignite using multiple technique[J]. Fuel,2015,153:176−182. doi: 10.1016/j.fuel.2015.02.117 [22] SONG H J, LIU G R, ZHANG J, WU J H. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Process Technol,2017,156:454−460. doi: 10.1016/j.fuproc.2016.10.008 -




 下载:
下载: