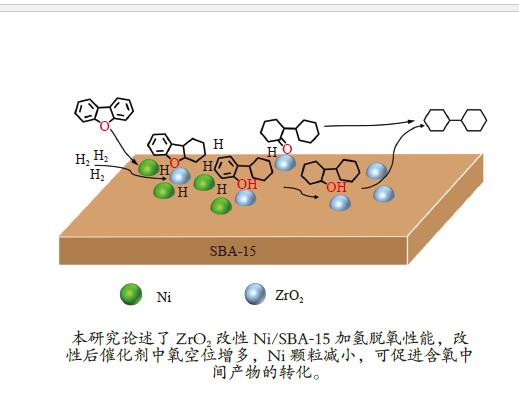
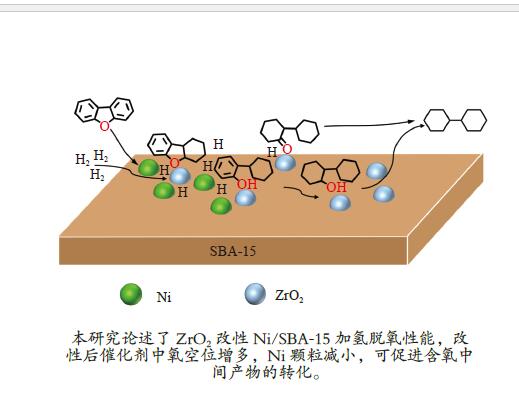
Promoting effects of ZrO2 modification on Ni/SBA-15 catalysts for dibenzofuran hydrodeoxygenation
-
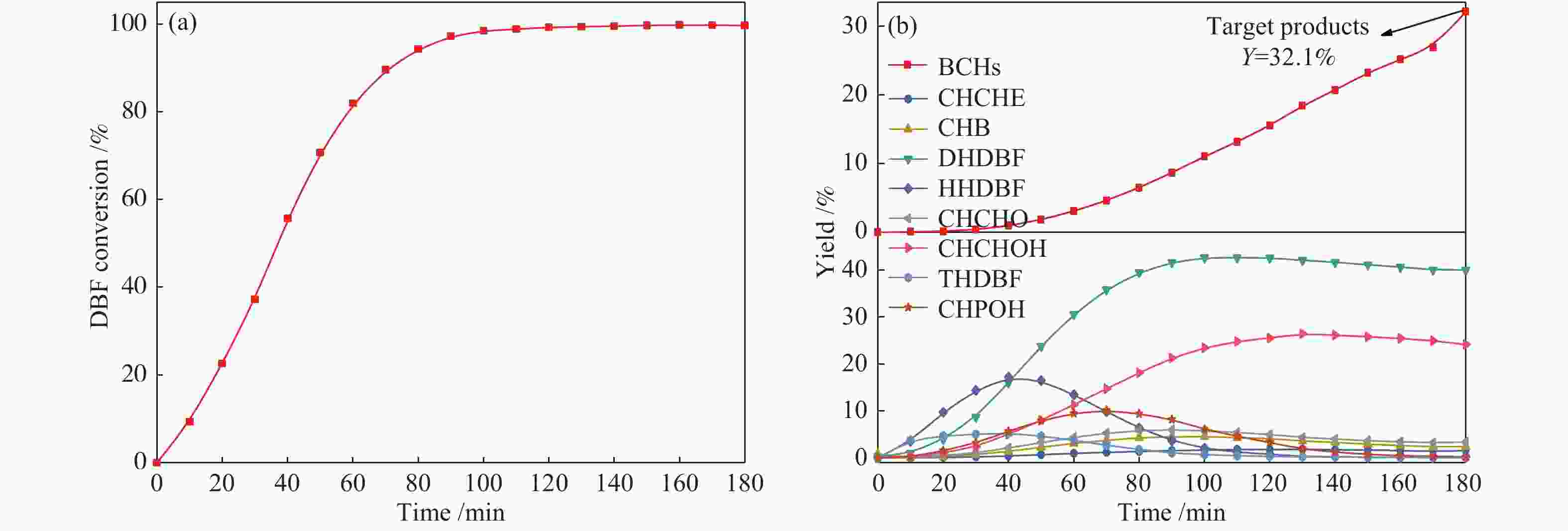
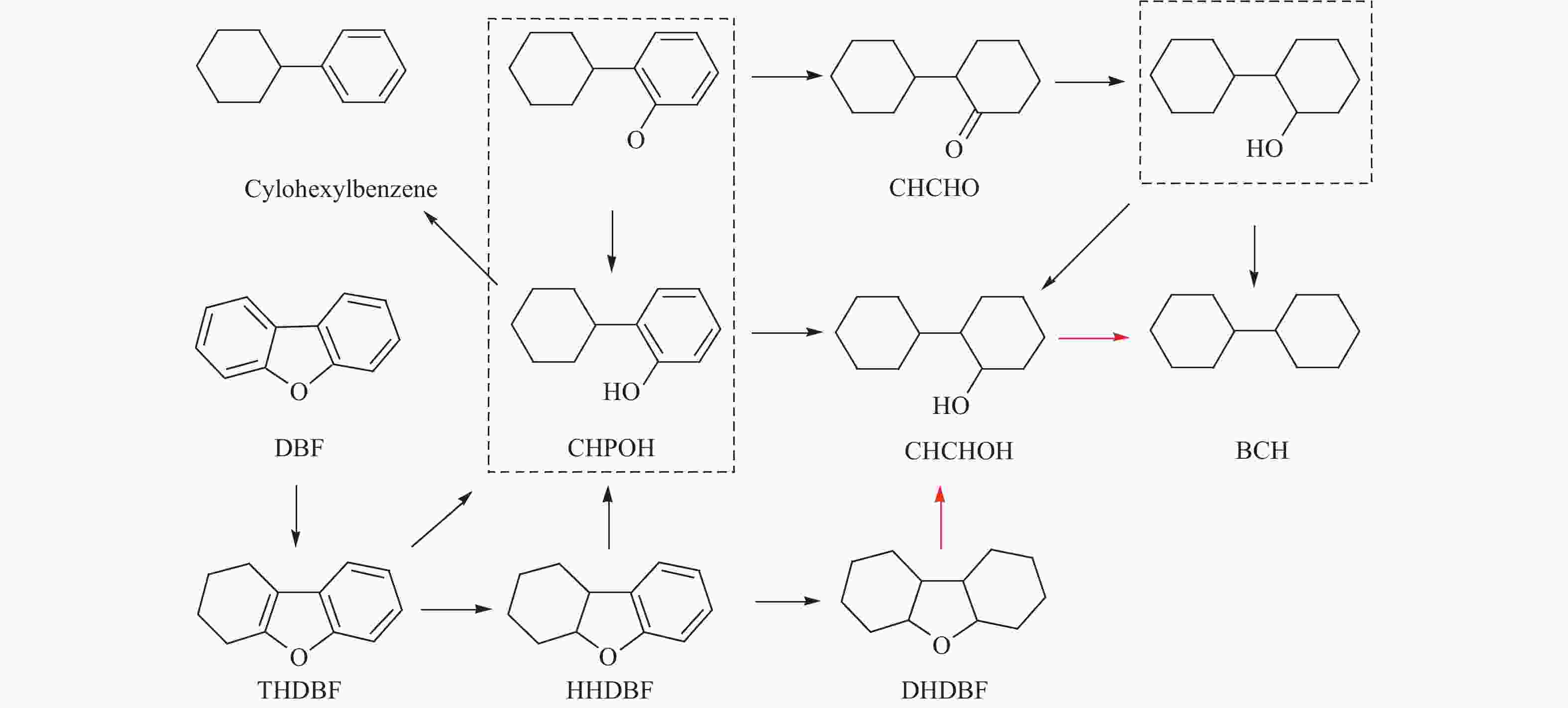
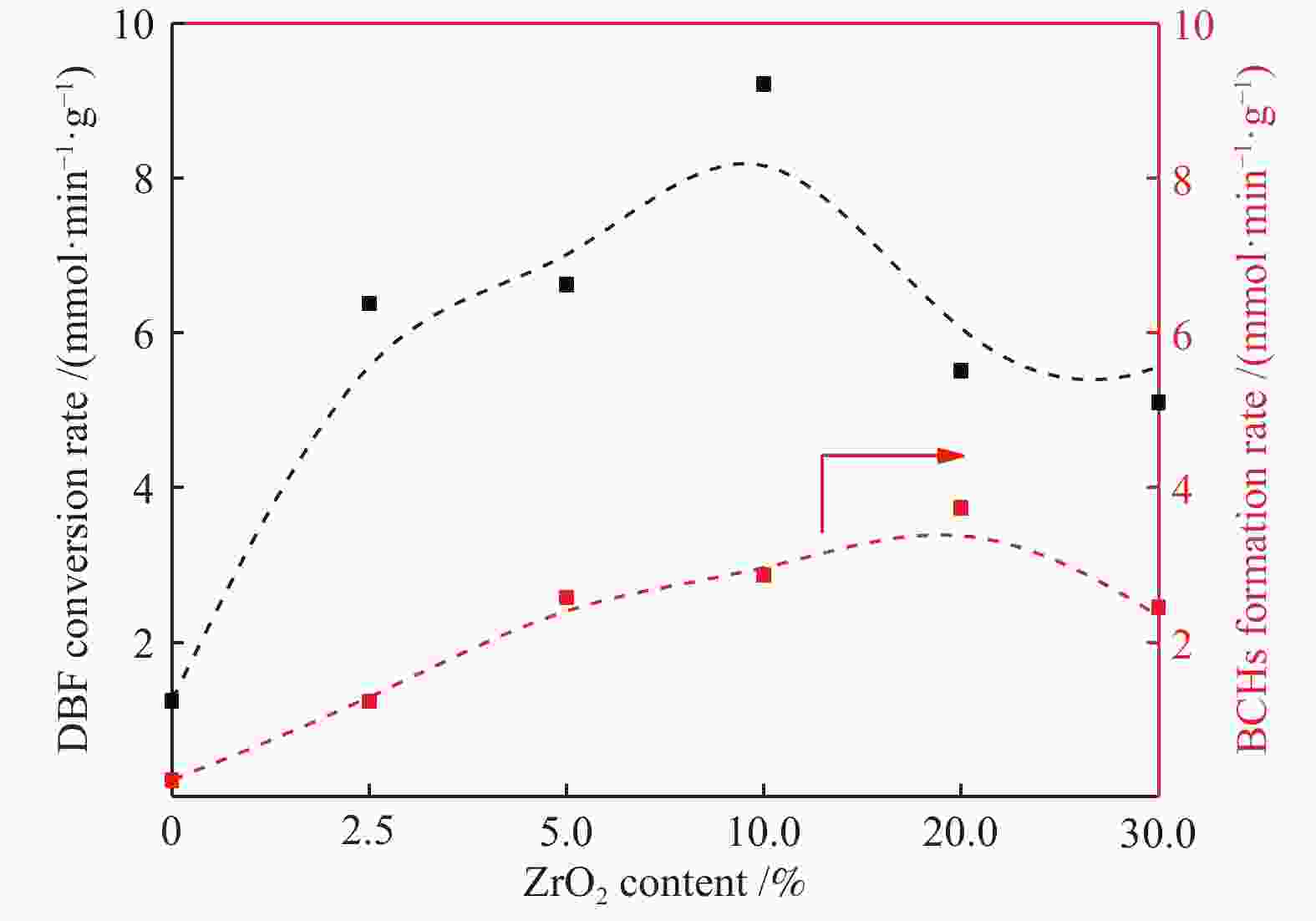
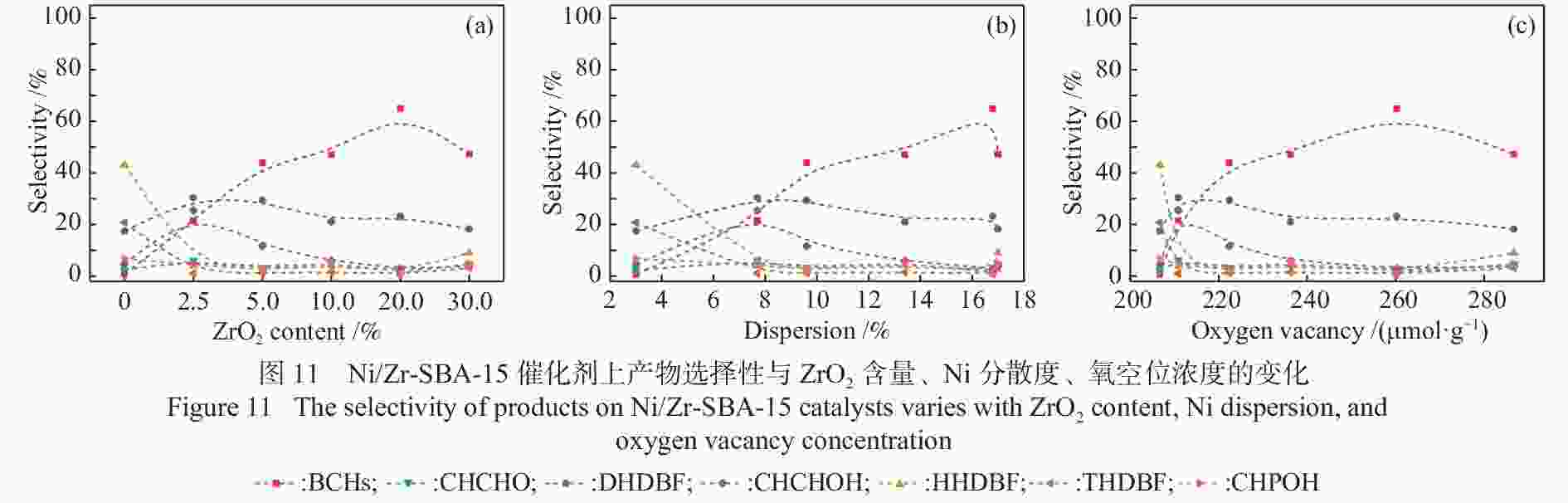
摘要: 以二苯并呋喃为煤焦油中含氧组分的模型化合物,在280 ℃、氢气压力6.5 MPa条件下于反应釜中考察了Ni/Zr-SBA-15催化剂的加氢脱氧性能,分析了ZrO2改性对Ni/SBA-15催化剂结构特性与反应活性的影响。结果表明,ZrO2的添加会增强活性金属Ni与载体间相互作用,促进Ni颗粒的分散,同时会在催化剂中引入氧空位,氧空位与活性金属Ni的协同作用可以促进含氧中间产物的转化,提高目标产物联环己烷的收率。但过量ZrO2(30%)的添加会降低催化剂的比表面积、孔容,不利于Ni颗粒分散,不利于反应的进行。研究发现,当ZrO2添加量为10%时,二苯并呋喃反应速率最高,为9.21 mmol/(min·g);当ZrO2添加量为20%时联环己烷生成速率最高,为3.74 mmol/(min·g);两者均高于未改性的Ni/SBA-15催化剂。Abstract: The performance of the ZrO2 modified Ni/SBA-15 catalysts was investigated for the hydrodeoxygenation of dibenzofuran as a coal tar model compound in an autoclave at 280 ℃ and a hydrogen pressure of 6.5 MPa. The effects of ZrO2 modification on the structural characteristics and reaction activity of Ni/Zr-SBA-15 catalysts were analyzed. The results showed that the addition of ZrO2 increased the interaction between the active metal Ni and the SBA-15 support, promoted the dispersion of Ni particles, and introduced oxygen vacancies in the catalysts. As a result, the conversion of oxygen-containing intermediates was enhanced, and thereby the yield of the target product bicyclohexane was improved. However, the addition of excessive ZrO2 (30%) could reduce the specific surface area and pore volume of the catalysts and cover Ni surface, which was not conducive to the reaction. The study found that the highest dibenzofuran conversion rate of 9.21 mmol/(min·g) and the highest bicyclohexane formation rate of 3.74 mmol/(min·g) were achieved when the addition amount of ZrO2 were 10% and 20%, respectively. Both values were much higher than those on the unmodified Ni/SBA-15 catalyst.
-
Key words:
- dibenzofuran /
- hydrodeoxygenation /
- Ni/SBA-15 /
- ZrO2 modification /
- oxygen vacancies
-
表 1 催化剂的化学性质
Table 1 Chemical properties of catalysts
Sample Crystallite
size/nm aH2 consumption
amount/(μmol·g−1) bNi dispersion/% c O2 consumption
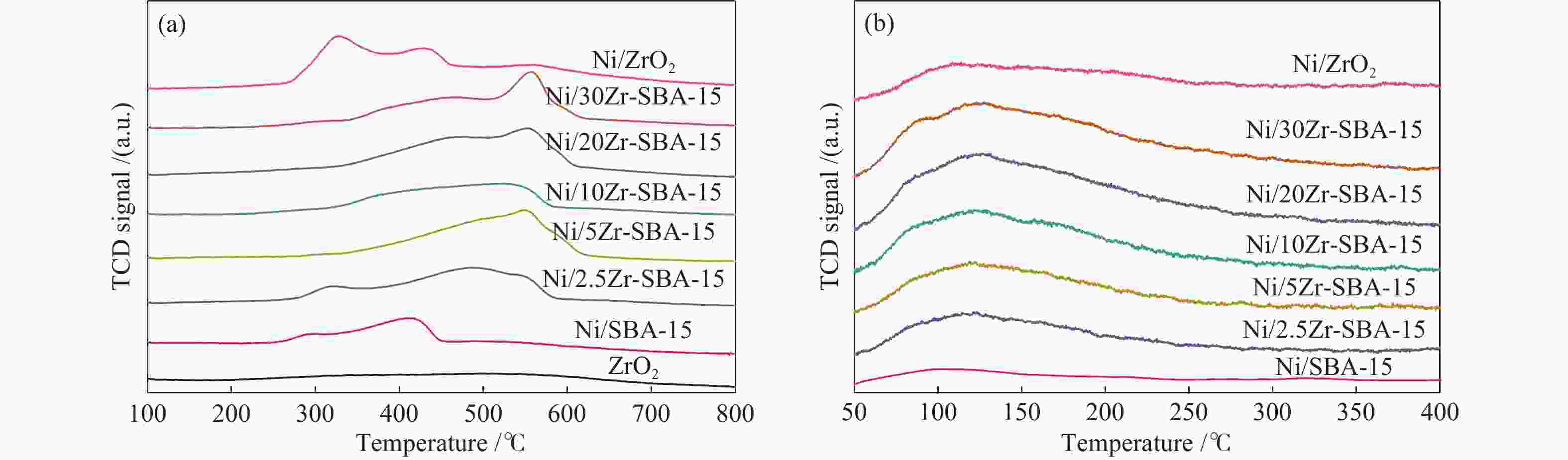
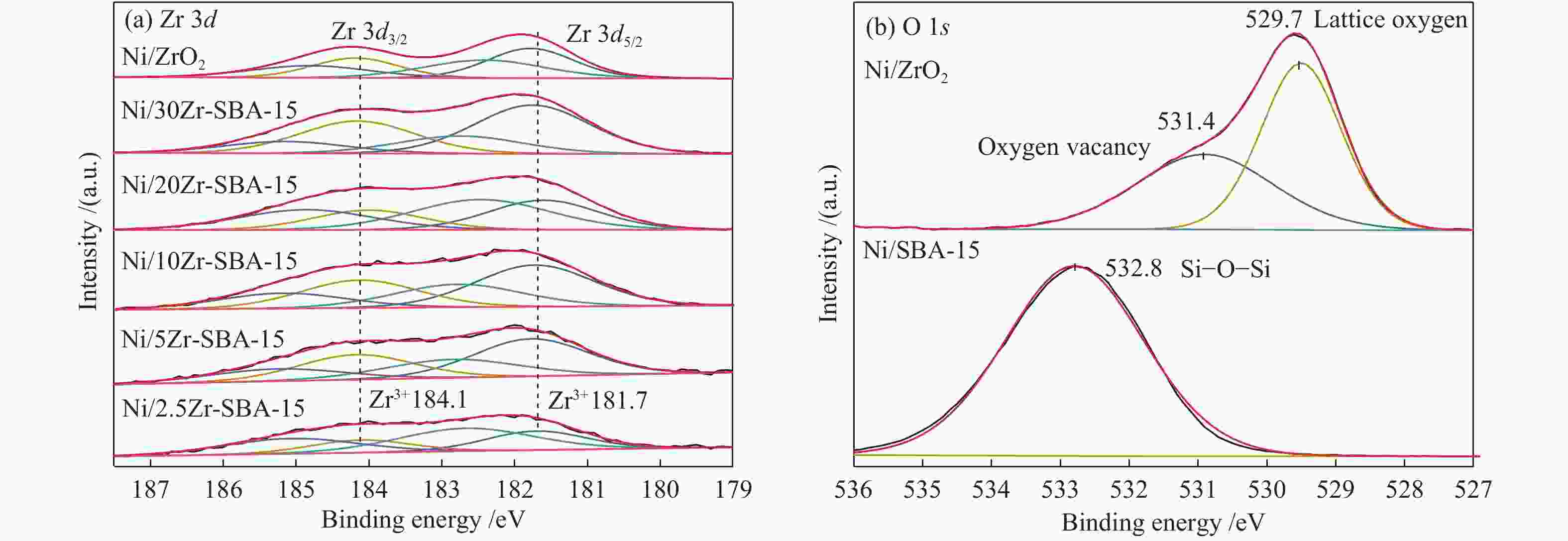
amount/(μmol·g−1) dZrO2 − 13.1 − − Ni/SBA-15 9.7 15.9 3.0 206.7 Ni/2.5Zr-SBA-15 8.2 29.3 7.7 210.9 Ni/5Zr-SBA-15 8.0 30.0 9.6 222.4 Ni/10Zr-SBA-15 7.5 31.4 13.4 236.2 Ni/20Zr-SBA-15 7.3 33.2 16.8 260.2 Ni/30Zr-SBA-15 6.7 34.4 17.0 286.7 Ni/ZrO2 6.8 43.5 6.6 288.1 a: calculated from the (111) reflections using the Scherrer equation; b: H2 consumption amount in H2-TPR; c: determined by H2-TPD;
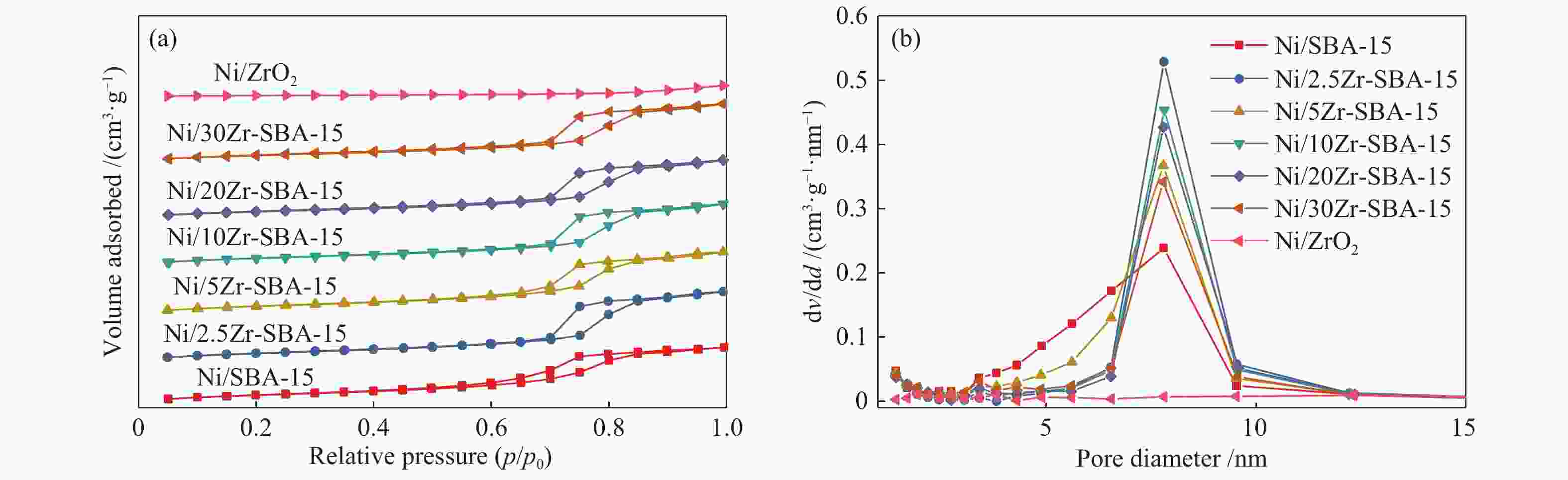
d: determined by O2-Pulse chemisorption表 2 催化剂的物理吸附性质
Table 2 Physical adsorption properties of catalysts
Sample Surface
areaa/(m2·g−1)Pore volumeb/
(cm3·g−1)Most probable
pore sizec/nmSBA-15 498.8 1.41 7.8 Ni/SBA-15 493.6 1.11 7.8 Ni2.5Zr/SBA-15 464.2 1.08 7.8 Ni5Zr/SBA-15 450.2 0.96 7.8 Ni10Zr/SBA-15 414.9 0.94 7.8 Ni20Zr/SBA-15 390.6 0.89 7.8 Ni30Zr/SBA-15 360.0 0.77 7.8 Ni/ZrO2 46.9 0.13 − a: calculated in the range of relative pressure (p/p0) = 0.05−0.30; b: total pore volume measured at p/p0 = 0.99; c: calculated from the desorption branch with BJH method 表 3 催化剂的酸量
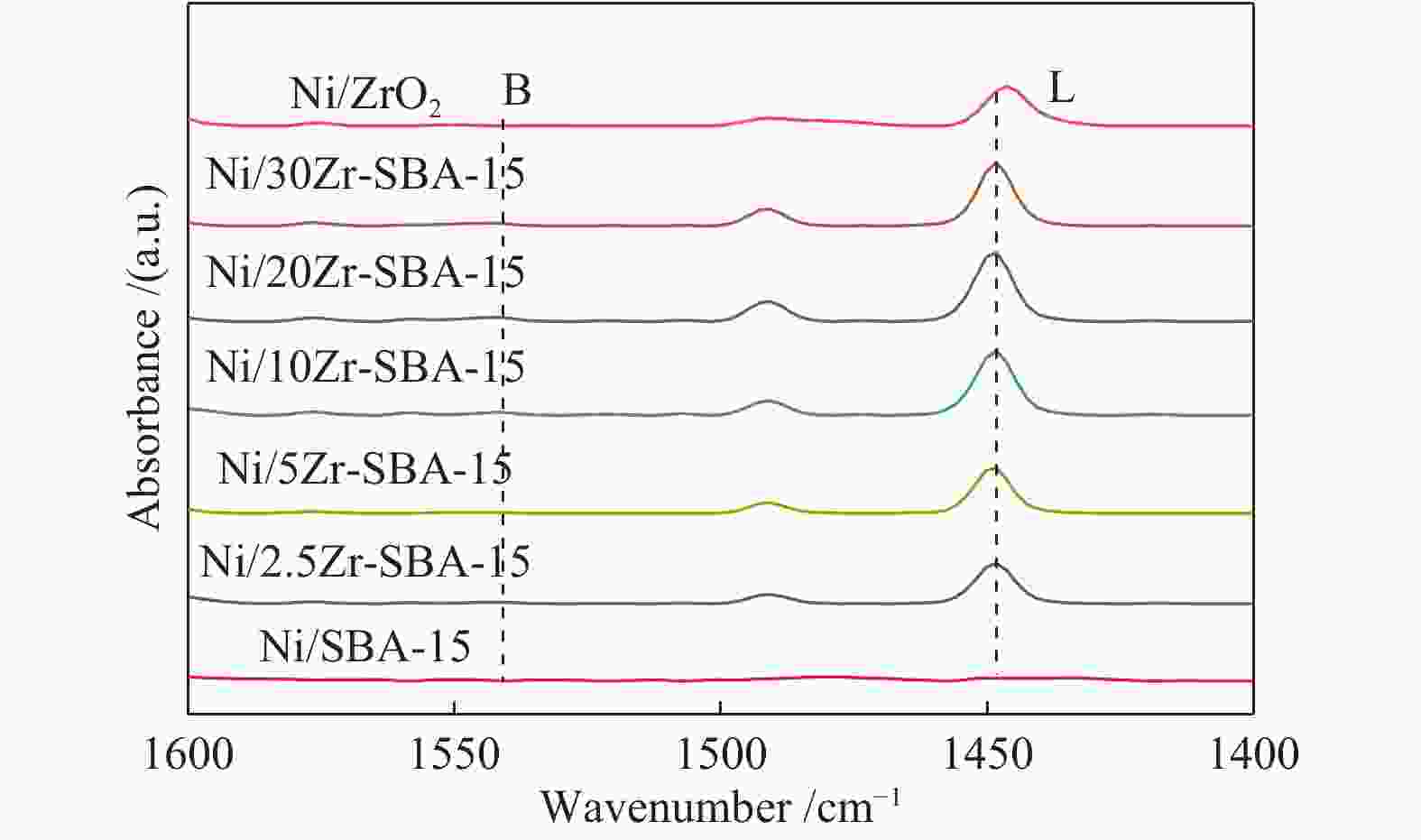
Table 3 The acid amount of catalysts
Catalyst Lewis acid amount/(μmol·g−1) Proportion of medium strong acid in total acid 150 ℃ 300 ℃ Ni/SBA-15 8.2 3.8 0.46 Ni/2.5Zr-SBA-15 43.1 20.1 0.46 Ni/5Zr-SBA-15 60.1 26.7 0.44 Ni/10Zr-SBA-15 84.5 30.5 0.36 Ni/20Zr-SBA-15 71.9 18.5 0.25 Ni/30Zr-SBA-15 68.3 14.2 0.20 Ni/ZrO2 52.8 21.4 0.40 -
[1] 赵振新, 朱书全, 马名杰. 中国褐煤的综合优化利用[J]. 洁净煤技术,2008,14(1):28−31. doi: 10.3969/j.issn.1006-6772.2008.01.008ZHAO Zhen-xin, ZHU Shu-quan, MA Ming-jie. Comprehensive and optimal utilization of lignite in China[J]. Clean Coal Technol,2008,14(1):28−31. doi: 10.3969/j.issn.1006-6772.2008.01.008 [2] HUBER G W, CORMA A. Synergies between bio- and oil refineries for the production of fuels from biomass[J]. Angew Chem Int Ed,2007,38(50):7184−7201. [3] 任洪凯, 邓文安, 李传, 崔文龙. 中/低温煤焦油酚类化合物的组成研究[J]. 煤炭转化,2013,36(2):67−70. doi: 10.3969/j.issn.1004-4248.2013.02.017REN Hong-kai, DENG Wen-an, LI Chuan, CUI Wen-long. Study on the composition of phenolic compounds in middle/low temperature coal tar[J]. Coal Convers,2013,36(2):67−70. doi: 10.3969/j.issn.1004-4248.2013.02.017 [4] 孙鸣, 陈静, 代晓敏, 马晓迅, 赵香龙, 刘科. 陕北中低温煤焦油减压馏分的GC-MS分析[J]. 煤炭转化,2015,38(1):58−63. doi: 10.3969/j.issn.1004-4248.2015.01.012SUN Ning, CHEN Jing, DAI Xiao-min, MA Xiao-xun, ZHAO Xiang-long, LIU Ke. Vacuum distillates and GC-MS Analysis of low temperature coal tar from northern Shaanxi[J]. Coal Convers,2015,38(1):58−63. doi: 10.3969/j.issn.1004-4248.2015.01.012 [5] WANG H, MALE J, WANG Y J A C. Recent advances in hydrotreating of pyrolysis bio-oil and its oxygen-containing model compounds[J]. ACS Catal,2013,3(5):1047−1070. doi: 10.1021/cs400069z [6] NI H, XU C, WANG R, GUO X, LONG Y, MA C, YAN L, LIU X, SHI Q. Composition and transformation of sulfur-, oxygen-, and nitrogen-containing compounds in the hydrotreating process of a low-temperature coal tar[J]. Energy Fuels,2018,32(3):3077−3084. doi: 10.1021/acs.energyfuels.7b03659 [7] 彭华伟. 负载Ni金属/有序介孔SiO2-Al2O3催化二苯并呋喃加氢脱氧反应研究[D]. 太原: 太原理工大学, 2018.PENG Hua-wei. Hydrodeoxygenation of dibenzofuran over Ni/ordered mesoporous SiO2-Al2O3 catalysts[D]. Taiyuan: Taiyuan University of Technology, 2018. [8] 王铷. Ni-Fe双金属催化剂用于二苯并呋喃加氢脱氧反应研究[D]. 太原: 太原理工大学, 2019.WANG Ru. Hydrodeoxygenation of dibenzofuran over bimetallic Ni-Fe catalysts[D]. Taiyuan: Taiyuan University of Technology, 2019. [9] WANG L, WAN H, JIN S, CHEN X, LI C, LIANG C. Hydrodeoxygenation of dibenzofuran over SiO2, Al2O3/SiO2 and ZrO2/SiO2 supported Pt catalysts[J]. Catal Sci Technol,2015,5(1):465−474. doi: 10.1039/C4CY00859F [10] WANG Y, FANG Y, HE T, HU H, WU J. Hydrodeoxygenation of dibenzofuran over noble metal supported on mesoporous zeolite[J]. Catal Commun,2011,12(13):1201−1205. doi: 10.1016/j.catcom.2011.04.010 [11] WANG L, LI C, JIN S, LI W, LIANG C. Hydrodeoxygenation of dibenzofuran over SBA-15 supported Pt, Pd, and Ru catalysts[J]. Catal Lett,2014,144(5):809−816. doi: 10.1007/s10562-014-1236-2 [12] DONG P, LU G, CAI C. Effective hydrodeoxygenation of dibenzofuran by a bimetallic catalyst in water[J]. New J Chem,2016,40(2):1605−1609. doi: 10.1039/C5NJ02164B [13] DE SOUZA P M, RABELO-NETO R C, BORGES L E P, JACOBS G, DAVIS B H, RESASCO D E, NORONHA F B. Hydrodeoxygenation of phenol over Pd catalysts. effect of support on reaction mechanism and catalyst deactivation[J]. ACS Catal,2017,7(3):2058−2073. doi: 10.1021/acscatal.6b02022 [14] ZHAO L, ZHAO J, WU T, ZHAO M, YAN W, ZHANG Y, LI H, WANG Y, XIAO T, ZHAO Y. Synergistic effect of oxygen vacancies and Ni species on tuning selectivity of Ni/ZrO2 catalyst for hydrogenation of maleic anhydride into succinic anhydride and gamma-butyrolacetone[J]. Nanomaterials-Basel,2019,9(3):2079−4991. [15] KRUK M, JARONIEC M, KO C H, RYOO R. Characterization of the porous structure of SBA-15[J]. Chem Mater,2000,12(7):1961−1968. doi: 10.1021/cm000164e [16] GUTIERREZ O, VALENCIA D, FUENTES G, KLIMOVA T. Mo and NiMo catalysts supported on SBA-15 modified by grafted ZrO2 species: Synthesis, characterization and evaluation in 4, 6-dimethyldibenzothiophene hydrodesulfurization[J]. J Catal,2007,249(2):140−153. doi: 10.1016/j.jcat.2007.04.014 [17] LINDO M, VIZCAíNO A J, CALLES J A, CARRERO A. Ethanol steam reforming on Ni/Al-SBA-15 catalysts: Effect of the aluminium content[J]. Int J Hydrogen Energy,2010,35(11):5895−5901. doi: 10.1016/j.ijhydene.2009.12.120 [18] OCHOA-HERNÁNDEZ C, YANG Y, PIZARRO P, DE LA PEÑA O'SHEA V A, CORONADO J M, SERRANO D P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15[J]. Catal Today,2013,210:81−88. doi: 10.1016/j.cattod.2012.12.002 [19] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. J Catal,1993,141(2):347−354. doi: 10.1006/jcat.1993.1145 [20] LIU C, WANG W, XU Y, LI Z, WANG B, MA X. Effect of zirconia morphology on sulfur-resistant methanation performance of MoO3/ZrO2 catalyst[J]. Appl Surf Sci,2018,441:482−490. doi: 10.1016/j.apsusc.2018.02.019 [21] CAMPA M C, FERRARIS G, GAZZOLI D, PETTITI I, PIETROGIACOMI D. Rhodium supported on tetragonal or monoclinic ZrO2 as catalyst for the partial oxidation of methane[J]. Appl Catal B: Environ,2013,142−143(5):423−431. [22] LI H, REN J, QIN X, QIN Z, LIN J, LI Z. Ni/SBA-15 catalysts for CO methanation: Effects of V, Ce, and Zr promoters[J]. RSC Adv,2015,5(117):96504−96517. doi: 10.1039/C5RA15990C [23] JIA X, ZHANG X, RUI N, HU X, LIU C-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity[J]. Appl Catal B: Environ,2019,244:159−169. doi: 10.1016/j.apcatb.2018.11.024 [24] NI J, LENG W, MAO J, WANG J, LIN J, JIANG D, LI X. Tuning electron density of metal nickel by support defects in Ni/ZrO2 for selective hydrogenation of fatty acids to alkanes and alcohols[J]. Appl Catal B: Environ,2019,253:170−178. doi: 10.1016/j.apcatb.2019.04.043 [25] SHUTTHANANDAN V, NANDASIRI M, ZHENG J, ENGELHARD M H, XU W, THEVUTHASAN S, MURUGESAN V. Applications of XPS in the characterization of Battery materials[J]. J Electron Spectrosc,2019,231:2−10. doi: 10.1016/j.elspec.2018.05.005 [26] BETTINELLI M, SPEGHINI A, FALCOMER D, DALDOSSO M, DALLACASA V, ROMANò L. Photocatalytic, spectroscopic and transport properties of lanthanide-doped TiO2 nanocrystals[J]. J Phys-Condens Mat,2006,18(33):2149−2160. doi: 10.1088/0953-8984/18/33/S30 [27] LIU B, LI C, ZHANG G, YAO X, CHUANG S S C, LI Z. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-Doped CeO2 nanorods[J]. ACS Catal,2018,8(11):10446−10456. doi: 10.1021/acscatal.8b00415 [28] WANG Y, CAI J, WU M, CHEN J, ZHAO W, TIAN Y, DING T, ZHANG J, JIANG Z, LI X. Rational construction of oxygen vacancies onto tungsten trioxide to improve visible light photocatalytic water oxidation reaction[J]. Appl Catal B: Environ,2018,239:398−407. doi: 10.1016/j.apcatb.2018.08.029 [29] 谢子铮. 二苯并呋喃加氢脱氧反应中Pt、Ni基催化剂的理论设计与实验[D]. 太原: 太原理工大学, 2020.XIE Zi-zheng. Theoretical design and experiments of dibenzofuran hydrodeoxygenation over Pt and Ni-based catalysts[D]. Taiyuan: Taiyuan University of Technology, 2020. [30] WANG X B, XIE Z Z, GUO L, DU Z Y, LI W Y J C T. Mechanism of dibenzofuran hydrodeoxygenation on the surface of Pt(111): A DFT study[J]. Catal Today,2021,36(4):220−228. doi: 10.1016/j.cattod.2020.04.044 [31] BITTER J H, SESHAN K, LERCHER J A. On the contribution of X-ray absorption spectroscopy to explore structure and activity relations of Pt/ZrO2 catalysts for CO2/CH4 reforming[J]. Top Catal,2000,10(3/4):295−305. [32] ZHU X, XIE Y, LIU C J, ZHANG Y P. Stability of Pt particles on ZrO2 support during partial oxidation of methane: DRIFT studies of adsorbed CO[J]. J Mol Catal A: Chem,2008,282(1/2):67−73. doi: 10.1016/j.molcata.2007.11.020 [33] PENG B, YUAN X, ZHAO C, LERCHER J A. Stabilizing catalytic pathways via redundancy: Selective reduction of microalgae oil to alkanes[J]. J Am Chem Soc,2012,134(22):9400−9405. doi: 10.1021/ja302436q -




 下载:
下载: