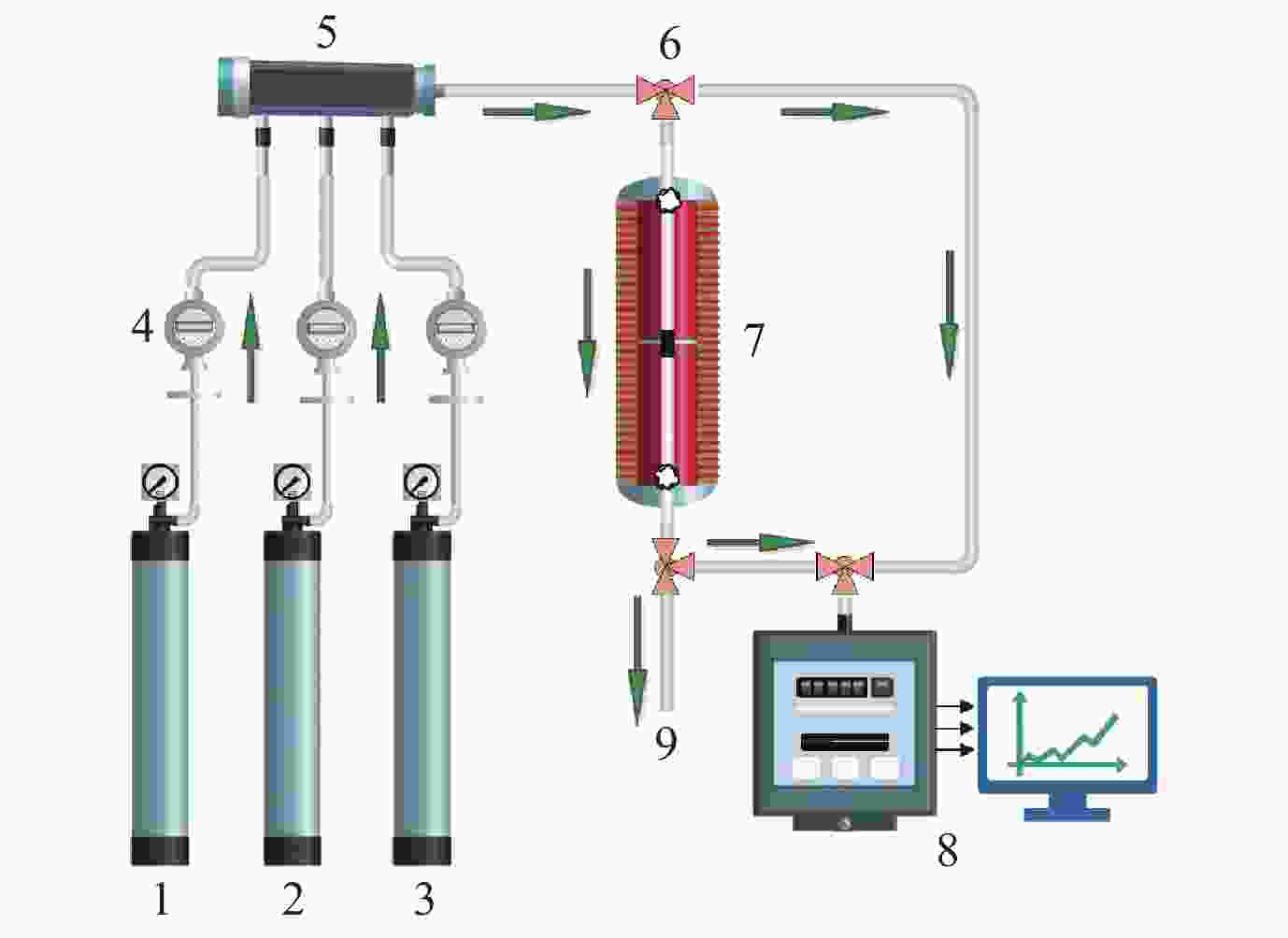
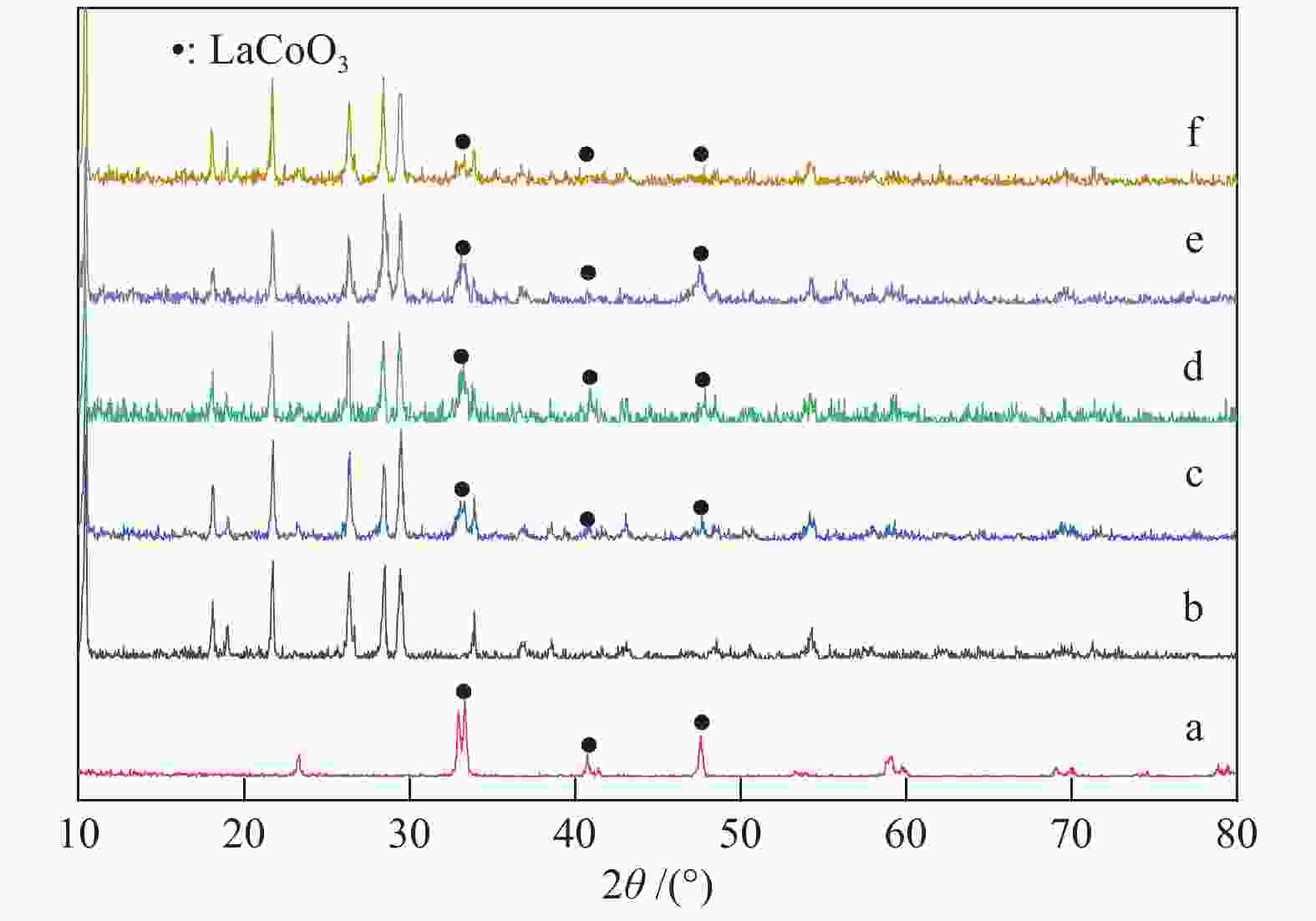
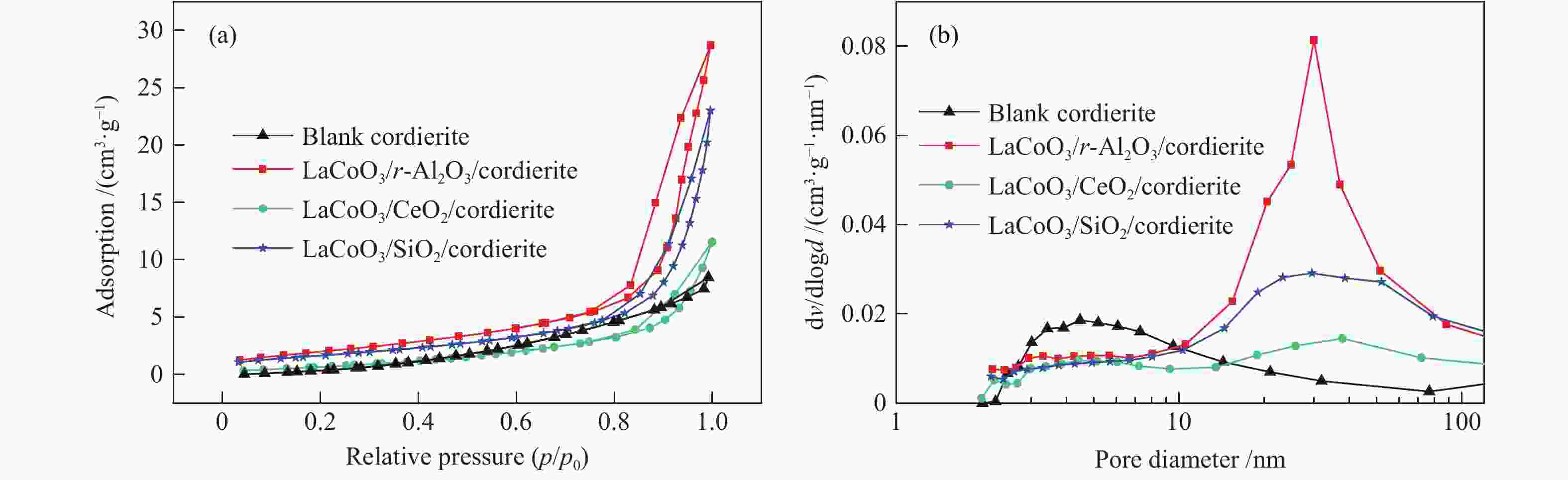
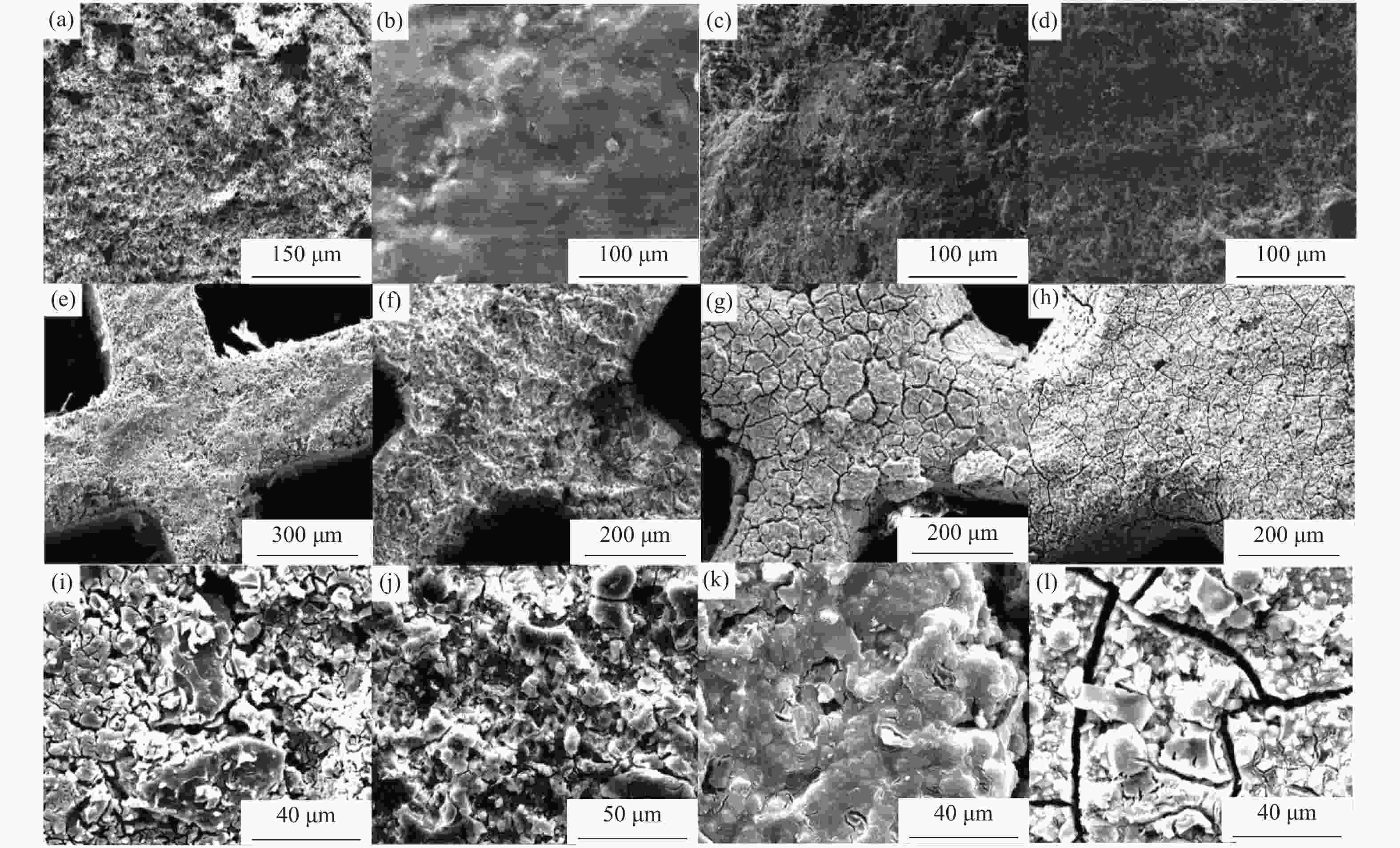
Effect of nano-coating on the catalytic combustion performance of perovskite-based monolithic catalysts for VOCs
-
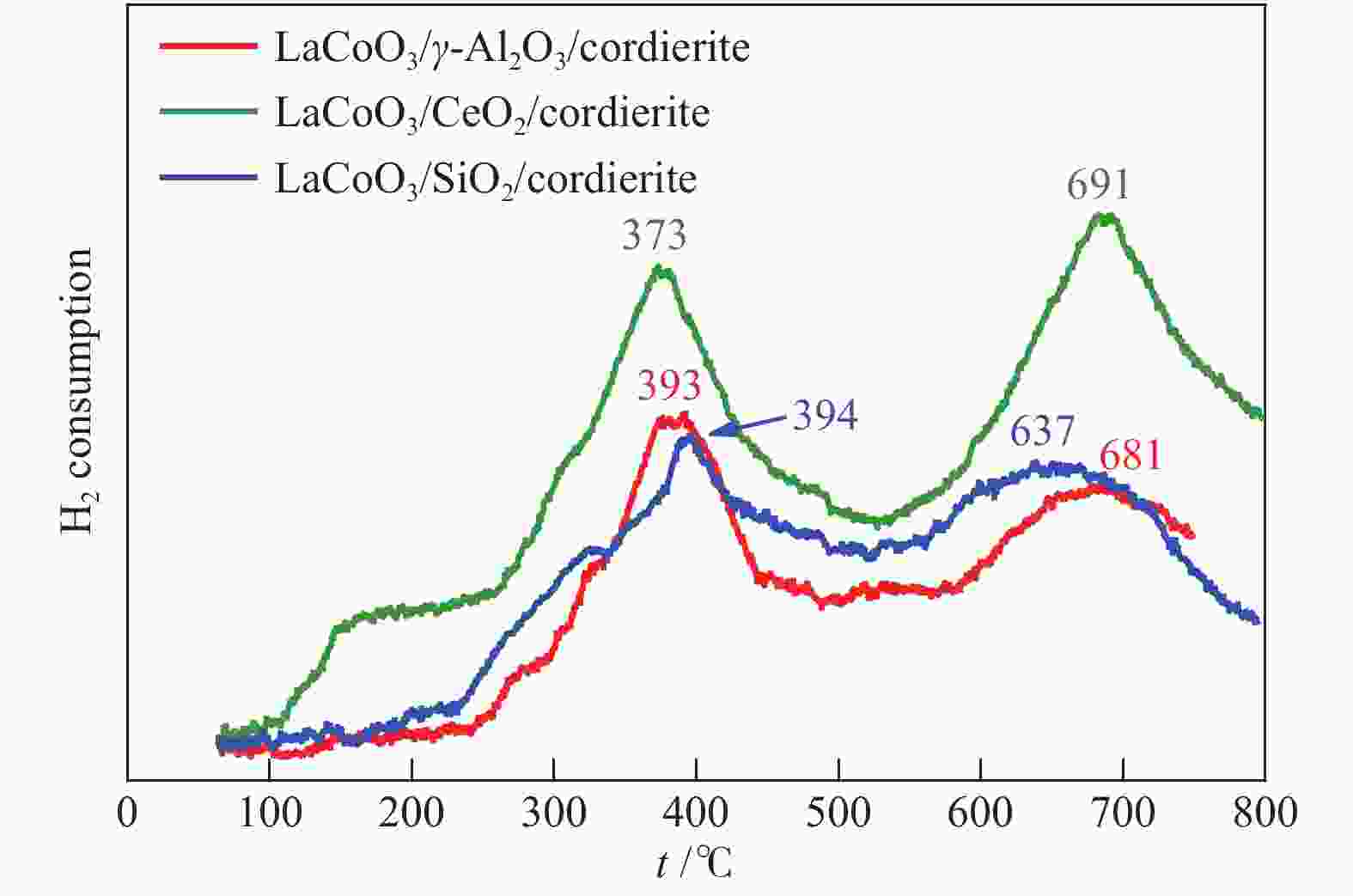
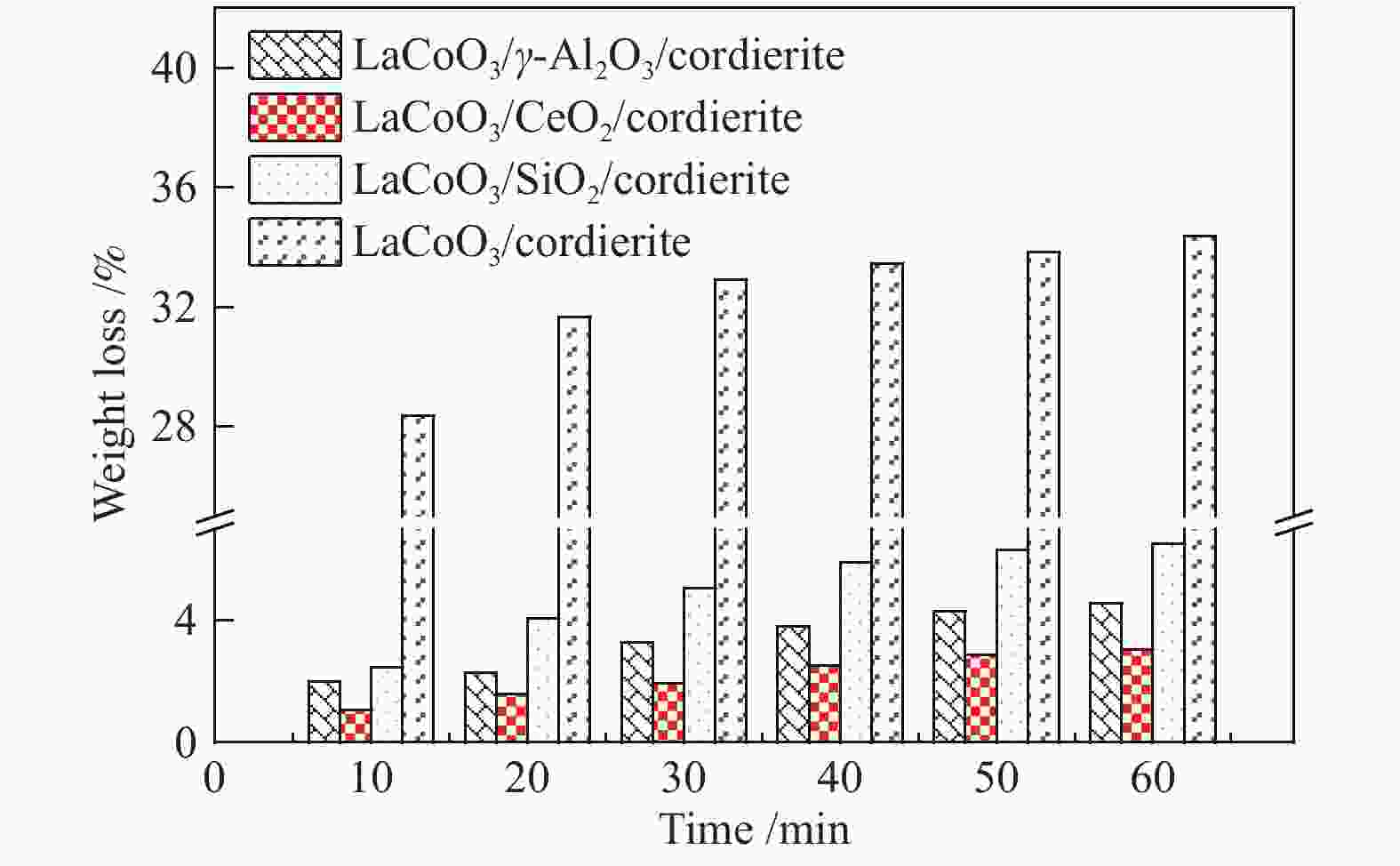
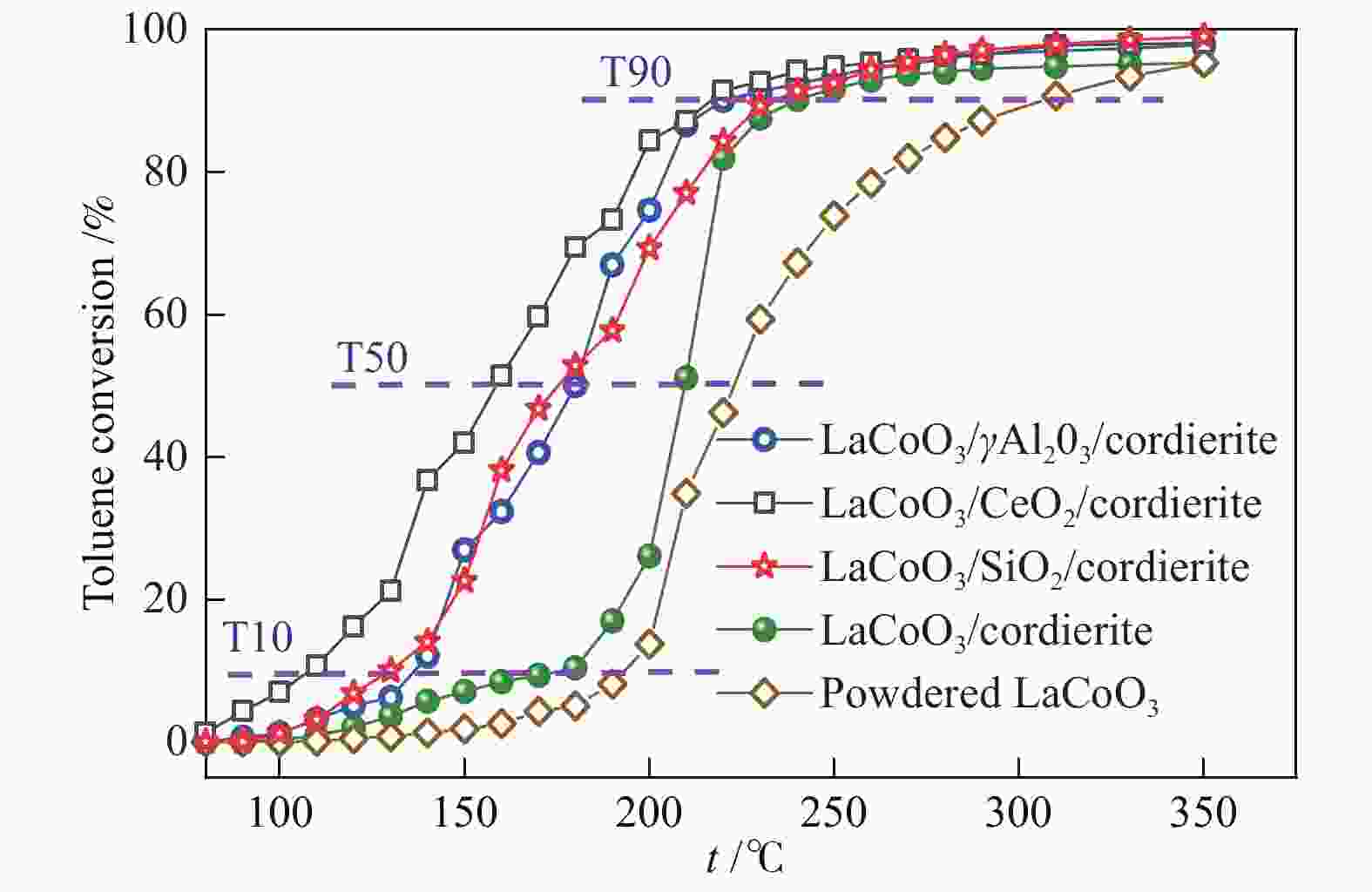
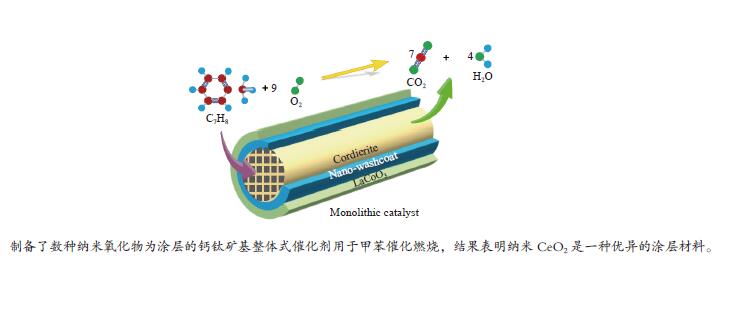
摘要: 以堇青石蜂窝陶瓷为基体,纳米氧化物(CeO2、γ-Al2O3、SiO2)为涂层载体,通过悬浮涂渍法和溶胶-凝胶法两步制得LaCoO3/CeO2/堇青石、LaCoO3/γ-Al2O3/堇青石、LaCoO3/SiO2/堇青石三种整体式催化剂。通过XRD、SEM、XPS、H2-TPR、UT和N2 吸附-脱附等技术,对样品的物相、微观形貌、元素成分、氧化还原性、黏附强度、质构性能进行了表征分析。通过催化燃烧VOCs实验评价了整体式催化剂的催化活性、高温稳定性、停机/重启循环稳定性和水汽稳定性。实验结果表明,三种催化剂均显示出良好的催化活性和稳定性,涂层类型会造成催化性能的差异。其中,LaCoO3/CeO2/堇青石催化剂表现出最优的性能。当甲苯体积分数为0.1%、空速为18000 mL/(g·h)时,其达到50%和90%转化率时温度分别为158和214 ℃;在转化率为90%的温度下经历72 h的稳定性测试(高温、停启循环、水汽)后,甲苯转换率仅降低7%。表征结果显示,CeO2纳米涂层有助于形成多孔蓬松结构的催化层, 同时催化剂具有较高的吸附氧比例、较强的低温还原性以及良好的黏附性。Abstract: The LaCoO3/CeO2/cordierite, LaCoO3/γ-Al2O3/cordierite, LaCoO3/SiO2/cordierite monolithic catalysts were synthesized by the suspension coating method and sol-gel method in two steps using cordierite honeycomb ceramic as the substrate and nano-oxides (CeO2, γ-Al2O3, SiO2) as the coating carrier. The phase, microscopic morphology, element composition, redox property, adhesion strength, and texture properties of the samples were characterized and analyzed by XRD, SEM, XPS, H2-TPR, UT and N2 adsorption-desorption techniques. The catalytic activity, high-temperature stability, shutdown/restart cycle stability, and water vapor stability of the monolithic catalyst were evaluated through the catalytic combustion performance of VOCs. Experimental results show that three catalysts exhibit good catalytic activity and stability, and the type of coating can cause a difference in catalytic performance. Among three cattalysts, the LaCoO3/CeO2/cordierite catalyst has the best performance. When the volume fraction of toluene is 0.1% and the space velocity is 18000 mL/(g·h), the tempereratures for the conversion rates of 50% and 90% are 158 and 214 ℃, respectively. The toluene conversion rate is only reduced by 7% after 72 h of stability testing (high temperature, stop/restart cycle, water vapor). Characterization results show that the CeO2 nano-coating helps to form a catalytic layer with a porous and fluffy structure, which makes the catalyst have a higher ratio of adsorbed oxygen, strong low-temperature reducibility and good adhesion.
-
Key words:
- coating /
- monolithic catalyst /
- catalytic combustion /
- perovskite /
- VOCs
-
表 1 样品的质构性质
Table 1 Textural properties of the samples
Sample $S_{{\rm{BET}}}^{\rm{a}} $/(m2·g−1) $v_{ {\rm{p} } }^{\rm{b} }$/(cm3·g−1) $D_{{\rm{p}}}^{\rm{c}} $/nm Cordierite 1.2 0.013 7.2 Al2O3 138.1 0.771 11.1 γ-Al2O3/cordierite 11.7 0.068 13.9 LaCoO3/γ-Al2O3/cordierite 6.9 0.042 16.9 CeO2 48.7 0.198 81.3 CeO2/cordierite 3.3 0.016 10.0 LaCoO3/CeO2/cordierite 2.3 0.016 13.4 SiO2 187.0 0.060 50.0 SiO2/cordierite 11.9 0.080 21.5 LaCoO3/SiO2/cordierite 5.7 0.032 16.0 a: surface area, calculated by BET method, b: total pore volume, determined from the amount adsorbed at p/p0 = 0.99, c: average pore diameter, determined by BJH method of the desorption branch 表 2 XPS表征参数
Table 2 XPS characterization parameters
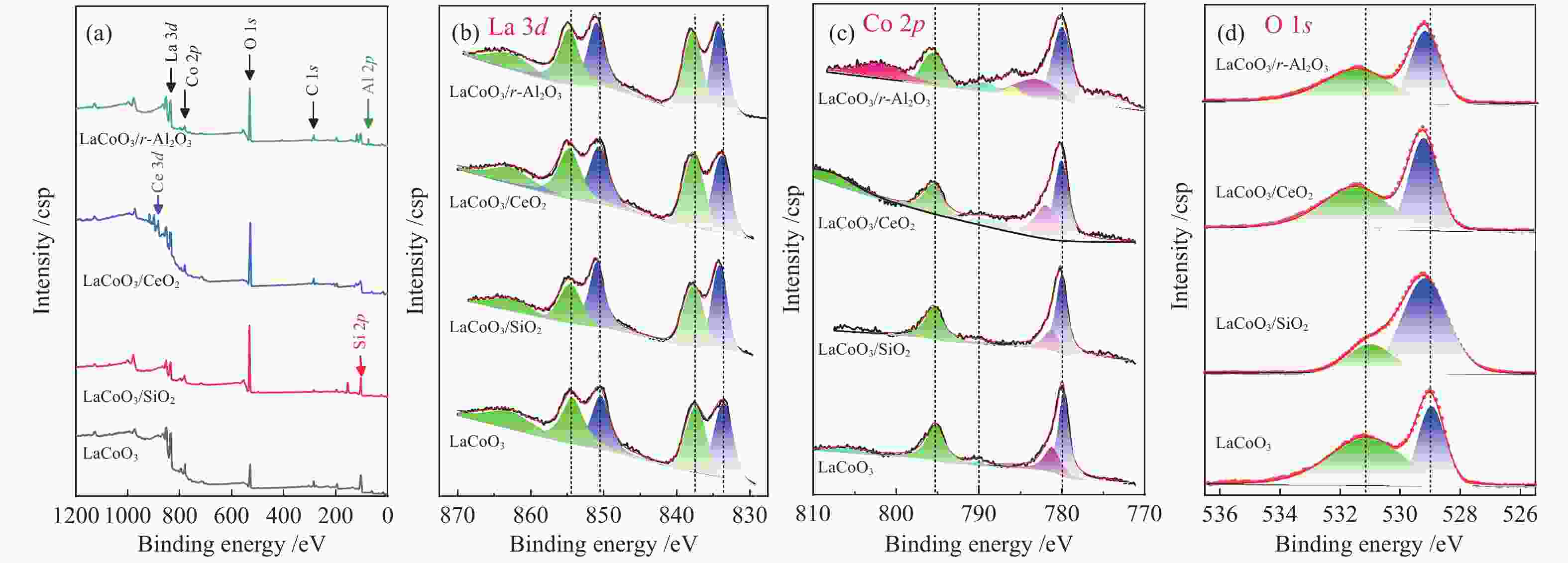
Sample Binding energy/eV Co/La Oads/Ototal La 3d5/2 La 3d3/2 Co 2p3/2 Co 2p1/2 Oads 1s Olatt 1s Ce 3d/Al 2p/Si 2p LaCoO3/γ-Al2O3 834.4/838 851.2/854.9 780.3 795.6 529.3 531.4 74.3 0.41 0.54 LaCoO3/CeO2 834.3/837.8 850.9/854.8 780.2 795.4 529.3 531.4 882.5 0.52 0.64 LaCoO3/SiO2 834.5/837.9 851.1/854.9 780.2 795.4 529.5 531.0 103.2 0.38 0.24 LaCoO3 833.7/837.5 850.6/854.4 779.9 795.2 529.0 531.1 − 0.55 0.651 表 3 本研究与其他文献中催化剂的甲苯催化燃烧性能比较
Table 3 Catalysts comparison of toluene catalytic combustion performance in this work and other literature
Catalyst Toluene GHSV T50/℃ T90/℃ Reference LaCoO3/CeO2/cordierite 0.1% 18000 mL/(g·h) 158 214 this work LaCoO3/γ-Al2O3/cordierite 180 219 LaCoO3/SiO2/cordierite 176 230 LaMnO3/TiO2 0.1% 18000 mL/(g·h) 278 303 [20] LaMnO3/YSZ 235 247 Co/La-CeO2/cordierite 0.1% 12000 mL/(g·h) 253 286 [38] Co/La-CeO2 229 258 0.05La-Co 0.1% 20000 mL/(g·h) 218 224 [39] LaMnO3-PL-2 0.1% 20000 mL/(g·h) 226 249 [40] 3DOM-La0.8Ce0.2MnO3/cordierite (600 ℃) 0.05% 6000 h−1 147 217 [19] 3DOM-La0.8Ce0.2MnO3/cordierite (700 ℃) 162 249 3DOM-La0.8Ce0.2MnO3/cordierite (800 ℃) 190 320 La0.75Sr0.25CoO3 0.05% 30000 h−1 212 228 [41] LaZn0.3Fe0.7O3 0.2% 15000 h−1 222 310 NiMnO3/Ce0.75Zr0.25O2/cordierit 500 mg/m3 15000 h−1 209 235 [42] 20%La0.8Ce0.2MnO3/ZSM-5 3 mg/L 20000 h−1 206 285 [43] -
[1] HE C, CHENG J, ZHANG X, ZHANG X, DOUTHWAITE M, HAO Z. Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources[J]. Chem Rev,2019,119(7):4471−4568. doi: 10.1021/acs.chemrev.8b00408 [2] MANISALIDIS I, STAVROPOULOU E, STAVROPOULOS A, BEZIRTZOGLOU E. Environmental and health impacts of air pollution: a review[J]. Front Public Health,2020,8:14. doi: 10.3389/fpubh.2020.00014 [3] GRANGER P. Challenges and breakthroughs in post-combustion catalysis: how to match future stringent regulations[J]. Catal Sci Technol,2017,7(22):5195−5211. doi: 10.1039/C7CY00983F [4] ZHU J, LI H, ZHONG L, XIAO P, XU X, YANG X, ZHAO Z, LI J. Perovskite oxides: preparation, characterizations, and applications in heterogeneous catalysis[J]. ACS Catal,2014,4(9):2917−2940. doi: 10.1021/cs500606g [5] 胡明江, 吕春旺, 杨师斌, 王忠. La1-xAlxFeO3催化剂降低甲醇/柴油二元燃料发动机非常规排放研究[J]. 环境科学学报,2018,38(4):1437−1445.HU Ming-jiang, LV Chun-wang, YANG Shi-bin, WANG Zhong. Research of effects on irregular exhaust emissions control from methanol-diesel engine with La1−xAlxFeO3 catalysts[J]. Acta Sci Circum,2018,38(4):1437−1445. [6] ZANG M, ZHAO C, WANG Y, CHEN S. A review of recent advances in catalytic combustion of VOCs on perovskite-type catalysts[J]. J Saudi Chem Soc,2019,23(6):645−654. doi: 10.1016/j.jscs.2019.01.004 [7] 胡明江, 刘海燕, 吕春旺, 赵丽霞, 张志远. 基于LaFe1−xPdxO3纳米纤维的三明治结构甲苯传感器研究[J]. 分析化学,2020,48(1):66−73.HU Ming-jiang, LIU Hai-yan, LV Chun-wang, ZAHO Li-xia, ZHANG Zhi-yuan. Study on Sandwich Structure Toluene Sensor Based on LaFe1−xPdxO3 Nanofibers[J]. Chin J Anal Chem,2020,48(1):66−73. [8] HOSSEINI S, MOGHADDAS H, MASOUDI SOLTANI S, KHEAWHOM S. Technological applications of honeycomb monoliths in environmental processes: A review[J]. Process Saf Environ,2020,133:286−300. doi: 10.1016/j.psep.2019.11.020 [9] KEAV S, MATAM S, FERRI D, WEIDENKAFF A. Structured perovskite-based catalysts and their application as three-way catalytic converters-a review[J]. Catalysts,2014,4(3):226−255. doi: 10.3390/catal4030226 [10] TOMAŠIĆ V, JOVIĆ F. State-of-the-art in the monolithic catalysts/reactors[J]. Appl Catal A: Gen,2006,311:112−121. doi: 10.1016/j.apcata.2006.06.013 [11] YUAN J, ZHAO K, CAI T, GAO Z, YANG L, HE D. One-step dip-coating of uniform γ-Al2O3 layers on cordierite honeycombs and its environmental applications[J]. Ceram Int,2016,42(13):14384−14390. doi: 10.1016/j.ceramint.2016.06.003 [12] ALMOHAMADI H, SMITH K J. Beneficial effect of adding γ-AlOOH to the γ-Al2O3 washcoat of a PdO catalyst for methane oxidation[J]. Can J Chem Eng,2019,98(1):281−293. [13] ZHOU T, LI L, CHENG J, HAO Z. Preparation of binary washcoat deposited on cordierite substrate for catalytic applications[J]. Ceram Int,2010,36(2):529−534. doi: 10.1016/j.ceramint.2009.09.027 [14] SOLOVIEV S O, KYRIIENKO P I, POPOVYCH N O. Effect of CeO2 and Al2O3 on the activity of Pd/Co3O4/cordierite catalyst in the three-way catalysis reactions (CO/NO/CnHm)[J]. J Environ Sci,2012,24(7):1327−1333. doi: 10.1016/S1001-0742(11)60930-3 [15] NIKOOFAR K, SHAHEDI Y, CHENARBOO F J J M-R I O C. Nano alumina catalytic applications in organic transformations[J]. Mini-Rev Org Chem, 2019, 16(2): 102−110. [16] 李淑婵. 定—转子反应器中制备Ce0.5Zr0.5O2催化剂载体[D]. 北京: 北京化工大学, 2016.LI Shu-can. Preparation of Ce0.5Zr0.5O2 nano-support in rotor-stator reactor[D]. Beijing: Beijing University of Chemical Technology, 2016. [17] 张庆, 张洪泉, 白涛. 基于γ-A12O3-ZrO2-ThO2的Pd基催化剂载体制备及应用[J]. 实验技术与管理,2020,37(1):56−58+70.ZHANG Qing, ZHANG Hong-quan, BAI Tao. Preparation and application experiment for Pd catalyst carrier based on γ-A12O3-ZrO2-ThO2[J]. Exp Technol Manage,2020,37(1):56−58+70. [18] PRIETO G, TUYSUZ H, DUYCKAERTS N, KNOSSALLA J, WANG G, SCHÜTH F. Hollow nano- and microstructures as catalysts[J]. Chem Rev,2016,116(22):14056−14119. doi: 10.1021/acs.chemrev.6b00374 [19] TANG L, ZHAO Z, WEI Y, LIU J, PENG Y, LI K. Study on the coating of nano-particle and 3DOM LaCoO3 perovskite-type complex oxide on cordierite monolith and the catalytic performances for soot oxidation: The effect of washcoat materials of alumina, silica and titania[J]. Catal Today,2017,297:131−142. doi: 10.1016/j.cattod.2017.06.016 [20] GIROIR-FENDLER A, ALVES-FORTUNATO M, RICHARD M, WANG C, ANTONIODÍAZA J, GIL S, ZHANG C, CAN F, BION N, GUO Y. Synthesis of oxide supported LaMnO3 perovskites to enhance yields in toluene combustion[J]. Appl Catal B: Environ,2016,180:29−37. doi: 10.1016/j.apcatb.2015.06.005 [21] VILLORIA J A, ALVAREZ-GALVAN M C, AL-ZAHRANI S M, PALMISANOC P, SPECCHIA S, SPECCHIA V, FIERROA J L G, NAVARRO R M. Oxidative reforming of diesel fuel over LaCoO3 perovskite derived catalysts: Influence of perovskite synthesis method on catalyst properties and performance[J]. Appl Catal B-Environ,2011,105(3/4):276−288. doi: 10.1016/j.apcatb.2011.04.010 [22] GUIOTTO M, PACELLA M, PERIN G, IOVINO A, MICHELON N, NATILE M M, GLISENTI A, CANU P. Washcoating vs. direct synthesis of LaCoO3 on monoliths for environmental applications[J]. Appl Catal A: Gen,2015,499:146−157. doi: 10.1016/j.apcata.2015.04.013 [23] HAGELIN-WEAVER H A E, HOFLUND G B, MINAHAN D M, SALAITA G N. Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar+ bombardment[J]. Appl Surf Sci,2004,235(4):420−448. doi: 10.1016/j.apsusc.2004.02.062 [24] CARLEY A F, ROBERTS M W, SANTRA A K. Interaction of oxygen and carbon monoxide with csau surfaces[J]. J Phys Chem B,1997,101(48):9978−9983. doi: 10.1021/jp971780+ [25] KALIAGUINE S, NESTE A V, SZABO V, GALLOT J E, MUZYCHUK R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization[J], Appl Catal A-Gen, 2001, 209(1–2): 345−358. [26] ASHOK J, DAS S, DEWANGAN N, KAWI S. H2S and NOx tolerance capability of CeO2 doped La1−xCexCo0.5Ti0.5O3−δ perovskites for steam reforming of biomass tar model reaction[J]. Energ Convers Manage,2019,1:10003. [27] LI Y, WU L, WANG Y, KE P, GUAN B. γ-Al2O3 doped with cerium to enhance electron transfer in catalytic ozonation of phenol[J]. J Water Process Eng,2020,36:101313. [28] WANG L, CHEN M, YU X, ZHAO Z, FAN X,WEI Y, LIU J. Preparation, characterization and catalytic performance of ordered macroporous-mesoporous SiO2-supported MnMOx catalysts for soot combustion[J]. Catal Today,2020,364:21−34. [29] TIAN M, HE C, YU Y, PAN H, SMITH L, JIANG Z, GAO N, JIAN Y, HAO Z, ZHU Q. Catalytic oxidation of 1, 2-dichloroethane over three-dimensional ordered meso-macroporous Co3O4/La0.7Sr0.3Fe0.5Co0.5O3: Destruction route and mechanism[J]. Appl Catal A: Gen,2018,553:1−14. doi: 10.1016/j.apcata.2018.01.013 [30] ROYER S, BÉRUBÉ F, KALIAGUINE S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1−xFexO3[J]. Appl Catal A: Gen,2005,282(1/2):273−284. doi: 10.1016/j.apcata.2004.12.018 [31] BRACKMANN R, PEREZ C A, SCHMAL M. LaCoO3 perovskite on ceramic monoliths – Pre and post reaction analyzes of the partial oxidation of methane[J]. Int J Hydrogen Energy,2014,39(26):13991−14007. doi: 10.1016/j.ijhydene.2014.07.027 [32] LIANG H, HONG Y, ZHU C, LI S, CHEN Y, LIU Z, YE D. Influence of partial Mn-substitution on surface oxygen species of LaCoO3 catalysts[J]. Catal Today,2013,201:98−102. doi: 10.1016/j.cattod.2012.04.036 [33] ZHENG Y, LI K, WANG H, TIAN D, WANG Y, ZHU X, WEI Y, ZHENG M, LUO Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane[J]. Appl Catal B: Environ,2017,202:51−63. doi: 10.1016/j.apcatb.2016.08.024 [34] HWANG J, HA H J, RYU J H, CHOI J J, AHN C W, KIM J W, HAHN D B, YOON W H, LEE H, CHOI J J. Enhancement of washcoat adhesion for SCR catalysts to convert nitrogen oxide using powder spray coating of TiO2 on metallic honeycomb substrate[J]. Catal Commun,2017,94:1−4. doi: 10.1016/j.catcom.2017.02.002 [35] TARJOMANNEJAD A, FARZI A, NIAEI A, SALARI D. An experimental and kinetic study of toluene oxidation over LaMn1−xBxO3 and La0.8A0.2Mn0.3B0.7O3 (A=Sr, Ce and B=Cu, Fe) nano-perovskite catalysts[J]. Korean J Chem Eng,2016,33(9):2628−2637. doi: 10.1007/s11814-016-0108-4 [36] 张巍, 汤云灏, 尹艳山, 龚蔚成, 宋健, 马英, 阮敏, 徐慧芳, 陈冬林. 改性镧系钙钛矿催化剂强化挥发性有机物催化氧化的研究进展[J]. 化工进展,2021,40(3):1425−1437.ZHANG Wei, TANG Yun-hao, YIN Yan-shan, GONG Wei-cheng, SONG Jian, MA Ying, RUAN Min, XU Hui-fang, CHEN Dong-lin. Research progress in enhanced catalytic oxidation of VOCs by modified La-based perocskite catalyst[J]. Chem Ind Eng Process,2021,40(3):1425−1437. [37] 刘晓刚, 闫梦雪, 许茹雯, 孙巾茹, 王虹, 迟姚玲, 李翠清, 宋永吉. La1−xRbxMnO3钙钛矿催化剂同时消除NO和碳烟的催化性能[J]. 燃料化学学报,2019,47(9):1053−1066. doi: 10.3969/j.issn.0253-2409.2019.09.004LIU Xiao-gang, YAN Meng-xue, XU Ru-wen, SUN Jin-ru, WANG Hong, CHI Yao-ling, LI Cui-qing, SONG Yong-ji. Catalytic performance of La1−xRbxMnO3 perovskite in the simultaneous removal of NO and soot[J]. J Fuel Chem Technol,2019,47(9):1053−1066. doi: 10.3969/j.issn.0253-2409.2019.09.004 [38] GÓMEZ D M, GATICA J M, HERNáNDEZ-GARRIDO J C, CIFREDO G, MONTES M, SANZ O, REBLED J M, VIDAL H. A novel CoOx/La-modified-CeO2 formulation for powdered and washcoated onto cordierite honeycomb catalysts with application in VOCs oxidation[J]. Appl Catal B: Environ,2014,144:425−434. doi: 10.1016/j.apcatb.2013.07.045 [39] WU M, CHEN S, SOOMRO A, MA S, ZHU M, HUA X, XIANG W. Investigation of synergistic effects and high performance of La-Co composite oxides for toluene catalytic oxidation at low temperature[J]. Environ Sci Pollut Res,2019,26(12):12123−12135. [40] ZHANG C, GUO Y, GUO Y, LU G, BOREAVE A, RETAILLEAU L, BAYLET A, GIROIR-FENDLER A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene[J]. Appl Catal B: Environ,2014,148−149. [41] PEREÑÍGUEZ R, HUESO J L, GAILLARD F, HOLGADO J, CABALLERO A. Study of oxygen reactivity in La1−xSrxCoO3−δ perovskites for total oxidation of toluene[J]. Catal Lett,2012,142(4):408−416. doi: 10.1007/s10562-012-0799-z [42] DENG L, HUANG C, KAN J, LI B, CHEN Y, ZHU S, SHEN S. Effect of coating modification of cordierite carrier on catalytic performance of supported NiMnO3 catalysts for VOCs combustion[J]. J Rare Earths,2018,36(3):265−272. doi: 10.1016/j.jre.2017.07.015 [43] WANG Y, XIAO L, ZHAO CC, LIU F, LI S. Catalytic combustion of toluene with Pd/La0.8Ce0.2MnO3 supported on different zeolites[J]. Environ Prog Sustainable Energy,2018,37(1):101313. -





 下载:
下载: