Preparation of TS-1 by dynamic method and study on the performance of thiophene sulfur removal
-
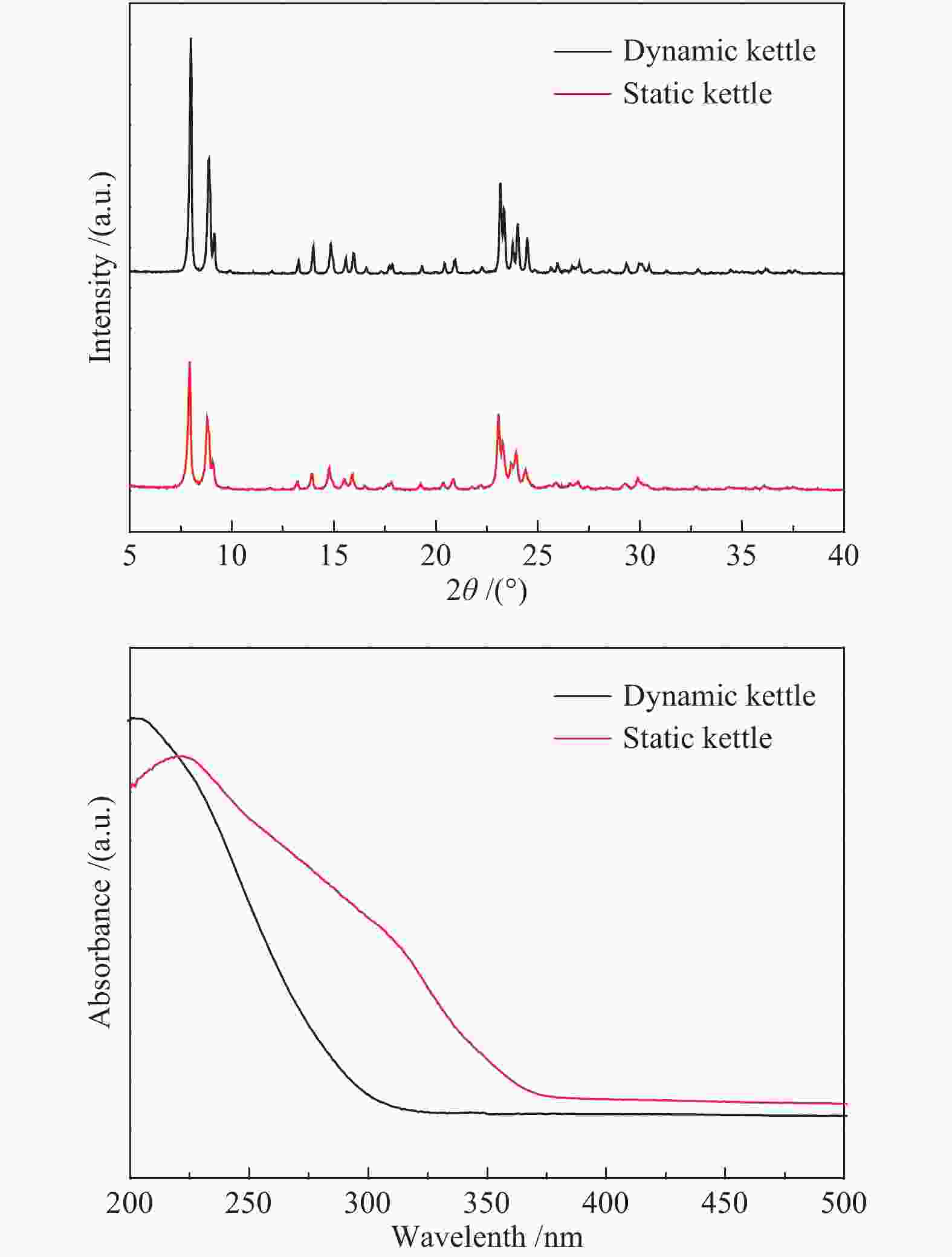
摘要: 本研究以四丙基氢氧化铵(TPAOH)为模板剂,正硅酸乙酯(TEOS)为硅源,钛酸四丁酯(TBOT)为钛源,在动态晶化釜内合成形貌规则且粒径约为600 nm的TS-1分子筛。通过改变TBOT的用量,进而改变初始合成液中的硅钛比,考察硅钛比对TS-1分子筛的影响,并采用SEM、TEM、XRD、FT-IR、UV-vis、XPS、N2吸附-脱附技术对制得的催化剂进行表征。将合成得到的TS-1分子筛应用于以噻吩正辛烷为模拟油的体系中,考察TS-1分子筛催化氧化脱硫性能。Abstract: Using TPAOH as the template, TEOS as the silicon source and TBOT as the titanium source, the as-prepared samples utilizing a dynamic crystallizating kettle possessed regular morphology and the average particle size of 600 nm. By modulating the dosage of TBOT, and then changing the molar ratio of titanium to silicon in the initial synthesis solution, the effect of silicon to titanium ratio on TS-1 molecular sieve was investigated. TS-1 zeolite properties were characterized by means of SEM, TEM, XRD, FT-IR, UV-vis, XPS, N2 adsorption and desorption technology. Finally, using a simulated system of thiophene n-octane dissolved in octane, the catalytic oxidative desulfurization performance of TS-1 zeolite was investigated.
-
表 1 合成TS-1分子筛初始合成液组成
Table 1 Initial gel composition of TS-1 synthesis
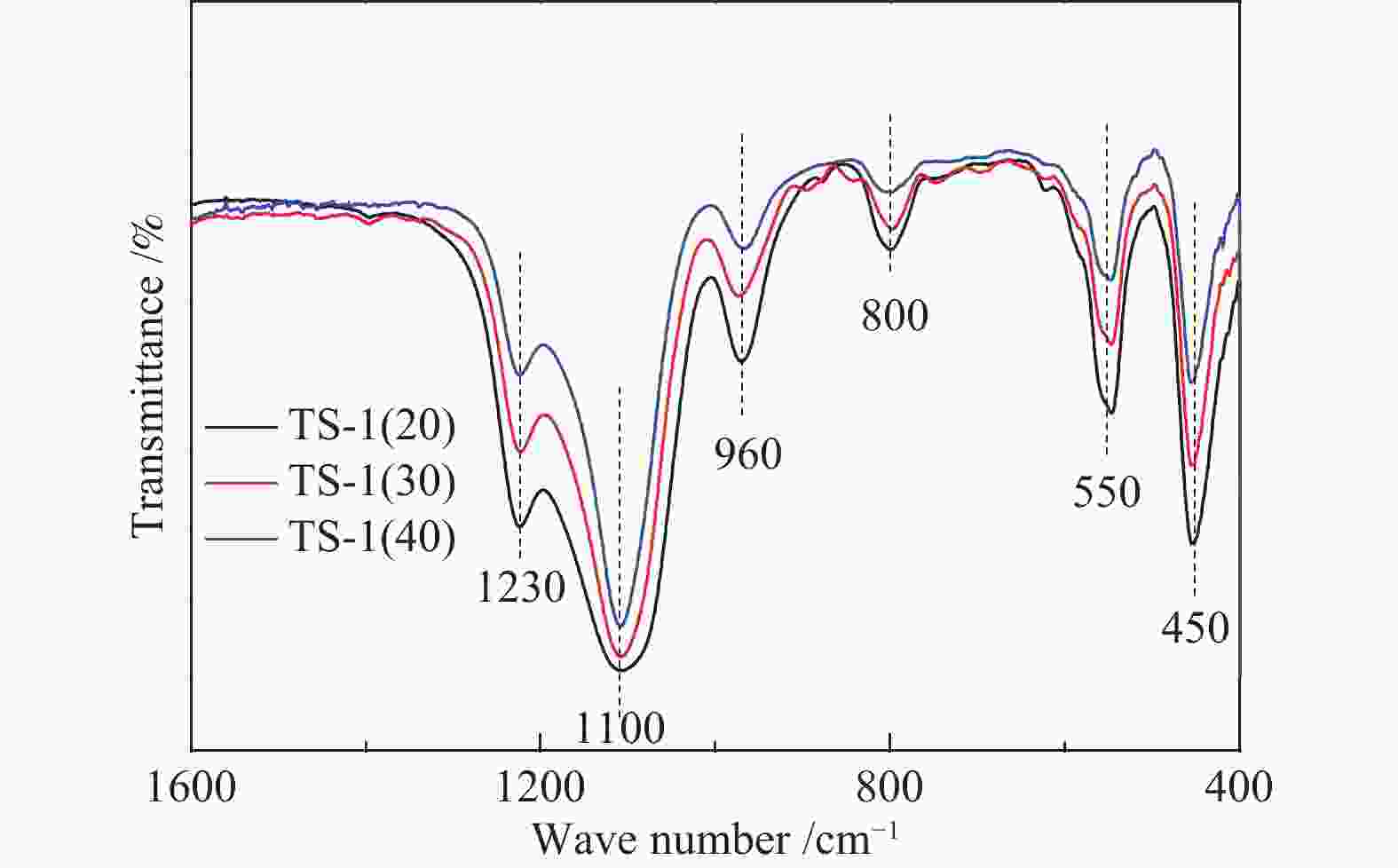
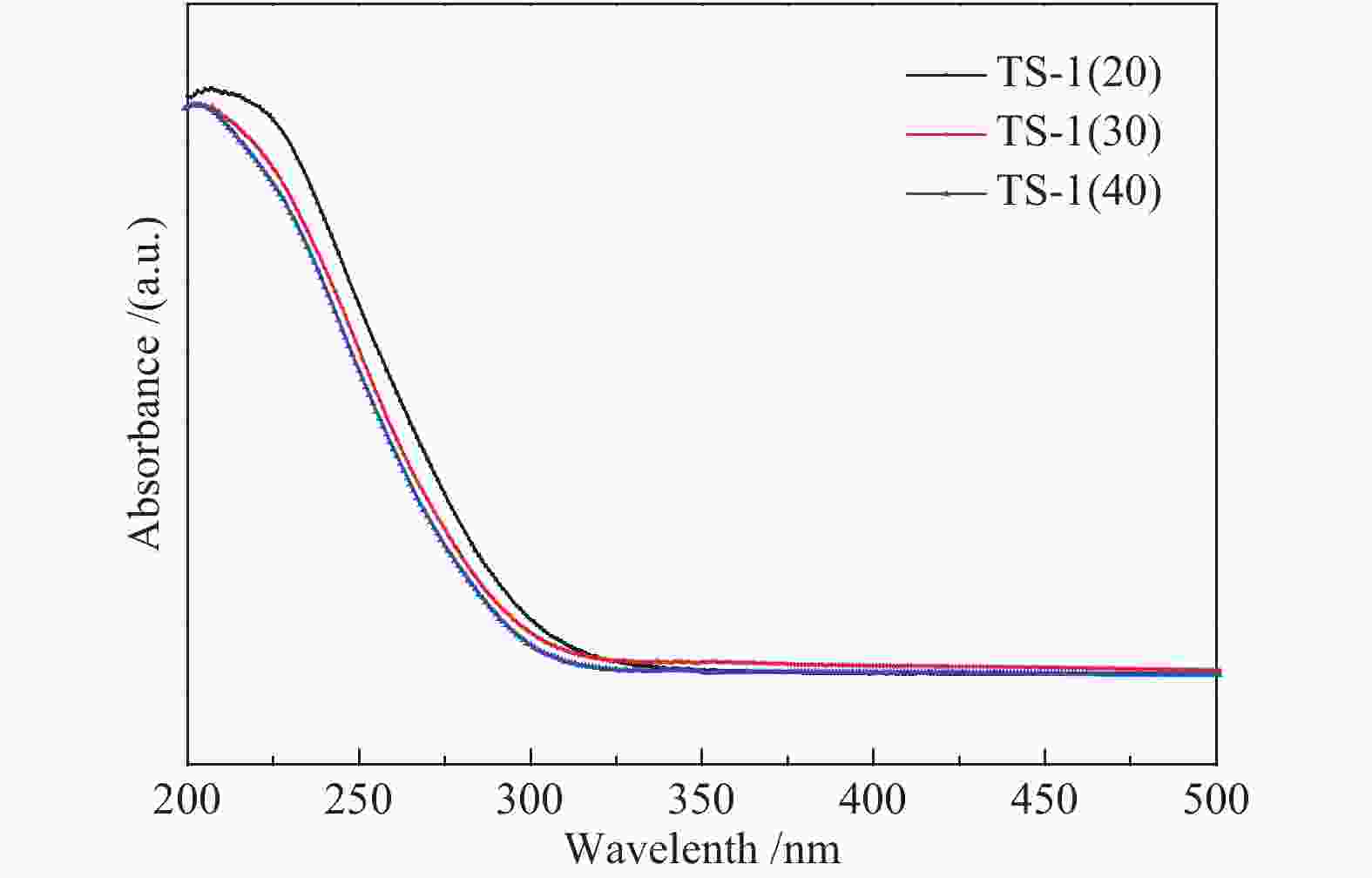
Sample Initial gel composition TS-1(20) 1.0SiO2∶0.050TiO2∶0.3TPAOH∶50H2O TS-1(30) 1.0SiO2∶0.033TiO2∶0.3TPAOH∶50H2O TS-1(40) 1.0SiO2∶0.025TiO2∶0.3TPAOH∶50H2O 表 2 不同硅钛比TS-1体相的元素原子百分比
Table 2 Elements atomic percentage of overall TS-1 samples with different ratios of silicon to titanium
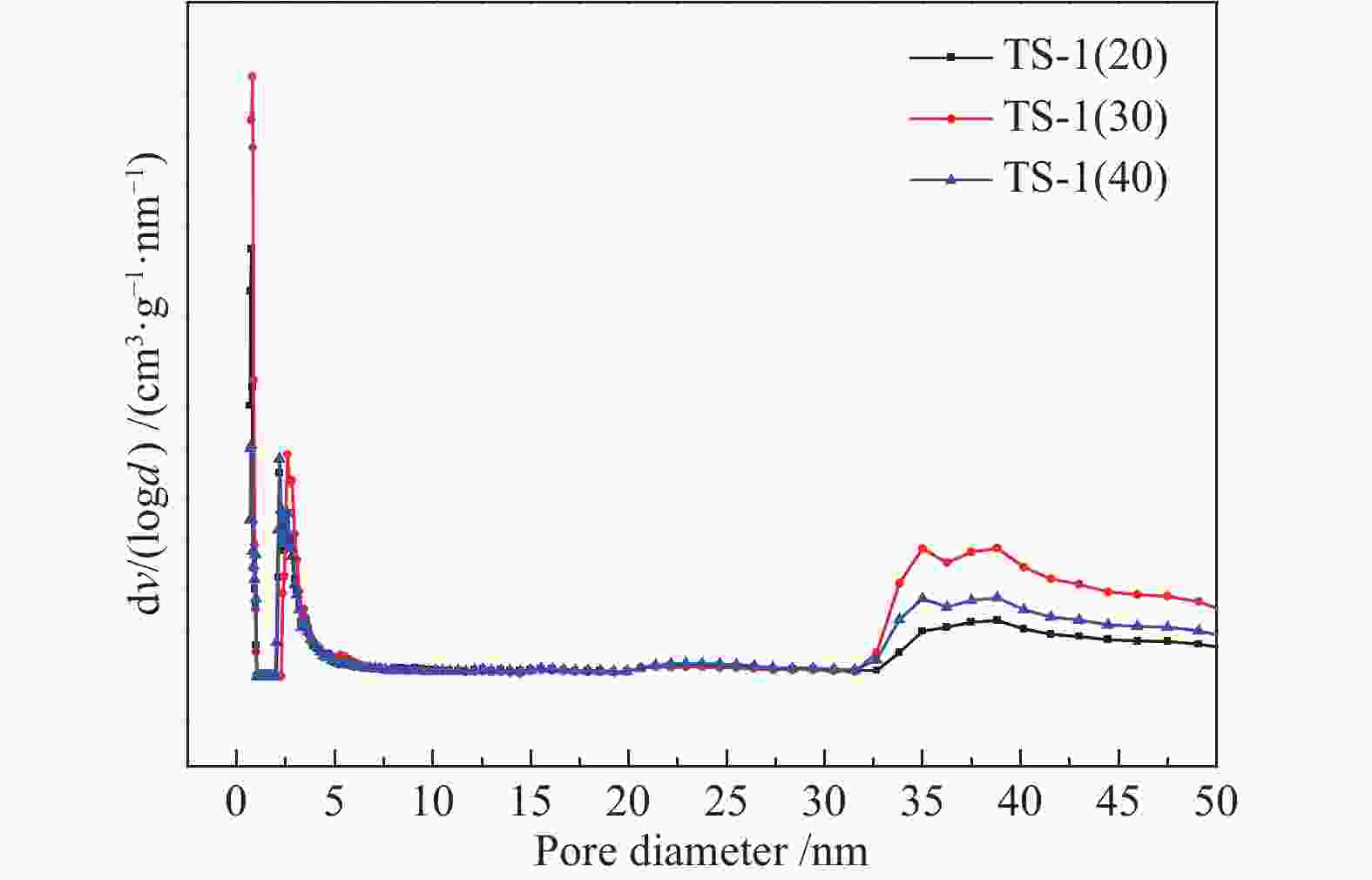
Sample Element atomic percentage/% TS-1(20) 62.52 36.78 0.69 TS-1(30) 60.01 39.26 0.73 TS-1(40) 60.58 38.95 0.47 表 3 不同硅钛比TS-1的孔结构性质
Table 3 Pore structure properties of TS-1 samples with different ratios of silicon to titanium
Sample St/
(m2·g−1)Sm/
(m2·g−1)dp/
nmvm/
(cm3·g−1)vt/
(cm3·g−1)TS-1(20) 512.4 483.6 2.154 0.21 0.28 TS-1(30) 513.9 483.4 2.275 0.21 0.29 TS-1(40) 464.0 418.6 2.168 0.18 0.25 表 4 不同物质光电能谱峰处结合能
Table 4 Binding energy of different samples
Sample Ti 2p3/2 E/eV α β TS-1 460.0 ± 0.2 457.9 ± 0.2 TiO2 + SiO2 − 458.0 TiO2 − 458.8 α represents the binding energy at the spectral peak of framework titanium; β represents the binding energy at the spectral peak of non-framework titanium (TiO2) 表 5 不同硅钛比TS-1表相的元素原子百分比
Table 5 Elements atomic percentage of surface TS-1 samples with different ratios of silicon to titanium
Sample Element atomic percentage/% TS-1(20) 60.17 29.91 0.68 TS-1(30) 59.42 29.90 0.68 TS-1(40) 59.51 30.46 0.29 -
[1] TARAMASSO M, PEREGO G, NOTARI B. Preparation of porous crystalline synthetic; material comprised of silicon and titanium oxides: US, 4410501[P]. 1983-10-18. [2] CLERIOI M G, INGALLINA P. Epoxidation of lower olefins with hydrogen peroxide and titanium silicalite[J]. J Catal,1993,140(1):71−83. doi: 10.1006/jcat.1993.1069 [3] RATNASAMY P, SRINIVAS D, KNOZINGER H. Active sites and reactive intermediates in titanium slilicate molecular sieves[J]. Adv Catal,2004,48:164−169. [4] PEREGO C, CARATI A, INGALLINA P, MANTEGAZZA M A, BELLUSSI G. Production of titanium containing molecular sieves and their application in catalysis[J]. Appl Catal A: Gen,2001,221(1/2):63−72. doi: 10.1016/S0926-860X(01)00797-9 [5] 史春风, 朱斌, 林民. 空心钛硅分子筛在环己烷温和氧化反应中的催化性能[J]. 石油学报(石油加工),2014,30(5):792−797.SHI Chun-feng, ZHU Bin, LIN Min. Catalytic performance of hollow titanium silicalite in cyclohexane mild oxidation reaction[J]. Acta Pet Sin (Pet Process Sect),2014,30(5):792−797. [6] 史春风, 林民, 朱斌, 龙军. HPPO工艺中试装置中钛硅催化剂的失活及再生[J]. 石油学报(石油加工),2013,29(5):864−869.SHI Chun-feng, LIN Min, ZHU Bin, LONG Jun. Deactivation and regeneration of titanosilicate catalyst used in HPPO pilot process[J]. Acta Pet Sin (Pet Process Sect),2013,29(5):864−869. [7] 周琳琳. 钛硅分子筛(TS-1)的合成与表征[D]. 大连: 大连理工大学, 2015.ZHOU Lin-lin. The synthesis and characterization of TS-1[D]. Dalian: Dalian University of Technology, 2015. [8] BRECK D W, FLANIGEN E M. Molecular sieves[J]. J Soc Chem Ind London, 1968: 47. [9] GEORGE T K. Chemistry of crystalline aluminosilicates. I. Factors affecting the formation of zeolite A[J]. J Phys Chem,1966,70(4):1047−1050. [10] ZELIMIR G, NIELS B, ERIC G, DEROUANE. Synthesis and characterization of zsm-5 type zeolites: III. A critical evaluation of the role of alkali and ammonium cations[J]. Appl Catal,1981,5(2):227−248. [11] SONG C, MA X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization[J]. Appl Catal B: Environ,2003,41(1/2):207−238. doi: 10.1016/S0926-3373(02)00212-6 [12] OTSUKI S, NONAKA T, TAKASHIMA N, QIAN W, ISHIHAR A, IMAI T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels,2000,14(6):1232−1239. [13] TE M, FAIRBRIDGE C, RING Z. Oxidation reactivities of dibenzothiophenes inpolyoxometalate/H2O2 and formic acid/H2O2 systems[J]. Appl Catal A: Gen,2001,219(1/2):267−280. [14] 孙刚, 刘瑞婷, 朱根权, 项玉芝, 夏道宏. 正交设计法选择最佳氧化抽提脱硫条件[J]. 石油化工高等学校学报,2001,14(4):31−34, 39.SUN Gang, LIU Rui-ting, ZHU Gen-quan, XIANG Yu-zhi, XIA Dao-hong. Orthogonal design method to select the best oxidative extraction desulfurization conditions[J]. J Petrochen Univ,2001,14(4):31−34, 39. [15] SHIRAISHI Y, TACHIBANA K, HIRAI T, KOMASAWA I. Desulfurization and denitrogenation process for lightoils based on chemical oxidation followed by liquid-liquid extraction[J]. Ind Eng Chem Res,2002,41(17):4362−4375. doi: 10.1021/ie010618x [16] CORE W, BONDE S E, DOLBEAR G E, SKOV E R. Method of desulfurization and dearomatization of petroleum liquids by oxidation and solvent extraction: US, 6596914[P]. 2003-07-22. [17] SHIRAISHI Y, KOMASAWA I, HIRAI T. TiO2-mediated photocatalytic desulfurization process for light oils using an organic two-phase system[J]. J Chem Eng Jpn,2002,35(5):489−492. doi: 10.1252/jcej.35.489 [18] REDDY R S, REDDY J S, KUMAR R, KUMAR P. Sulfoxidation of thioethers using titanium silicate molecular sieve catalyst[J]. J Chem Soc Chem Commun,1992,(1):84−85. [19] HULEA V, FAJULA F, BOUSQUET J. Mildoxidation with H2O2 over Ti-containing molecular sieves a very efficient method for removing aromatic sulfur compounds from fuels[J]. J Catal,2001,198(2):179−186. doi: 10.1006/jcat.2000.3149 [20] KONG L Y, LI G, WANG X S. Kinetics and mechanism of liquid-phase oxidation of thiophene over TS-1 using H2O2 under mildconditions[J]. Catal Lett,2004,92(3/4):l63−167. [21] KONG L Y, LI G, WANG X S. Thiophene oxidation over titanium silicalite using hydrogen peroxide[J]. Chin J Catal,2004,25(2):89−90. [22] KONG L Y, LI G, WANG X S. Mild oxidation of thiophene over TS-l/H2O2[J]. Catal Today,2004,93−95:341−345. doi: 10.1016/j.cattod.2004.06.016 [23] 孔令艳, 李钢, 王祥生, 王云. TS-1/过氧化氢催化氧化体系中有机硫化物的选择氧化[J]. 催化学报,2004,25(10):775−778. doi: 10.3321/j.issn:0253-9837.2004.10.003KONG Ling-yan, LI Gang, WANG Xiang-sheng, WANG Yun. Selective oxidation of organic sulfur compounds over TS-1/H2O2[J]. J Catal,2004,25(10):775−778. doi: 10.3321/j.issn:0253-9837.2004.10.003 [24] SHAKERI M. Efficient synthesis of titaniosilicalite-1 zeolite nanoparticles under solvent-free conditions for the oxidation of dibenzothiophene: impact of silica precursor[J]. ChemistrySelect,2019,4(25):7566−7571. [25] PRAKASH A M, KEVAN L. Reducibility and adsorbate interactions of Ti in titanosilicate molecular sieve TS-1[J]. J Catal,1998,178(2):586−597. doi: 10.1006/jcat.1998.2180 [26] VAYSSILOV G N. Structural and physicochemical features of titanium silicalities[J]. Catal Rev,1997,39(3):209−251. doi: 10.1080/01614949709353777 [27] 杜起. 分级孔TS-1沸石分子筛的合成及其氧化脱硫性能的研究[D]. 上海: 上海交通大学, 2018.DU Qi. Synthesis of hierarchically porous TS-1 zeolites and their catalytic properties in oxidative desulfurization[D]. Shanghai: Shanghai Jiao Tong University, 2018. [28] GEOBALDO F, BORDIGA S, ZECCHINA A, GIAMELLO E, PETRINI G. DRS UV-vis and EPR spectroscopy of hydroperoxo and superoxo complexes in titanium silicalite[J]. Catal Lett,1992,16(1/2):109−115. [29] LV Q, LI G, SUN H. Synthesis of hierarchical TS-1 with convenient separation and the application for the oxidative desulfurization of bulky and small reactants[J]. Fuel,2014,130:70−75. [30] ZHANG T, ZUO Y, LIU M, SONG C S, GUO X W. Synthesis of titanium silicalite-1 with high catalytic performance for 1-butene epoxidation by eliminating the extraframework ti[J]. ACS Omega,2016,1(5):1034−1040. doi: 10.1021/acsomega.6b00266 [31] VETTER S, S SCHULA-EKLOFF G, KULAWIK K. On the para/ortho product ratio of phenol and anisole hydroxylation over titanium silicalite-1[J]. Chem Eng Techonol,1994,17(5):348−353. [32] HASEGAWA Y, AYAME A. Investigation of oxidation states of titanium in titanium silicalite-1 by X-ray photoelectron spectroscopy[J]. Catal Today,2001,71(1):177−187. [33] MILLINI R, MASSARAAND E P, PEREGOET G, BELLUSSI G J. Framework composition of titanium silicalite-1[J]. J Catal,1992,137:497. doi: 10.1016/0021-9517(92)90176-I -




 下载:
下载: