Microwave synthesis of ZIF-67 derived nickel-cobalt hydroxide and its electrochemical performance
-
摘要: 采用微波辅助加热法,以类沸石二甲基咪唑钴(ZIF-67)为模板和钴源,快速制备了三维中空结构的镍钴氢氧化物(Ni-Co LDH)。通过X射线衍射仪(XRD)、扫描电子显微镜(SEM)、X射线光电子能谱仪(XPS)、透射电子显微镜(TEM)和比表面积及孔径分析仪(BET)探究了微波反应时间对材料形貌、结构的影响;通过循环伏安法(CV)、恒电流充放电(GCD)曲线和电化学阻抗谱(EIS)分析了材料的电化学性能。结果显示,Ni-Co LDH-15 min电极材料的电化学性能最优:在0.5 A /g时,比电容高达2371.0 F/g;电流密度扩大20倍,材料具有较好的倍率性能(78.5%)。以镍钴氢氧化物为正极,活性炭为负极组装成非对称式超级电容器,在功率密度为448.05 W/kg时,能量密度高达19.17 W·h/kg,且循环5000圈后电容保持率高达88.7%,表明镍钴氢氧化物是一种具有优异电化学性能和实际应用潜力的超级电容器电极材料。Abstract: Microwave-assisted heating method was used to rapidly prepare three-dimensional hollow nickel-cobalt hydroxide (Ni-Co LDH) by using zeolite dimethyl imidazolium cobalt (ZIF-67) as template and cobalt source. The effect of microwave reaction time on the morphology and electrochemical properties of the materials was investigated. X-ray diffraction (XRD), scanning electron microscope (SEM), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and specific surface and aperture analyzer (BET) were used to investigate the effect of microwave reaction time on the structure and morphology of the samples. The electrochemical properties of Ni-Co LDH electrode materials were analyzed by cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS). The results showed that the electrochemical properties of Ni-Co LDH-15 min electrode were the best. The specific capacitance was 2371.0 F/g at 0.5 A/g. The Ni-Co LDH-15 min also possessed excellent capacity retention of 78.5% when the current density increased by 20 times. An asymmetric supercapacitor (Ni-Co LDH//AC) was assembled by using Ni-Co LDH as the positive electrode and AC as the negative electrode. The Ni-Co LDH//AC device delivered a high energy density of 19.17 W·h/kg at the power density of 448.05 W/kg. Furthermore, the capacitance retention rate still maintained 88.7% after 5000 cycles. These results showed that Ni-Co LDH was a kind of electrode material for supercapacitor with excellent electrochemical performance and practical application potential.
-
图 2 (a)ZIF-67,(b)Ni-Co LDH-5 min,(c)Ni-Co LDH-10 min,(d)Ni-Co LDH-15 min,(e)Ni-Co LDH-15 min,(f)Ni-Co LDH-20 min,(g)Ni-Co LDH-20 min,(h)Ni-Co LDH-30 min和(i)Ni-Co LDH-35 min的 SEM照片
Figure 2 SEM images of (a) ZIF-67, (b) Ni-Co LDH-5 min, (c) Ni-Co LDH-10 min, (d) Ni-Co LDH-15 min, (e) Ni-Co LDH-15 min, (f) Ni-Co LDH-20 min, (g) Ni-Co LDH-20 min, (h) Ni-Co LDH-30 min and (i) Ni-Co LDH-35 min
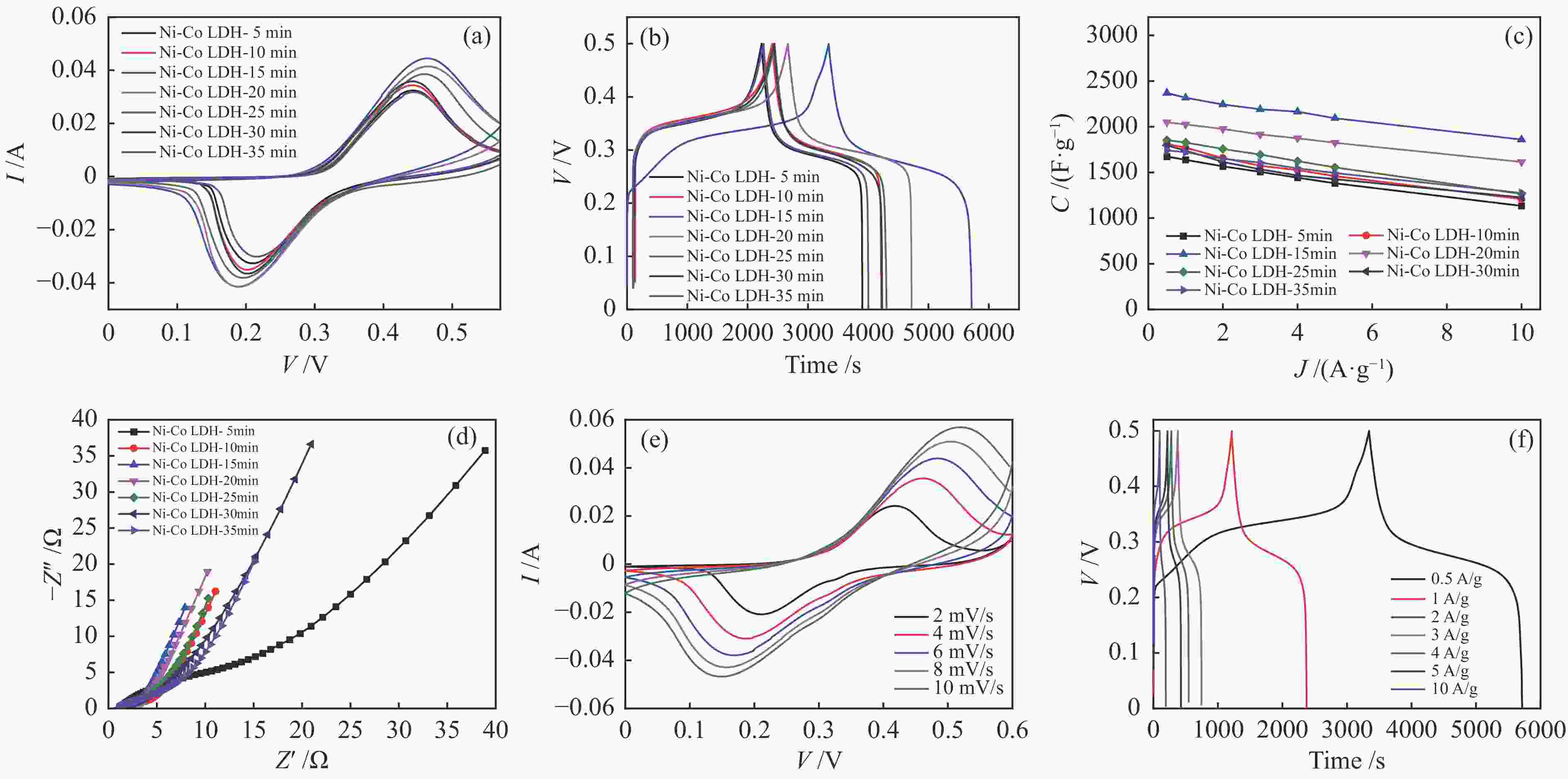
图 6 所有镍钴氢氧化物样品的(a)CV曲线(10 mV/s),(b)GCD曲线(0.5 A/g),(c)不同电流密度下的比电容关系,(d)EIS谱图,Ni-Co LDH-15 min的(e)CV曲线和(f)GCD曲线
Figure 6 CV curves (10 mV/s), (b) GCD curves (0.5 A/g), (c) specific capacity versus at different current densities, (d) EIS spectra of all Ni-Co LDH samples, (e) CV curves and (f) GCD curves of Ni-Co LDH-15 min
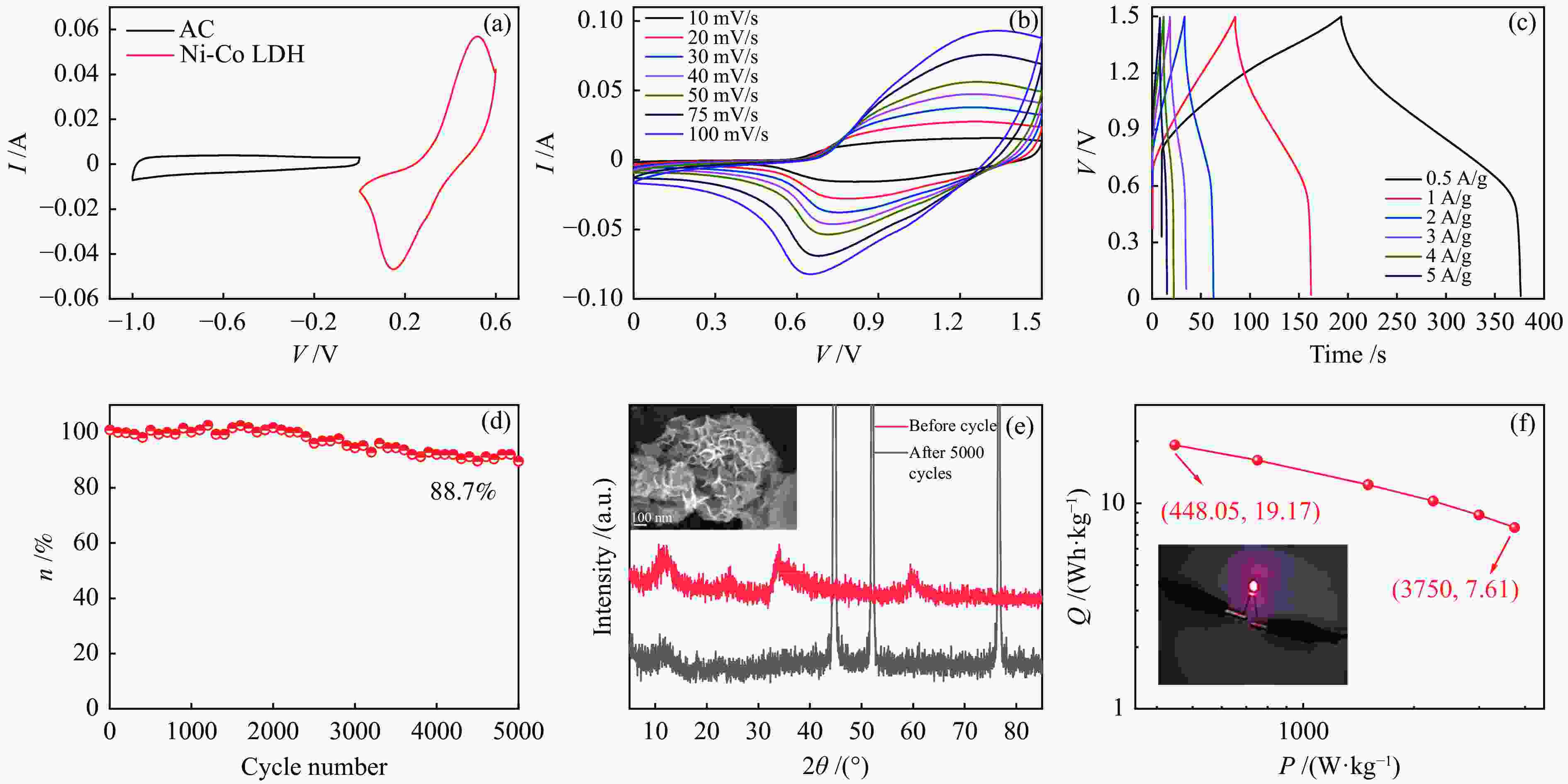
图 7 (a)活性炭和镍钴氢氧化物电极在10 mV/s下的CV曲线,(b)Ni-Co LDH//AC器件在不同扫速下的CV曲线,(c)Ni-Co LDH//AC器件在不同电流密度下的GCD曲线,(d)Ni-Co LDH//AC器件的循环稳定性,(e)Ni-Co LDH//AC器件循环5000圈前后的XRD谱图和SEM照片,(f)Ni-Co LDH//AC器件的拉贡图
Figure 7 (a) CV curves of AC and Ni-Co LDH electrode at 10 mV/s, (b) CV curves of Ni-Co LDH//AC at different scan rates, (c) GCD curves of Ni-Co LDH//AC at different current density, (d) cycling stability of Ni-Co LDH//AC, (e) before and after 5000 cycles of XRD patterns and SEM image, (f) Ragone plot of Ni-Co LDH//AC
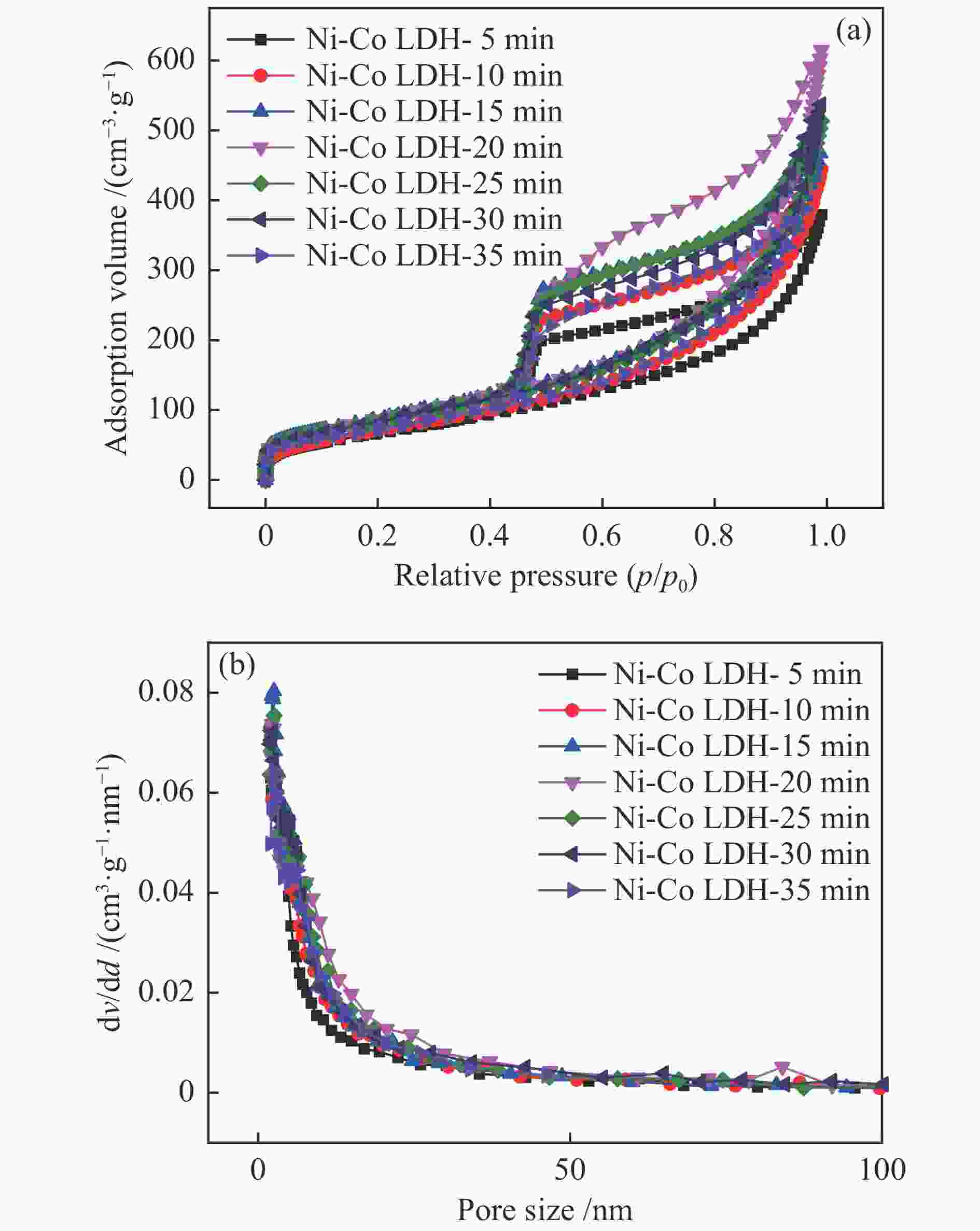
表 1 所有镍钴氢氧化物样品的BET比表面积和孔结构
Table 1 BET surface area and pore structure of all the Ni-Co LDH samples
Sample SBET/ (m2·g−1) Dave/nm vt/ (cm3·g−1) Ni-Co LDH-5 min 313 10.14 0.79 Ni-Co LDH-10 min 253 9.28 0.59 Ni-Co LDH-15 min 326 8.87 0.72 Ni-Co LDH-20 min 273 10.06 0.69 Ni-Co LDH-25 min 305 10.90 0.83 Ni-Co LDH-30 min 318 11.94 0.95 Ni-Co LDH-35 min 273 8.79 0.60 -
[1] LUO L, ZHOU Y L, YAN W, WU X, WANG S R, ZHAO W G. Two-step synthesis of B and N co-doped porous carbon composites by microwave-assisted hydrothermal and pyrolysis process for supercapacitor application[J]. Electrochim Acta,2020,360:137010. doi: 10.1016/j.electacta.2020.137010 [2] MA J M, XIA J L, LIANG Z, DU Y P, YAN C H. Layered double hydroxide hollowcages with adjustable layer spacing for high performance hybrid supercapacitor[J]. Small,2021,17(49):2104423. [3] MEMON J, SUN J H, MENG D L, QUYANG W Z, MEMON M A, HUANG Y, YAN S K, GENG J X. Synthesis of graphene/Ni-Al layered double hydroxide nanowires and their application as an electrode material for supercapacitors[J]. J Mater Chem,2014,2:50605067. [4] WAN H Z, LI L, XU Y, TAN Q Y, LIU X, ZHANG J, WANG H B, WANG H. Three-dimensional cotton-like nickel nanowire@Ni-Co hydroxide nanosheet arrays as binder-free electrode for high-performance asymmetric supercapacitor[J]. Nanotechnology,2018,29:194003. doi: 10.1088/1361-6528/aab129 [5] LI J, WEI M, CHU W, WANG N. High-stable α-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials[J]. Chem Eng J,2017,316:277−287. doi: 10.1016/j.cej.2017.01.057 [6] DONG T, ZHANG X, LI M, WANG P, YANG P. Hierarchical flower-like Ni-Co layered double hydroxide nanostructures: synthesis and super performance[J]. Inorg Chem Front,2018,5:3033−3041. doi: 10.1039/C8QI00931G [7] WANG Q H, GAO F, XU B Y, CAI F X, ZHAN F P, GAO F, WANG Q X. ZIF-67 derived amorphous CoNi2S4 nanocages with nanosheet arrays on the shell for a high-performance asymmetric supercapacitor[J]. Chem Eng J,2017,327:387−396. doi: 10.1016/j.cej.2017.06.124 [8] ZHONG G H, LIU D X, ZHANG J Y. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts[J]. J Mater Chem A,2018,6:1887−1899. doi: 10.1039/C7TA08268A [9] LIU Y X, WANG Y Z, SHI C J, CHEN Y J, LI D, HE Z F, WANG C, GUO L, MA J M. Co-ZIF derived porous NiCo-LDH nanosheets/N doped carbon foam for high-performance supercapacitor[J]. Carbon,2020,165:129−138. doi: 10.1016/j.carbon.2020.04.084 [10] YAN T, LI R Y, LI Z J. Nickel-cobalt layered double hydroxide ultrathin nanoflakes decorated on graphene sheets with a 3D nanonetwork structure as supercapacitive materials[J]. Mater Res Bull,2014,51:97−104. doi: 10.1016/j.materresbull.2013.11.044 [11] TANG J, SALUNKHE R R, LIU J, TORAD N L, IMURA M, FURUKAWA S, YAMAUCHI Y. Thermal conversion of core-shell metal-organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon[J]. J Am Chem Soc,2015,137:1572−1580. [12] QIN Q Q, OU D W, YE ChangjingY, CHEN L X, LAN B B, YAN J, WU Y C. Systematic study on hybrid supercapacitor of Ni-Co layered double hydroxide//activated carbons[J]. Electrochim Acta,2019,305:403−415. [13] ZHANG X D, YUE L C, ZHANG S G, FENG Y, AN L L, WANG M, MI J. Nickel-doped cobalt molybdate nanorods with excellent cycle stability for aqueous asymmetric supercapacitor[J]. Int J Hydrogen Energy,2020,45:8853−8865. doi: 10.1016/j.ijhydene.2020.01.127 [14] WANG M, AN L L, WU M M, ZHANG S G, FENG Y, ZHANG X D, MI J. Self-template synthesis of nickel cobalt sulfide hollow nanotubes for high-performance battery-type supercapacitors[J]. J Electrochem Soc,2021,168:060510. doi: 10.1149/1945-7111/ac0458 [15] WANG X H, HUANG F F, RONG F, HE P, QUE R H, JIANG S P. Unique MOF-derived hierarchical MnO2 nanotubes@NiCo-LDH/CoS2 nanocage materials as high performance supercapacitors[J]. J Mater Chem A,2019,7:12018−12028. doi: 10.1039/C9TA01951K [16] GUAN X H, HUANG M H, YANG L, WANG G S, GUAN X. Facial design and synthesis of CoSx/Ni-Co LDH nanocages with rhombic dodecahedral structure for high-performance asymmetric supercapacitors[J]. Chem Eng J,2019,372:151−162. doi: 10.1016/j.cej.2019.04.145 [17] YUE L C, ZHANG S G, ZHAO H Q, WANG M, MI J, FENG Y, WANG D F. Microwave-assisted one-pot synthesis of Fe2O3/CNTs composite as supercapacitor electrode materials[J]. J Alloy Compd,2018,765:1263−1266. doi: 10.1016/j.jallcom.2018.06.283 [18] ZHAO Y H, HE X Y, CHEN R R, LIU Q, LIU J Y, YU J, LI J Q, ZHANG H S, DONG H X, ZHANG M L, WANG J. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures[J]. Chem Eng J,2018,352:29−38. doi: 10.1016/j.cej.2018.06.181 -





 下载:
下载: