Preparation of Cu(I)NH4Y zeolite for adsorption and separation of ethylene and ethane
-
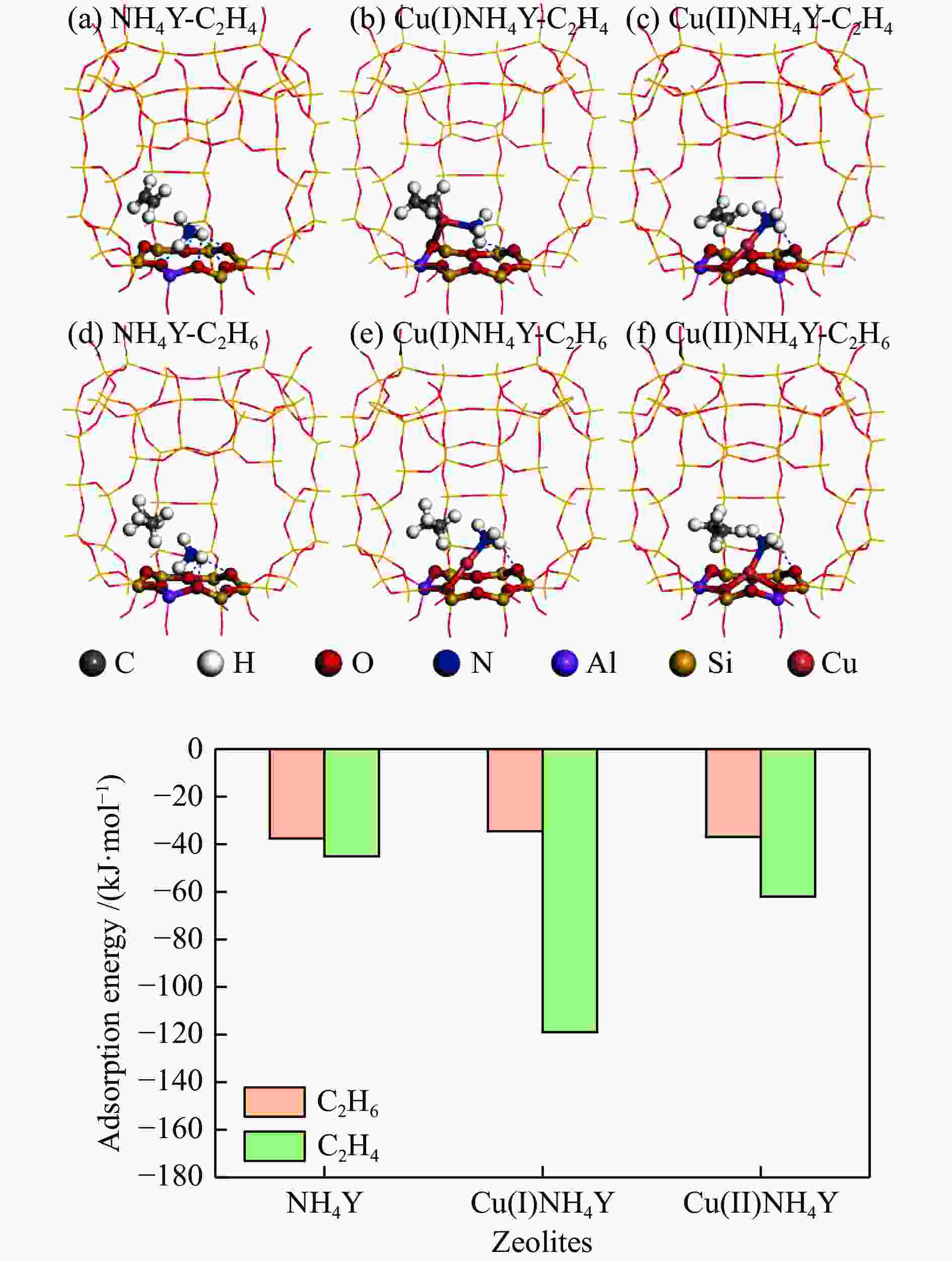
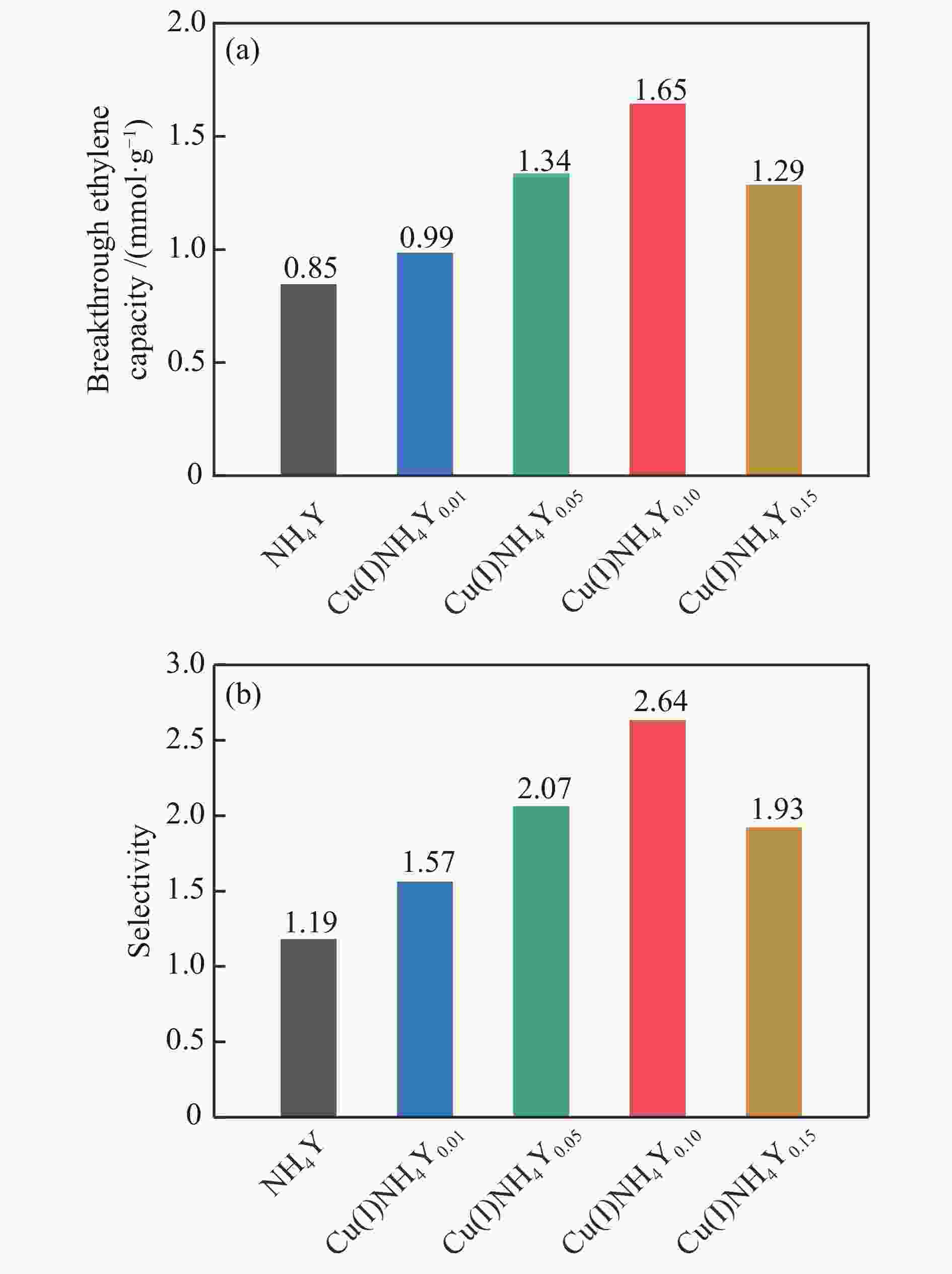
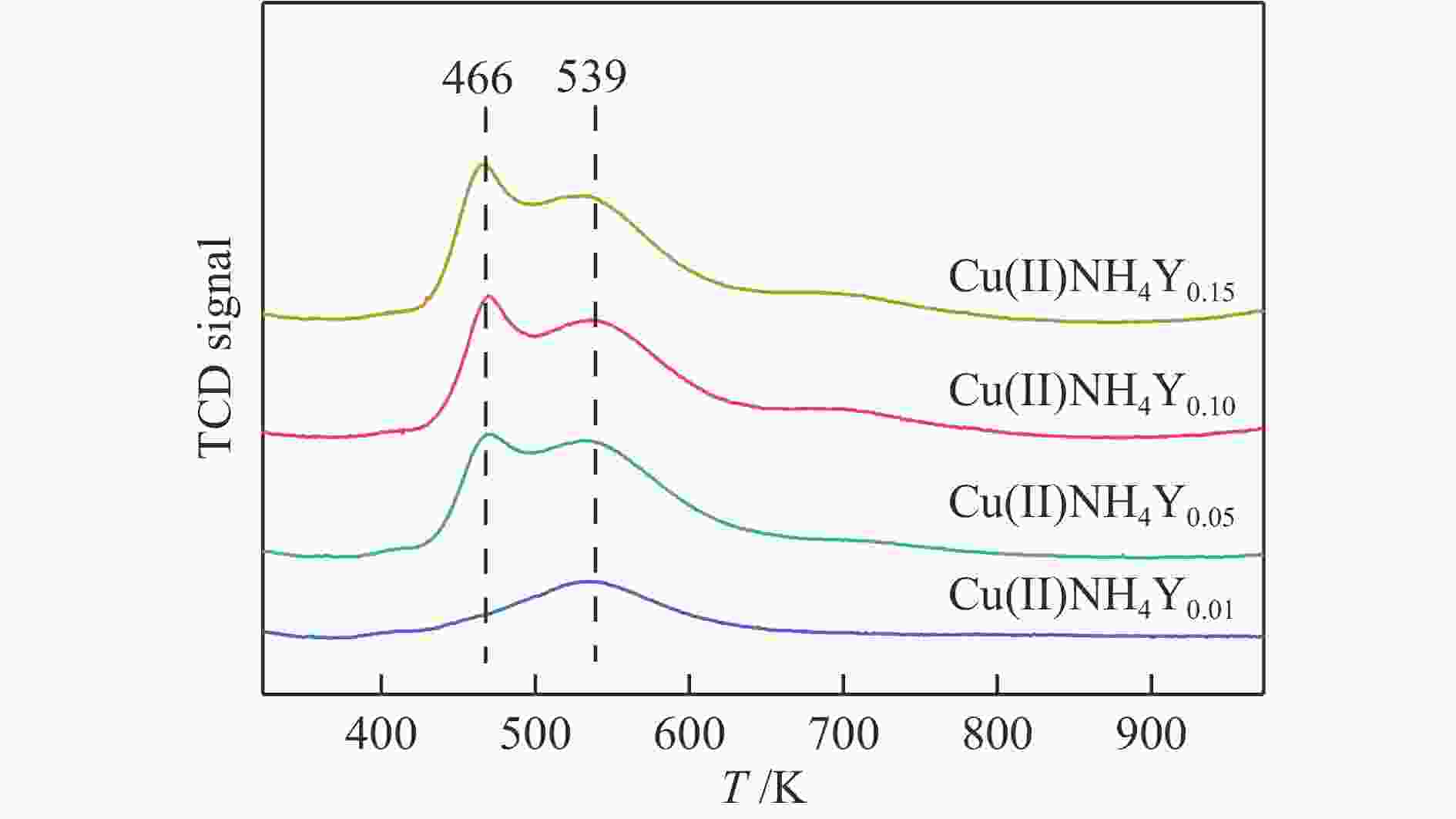
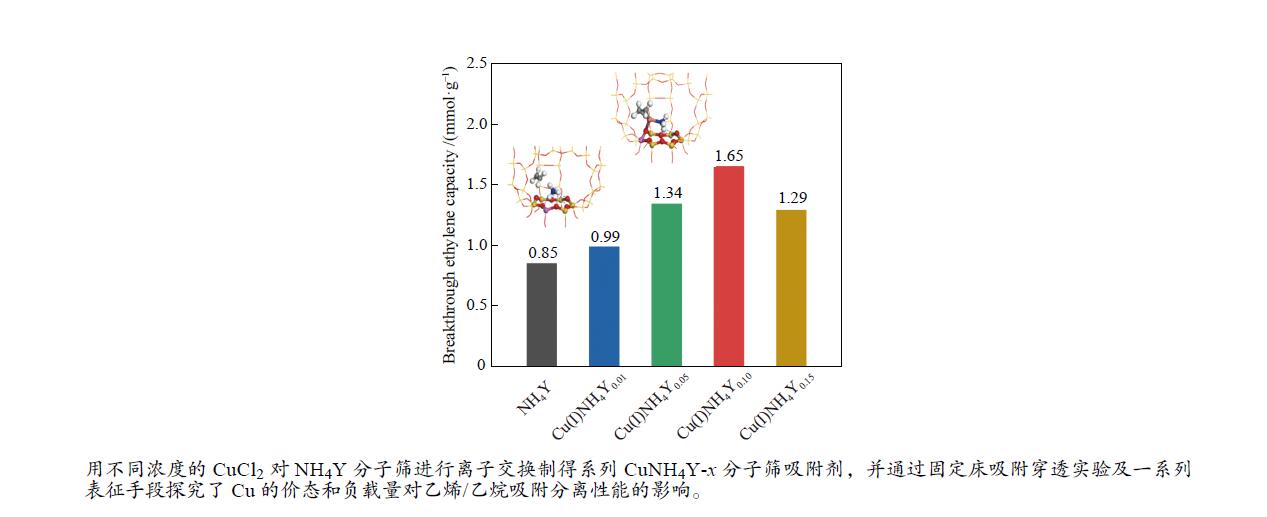
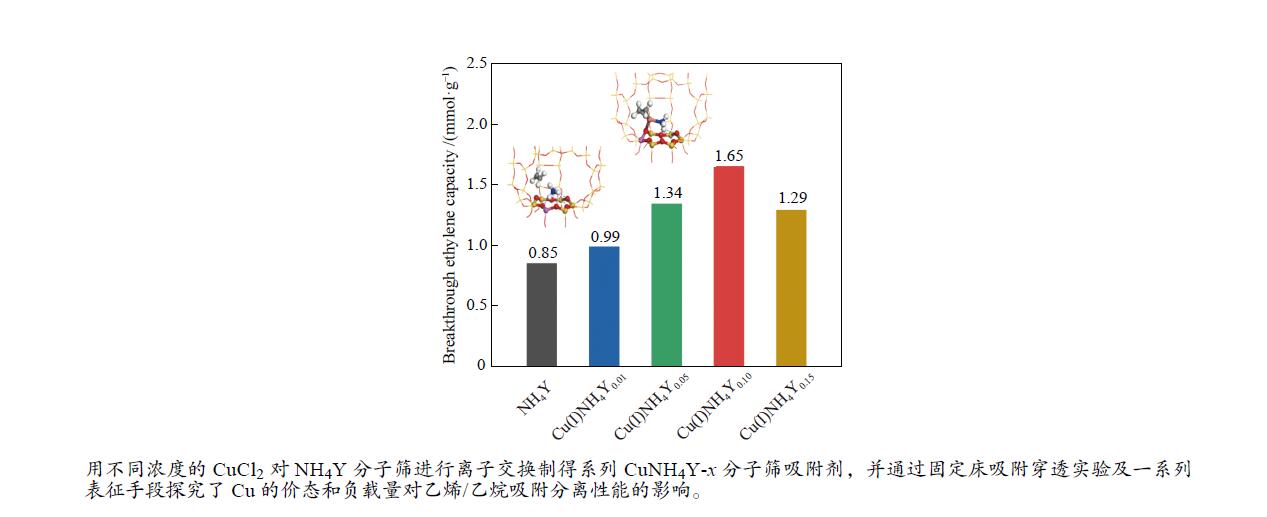
摘要: 本研究采用液相离子交换法,用不同浓度的CuCl2对NH4Y分子筛进行离子交换制得系列CuNH4Y-x分子筛吸附剂,并通过固定床吸附穿透实验及一系列表征手段探究了Cu的价态和负载量对乙烯/乙烷吸附分离性能的影响。吸附穿透实验结果表明,Cu(I)NH4Y0.10的乙烯吸附量明显高于Cu(II)NH4Y0.10,且Cu(I)NH4Y系列吸附剂的乙烯吸附量随着Cu负载量的增加呈现先增加后减小的趋势。H2-TPR和HRTEM表明,当Cu的负载量较低时,高度分散落位于Y分子筛超笼中的Cu(I)物种是乙烯有效吸附位点。然而,当Cu的负载量较高时,部分Cu物种发生团聚,造成对乙烯吸附能力的减弱。DFT密度泛函理论计算表明相比于Cu(II)NH4Y吸附剂,乙烯更容易吸附在Cu(I)NH4Y吸附剂上。该研究结果可为乙烯分离用高效Cu离子改性分子筛吸附剂的开发提供重要理论依据与指导。Abstract: In this work, a series of CuNH4Y-x zeolite adsorbents were prepared by ion exchange with different concentrations of CuCl2 and NH4Y zeolite. The effects of the valence and loading of Cu on the adsorption and separation of ethylene and ethane were investigated by fixed bed breakthrough adsorption experiment based on a series of characterization methods. The results of breakthrough adsorption experiments show that the ethylene adsorption capacity of Cu(I)NH4Y0.10 is significantly higher than that of Cu(II)NH4Y0.10, and the ethylene adsorption capacity of Cu(I)NH4Y series adsorbents increases first and then decreases with the increase of loading of Cu species. H2-TPR and HRTEM results show that monatomic Cu(I) species in the supercages of zeolite Y should be the effective active site when the loading amount of Cu is low. With the increase of Cu loading amount, the aggregation of Cu species results in the reduction of adsorption capacity of ethylene. The results of DFT calculation also confirm that the ethylene adsorption capacity of Cu(I)NH4Y adsorbent is significantly stronger than that of Cu(II)NH4Y adsorbent. These results can provide important theoretical basis and guidance for the development of high-efficiency Cu ion modified zeolite adsorbent for ethylene separation.
-
Key words:
- adsorption separation /
- ethylene /
- Cu(I)NH4Y zeolite /
- valence state of Cu /
- loading of Cu
-
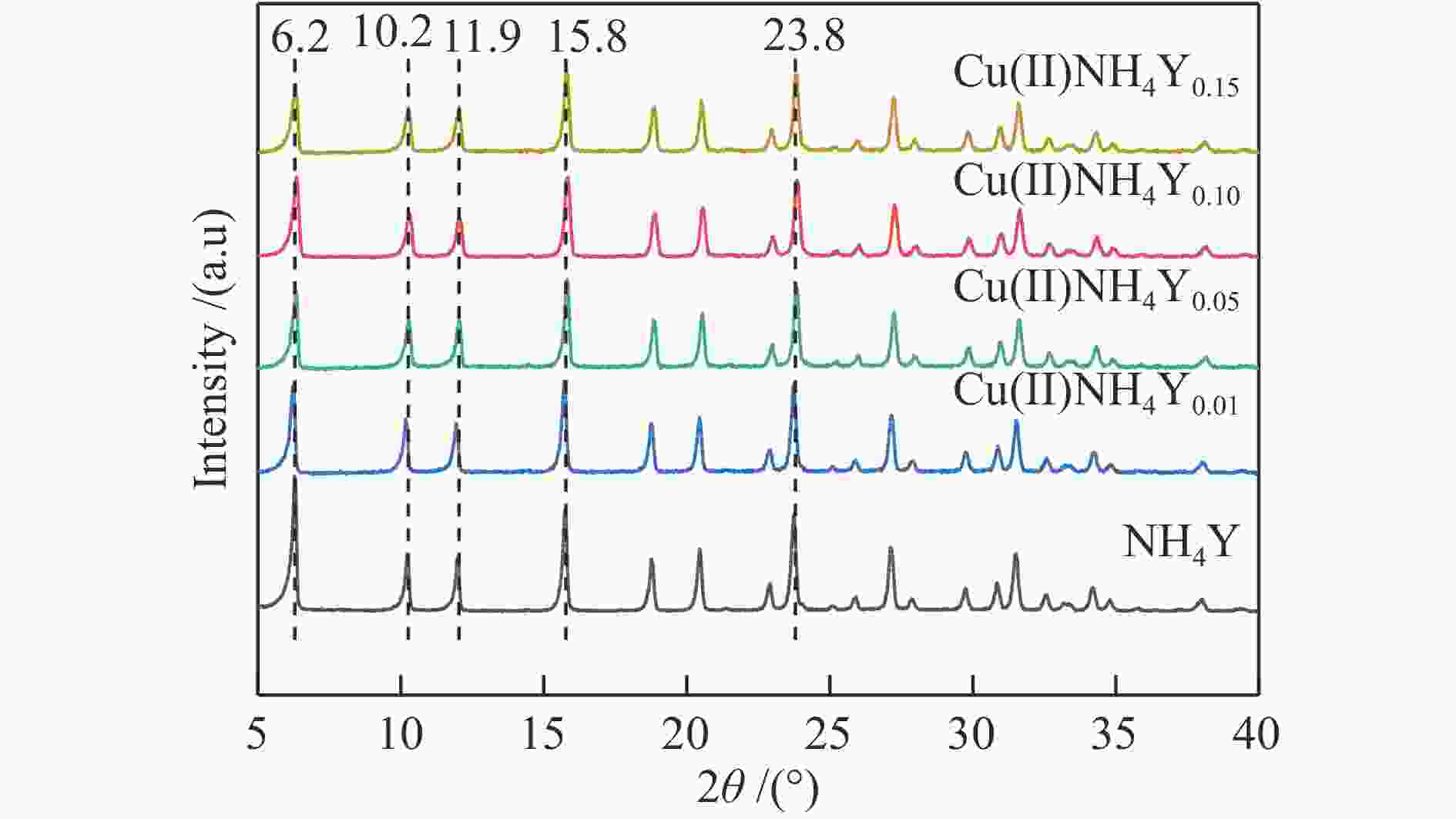
表 1 NH4Y和Cu(II)NH4Y分子筛的元素比及氨含量
Table 1 Element ratio and ammonia content of NH4Y and Cu(II)NH4Y zeolites
Sample Si/Al Cu/Al Ammonia content a /% NH4Y 2.69 0 100 Cu(II)NH4Y0.01 2.68 0.076 56.6 Cu(II)NH4Y0.05 2.67 0.165 17.5 Cu(II)NH4Y0.10 2.68 0.178 14.8 Cu(II)NH4Y0.15 2.67 0.191 11.4 a: ammonia content is measured by in situ infrared spectroscopy 表 2 Cu(II)NH4Y分子筛吸附剂的孔结构参数
Table 2 Pore structure parameters of Cu(II)NH4Y adsorbent
Sample $S _{\rm{BET}}^{\rm{a}}$ /
(m2·g−1)$S _{\rm{micro}}^{\rm{b}} $ /
(m2·g−1)$v _{\rm{micro} }^{\rm{c} }$ /
(cm3·g−1)$v _{\rm{meso} }^{\rm{d} }$ /
(cm3·g−1)NH4Y 485.6 436.5 0.21 0.08 Cu(II)NH4Y0.01 530.3 477.2 0.27 0.07 Cu(II)NH4Y0.05 537.4 486.4 0.28 0.06 Cu(II)NH4Y0.10 528.1 471.9 0.27 0.06 Cu(II)NH4Y0.15 532.7 479.5 0.27 0.07 a: BET surface area, b: micropore surface area, c: micropore pore volume, d: mesoporous pore volume -
[1] LI L B, LIN R B, KRISHNA R, LI H, XIANG S C, WU H, LI J P, ZHOU W, CHEN B L. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science,2018,362(6413):443−446. doi: 10.1126/science.aat0586 [2] AKAH A, WILLIAMS J, GHRAMI M. An overview of light olefins production via steam enhanced catalytic cracking[J]. Catal Tal Surv Asia,2019,23(4):265−276. doi: 10.1007/s10563-019-09280-6 [3] AMGHIZAR I, VANDEWALLE L A, VAN GEEM K M, GEEM V, MARIN G B. New trends in olefin production[J]. Engineering,2017,3(2):171−178. doi: 10.1016/J.ENG.2017.02.006 [4] ZHU H B, DONG H L, LAVEILLE P, SAIH Y, CAPS V, BASSET J M. Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene[J]. Catal Today,2014,228:58−64. doi: 10.1016/j.cattod.2013.11.061 [5] SHOLL D S, LIVELY R P. Seven chemical separations to change the world[J]. Nature,2016,532(7600):435−437. doi: 10.1038/532435a [6] LIU Y Z, WU Y, LIANG W W, PENG J J, LI Z, WANG H H, JANIK M J, XIAO J. Bimetallic ions regulate pore size and chemistry of zeolites for selective adsorption of ethylene from ethane[J]. Chem Eng Sci,2020,220:115636. doi: 10.1016/j.ces.2020.115636 [7] 崔希利, 邢华斌. 金属有机框架材料分离低碳烃的研究进展[J]. 化工学报,2018,69(6):2339−2352.CUI Xi-li, XING Hua-bin. Separation of light hydrocarbons with metal-organic frameworks[J]. CIESC J,2018,69(6):2339−2352. [8] 马士珍, 苏宝根, 鲍宗必, 苏云, 杨亦文, 任其龙. 干气中烷烃、烯烃新型分离吸附剂的研究进展[J]. 化工学报,2014,65(2):396−405. doi: 10.3969/j.issn.0438-1157.2014.02.005MA Shi-zhen, SU Bao-gen, BAO Zong-bi, SU Yun, YANG Yi-wen, REN Qi-long. Advances in new type adsorbent for separating alkene from dry gas[J]. CIESC J,2014,65(2):396−405. doi: 10.3969/j.issn.0438-1157.2014.02.005 [9] BANERJEE D, LIU J, THALLAPALLY P K. Separation of C2 hydrocarbons by porous materials: Metal organic frameworks as platform[J]. Comments Inorg Chem,2015,35(1):18−38. doi: 10.1080/02603594.2014.976704 [10] SAFARIK D J, ELDREDGE R B. Olefin/paraffin separations by reactive absorption: A review[J]. Ind Eng Chem Res,1998,37(7):2571−2581. doi: 10.1021/ie970897h [11] MIN J G, KEMP K C, HONG S B. Silver ZK-5 zeolites for selective ethylene/ethane separation[J]. Sep Purif Technol,2020,250:117146. doi: 10.1016/j.seppur.2020.117146 [12] SOLANKI V A, BORAH B. In-silico identification of adsorbent for separation of ethane/ethylene mixture[J]. J Mol Model,2021,64(4):666−672. [13] JIANG W J, SUN L B, YIN Y, SONG X L, LIU X Q. Ordered mesoporous carbon CMK-3 modified with Cu (I) for selective ethylene/ethane adsorption[J]. Sep Sci Technol,2013,48(6):968−976. doi: 10.1080/01496395.2012.712600 [14] CHEN L, LIU X Q. π-Complexation mesoporous adsorbents Cu-MCM-48 for ethylene-ethane separation[J]. Chin J Chem Eng,2008,16(4):570−574. doi: 10.1016/S1004-9541(08)60123-8 [15] GOLIPOUR H, MOKHTARANI B, MAFI M, MORADI A, GODINI H R. Experimental measurement for adsorption of ethylene and ethane gases on copper-exchanged zeolites 13X and 5A[J]. J Chem Eng Data,2020,65(8):3920−3932. doi: 10.1021/acs.jced.0c00251 [16] LI Y X, SHEN J X, PENG S S, ZHANG J K, WU J, LIU X Q, SUN L B. Enhancing oxidation resistance of Cu (I) by tailoring microenvironment in zeolites for efficient adsorptive desulfurization[J]. Nat Commun,2020,11(1):1−9. doi: 10.1038/s41467-019-13993-7 [17] 丁润东, 祖运, 周传行, 王焕, 莫周胜, 秦玉才, 孙兆林, 宋丽娟. CuNaY分子筛的有效吸附位与其脱硫性能的关联性研究[J]. 燃料化学学报,2018,46(4):451−458. doi: 10.3969/j.issn.0253-2409.2018.04.010DING Run-dong, ZU Yun, ZHOU Chuan-hang, WANG Huan, MO Zhou-sheng, QIN Yu-cai, SUN Zhao-lin, SONG Li-juan. Insight into the correlation between the effective adsorption sites and adsorption desulfurization performance of CuNaY zeolite[J]. J Fuel Chem Technol,2018,46(4):451−458. doi: 10.3969/j.issn.0253-2409.2018.04.010 [18] ZU Y, WANG S H, HUI Y, NI N, ZHANG X T, QIN Y C, ZHANG L, LIU H H, GAO X H, SONG L J. Facile fabrication of a superior Cu(I)-NH4Y zeolite adsorbent for improving thiophene adsorption selectivity in the presence of aromatics or olefins[J]. Chem Eng J,2020,401:126112. doi: 10.1016/j.cej.2020.126112 [19] ZU Y, GUO Z S, ZHENG J, HUI Y, WANG S H, QIN Y C, ZHANG L, LIU H H, GAO X H, SONG L J. Investigation of Cu(I)-Y zeolites with different Cu/Al ratios towards the ultra-deep adsorption desulfurization: Discrimination and role of the specific adsorption active sites[J]. Chem Eng J,2020,380:122319. doi: 10.1016/j.cej.2019.122319 [20] SUZUKI K, KATADA N, NIWA M. Detection and quantitative measurements of four kinds of OH in HY zeolite[J]. J Phys Chem C,2007,111(2):894−900. doi: 10.1021/jp065054v [21] SHY D S, CHEN S H, LIEVENS J, LIU S B, CHAO K J. Distribution of cations in lanthanum-exchanged NaY zeolites[J]. J Chem Soc, Faraday Transactions,1991,87(17):2855−2859. doi: 10.1039/ft9918702855 -




 下载:
下载: