Synthesis of sequential mesoporous HZSM-5 molecular sieve and its catalytic performance in methanol to aromatics
-
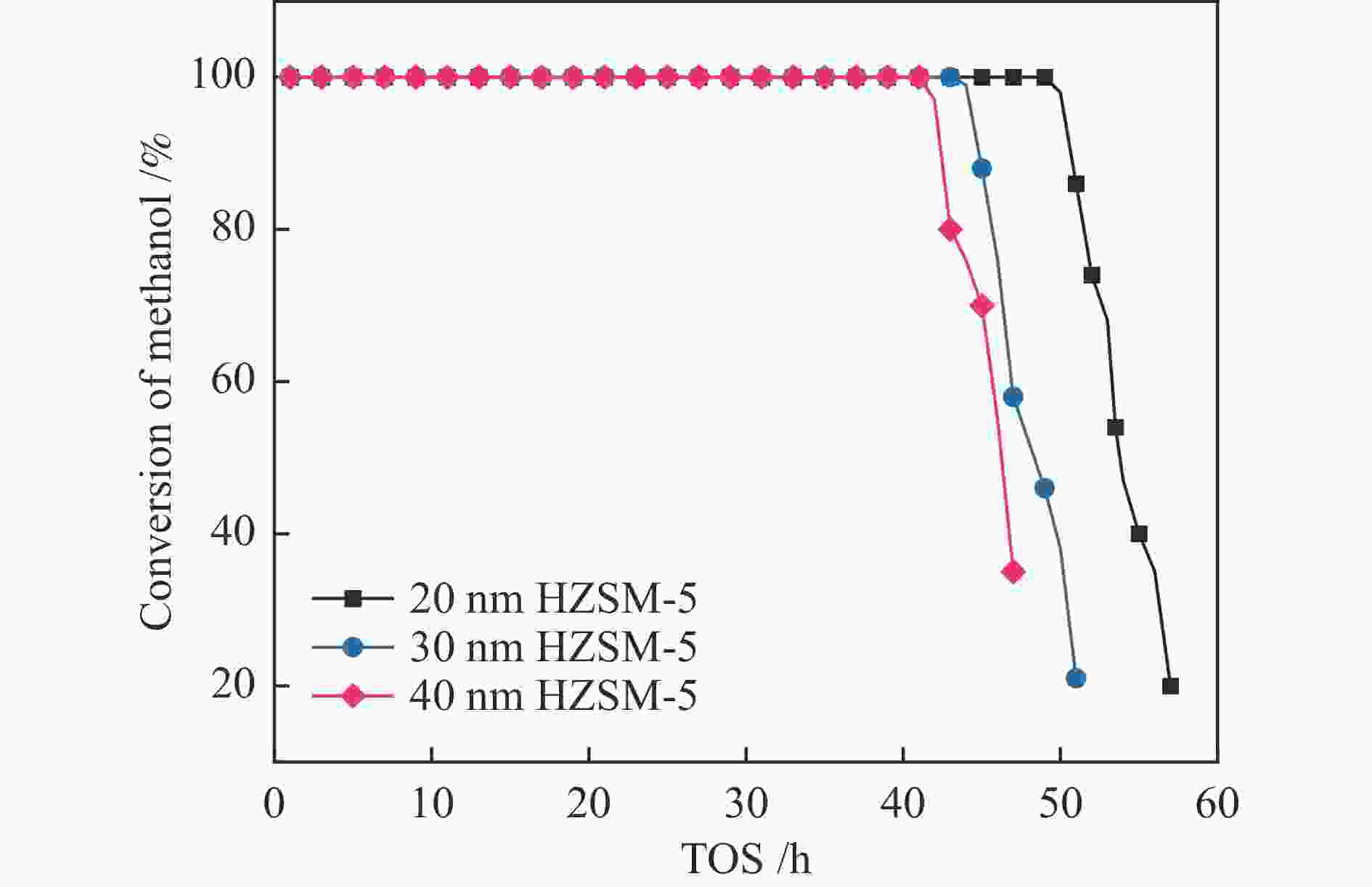
摘要: 本研究采用模板法以及水热法,成功制备了不同粒径(20、30和40 nm)、硅铝比约为50的有序介孔HZSM-5分子筛。采用XRD、SEM、TEM、N2等温吸附-脱附和Py-FTIR等手段对合成样品的结构、形貌和表面酸性等性质进行了表征,并在固定床反应器上测试了其在甲醇制芳烃过程中的催化活性。实验结果表明,不同粒径的有序介孔HZSM-5分子筛对甲醇制芳烃反应的催化性能不同,其中,20 nm的有序介孔HZSM-5分子筛展示了优异的催化性能,轻质芳烃的选择性高达60.0%,催化剂在连续运行51 h之后没有明显的失活现象。Abstract: In this paper, ordered mesoporous HZSM-5 zeolite with different particle sizes (20, 30 and 40 nm) and the Si/Al ratio of 50 was successfully prepared by the hydrothermal method. The structure, morphology and surface acidity of the synthesized samples were characterized by XRD, SEM, TEM, N2 isothermal adsorption/desorption and Py-FTIR, and their catalytic activities were tested in the methanol to aromatics process on a fixed-bed reactor. The experimental results showed that the catalytic performance of ordered mesoporous HZSM-5 zeolite with different particle sizes was different for the methanol to aromatics reaction. The ordered mesoporous HZSM-5 zeolite of 20 nm exhibited excellent catalytic performance with the selectivity of light aromatics up to 60.0% and no significant deactivation of the catalyst after 51 h of continuous operation.
-
Key words:
- ordered mesoporous zeolite /
- particle sizes /
- methanol to aromatics
-
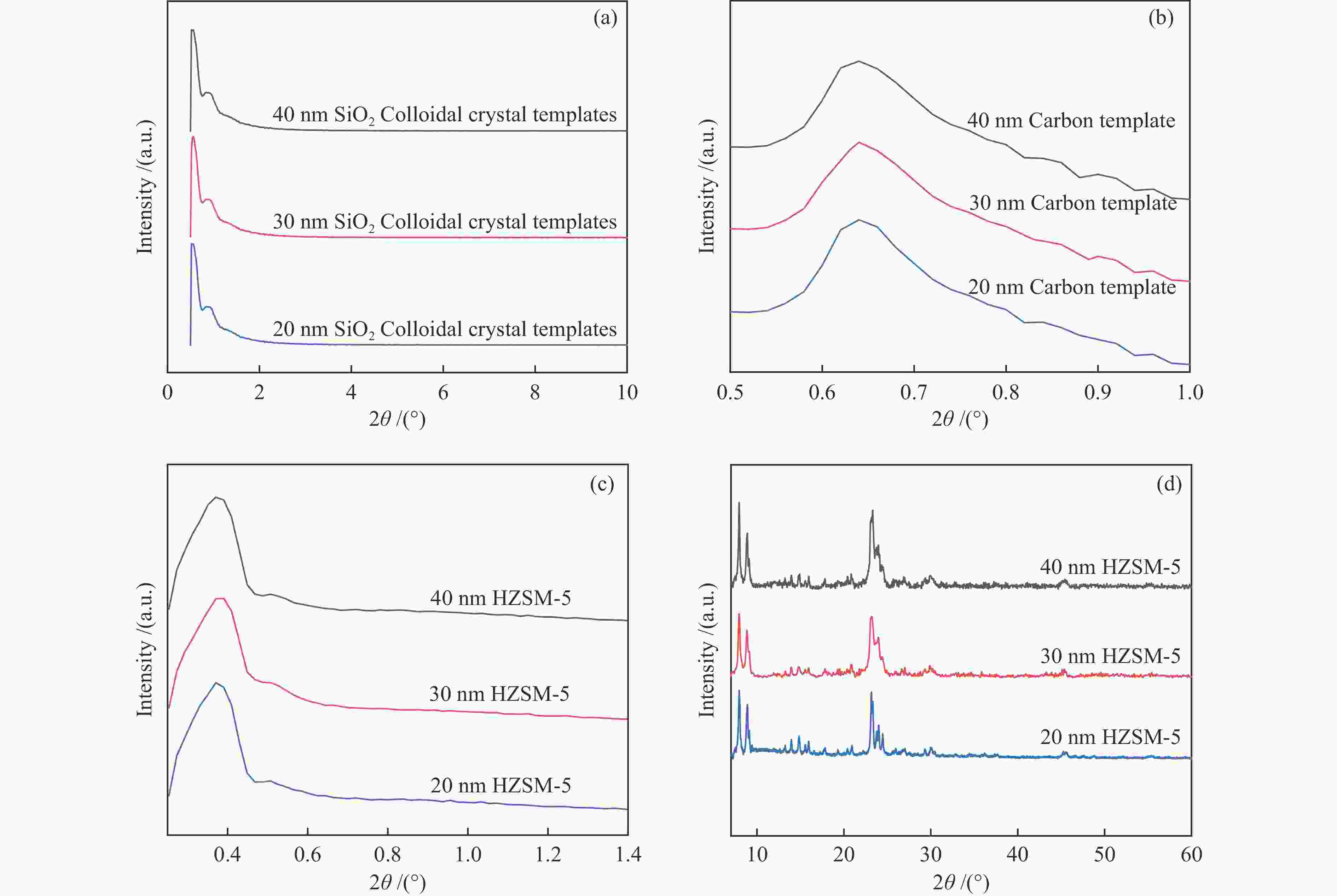
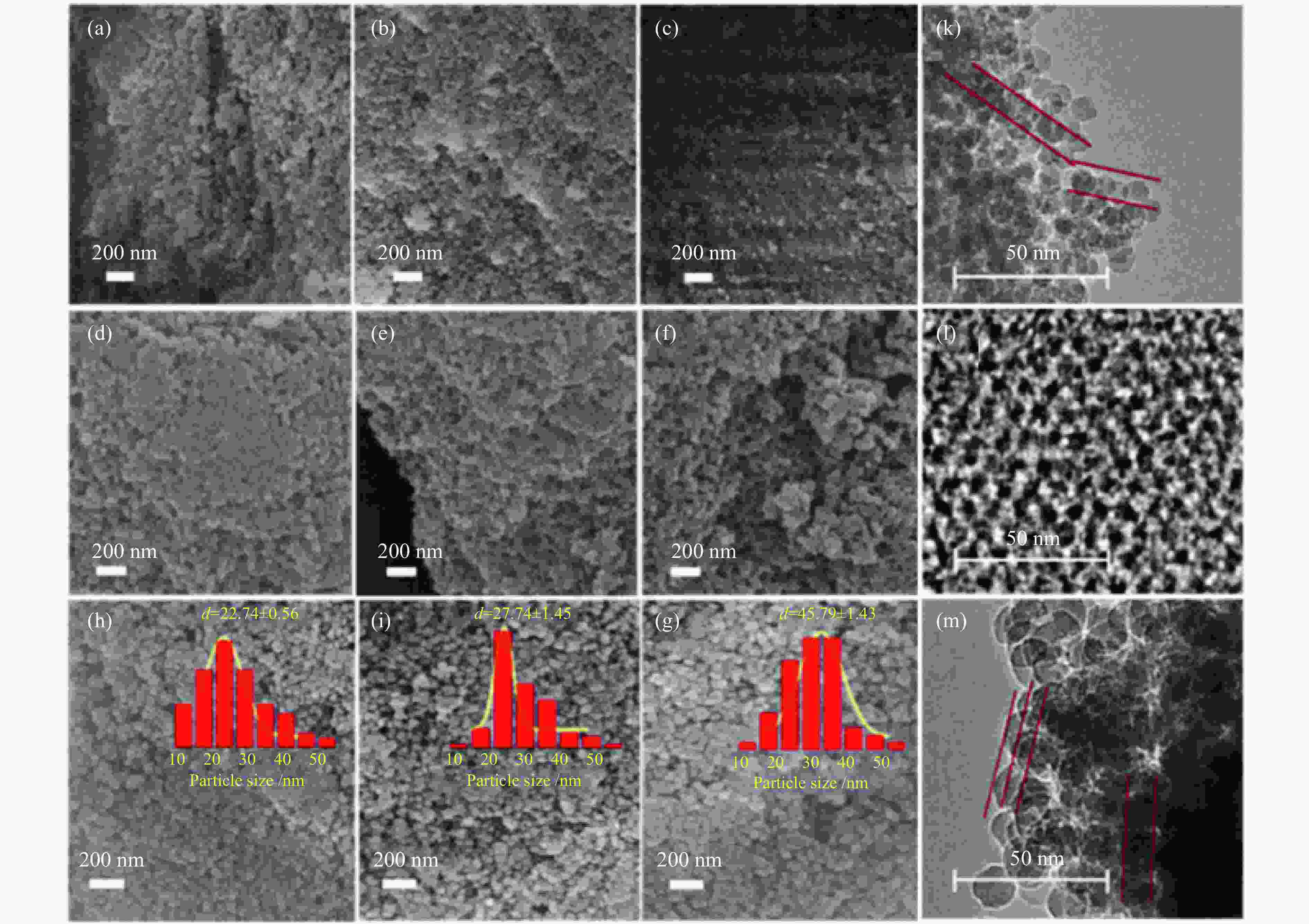
图 2 (a) 20 nm,(b) 30 nm,(c) 40 nm SiO2微球;(d) 20 nm,(e) 30 nm,(f) 40 nm有序介孔碳;(h) 20 nm,(i) 30 nm,(g) 40 nm有序介孔HZSM-5分子筛的SEM照片和(k) 20 nm SiO2微球;(l) 20 nm有序介孔碳;(m) 20 nm有序介孔HZSM-5分子筛的TEM照片
Figure 2 SEM images of (a) 20 nm, (b) 30 nm, (c) 40 nm SiO2 microspheres; (d) 20 nm, (e) 30 nm, (f) 40 nm ordered mesoporous carbon; (h) 20 nm, (i) 30 nm, (g) 40 nm ordered mesoporous HZSM-5 zeolite; TEM images of (k) 20 nm SiO2 microspheres; (l) 20 nm ordered mesoporous carbon; (m) 20 nm ordered mesoporous HZSM-5 zeolite
表 1 催化剂的结构性质
Table 1 Textural properties of the catalysts
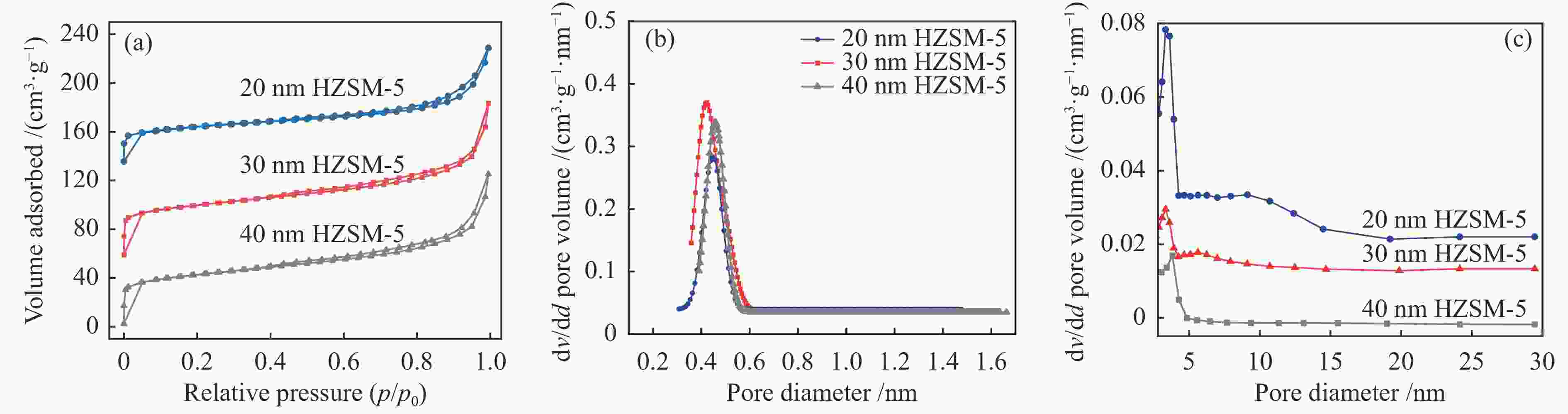
Sample SBETa
/(m2·g–1)Smicrob
/(m2·g–1)Smesoc
/(m2·g–1)vtotald
/(cm3·g–1)vmicroe
/(cm3·g–1)vmesof
/(cm3·g–1)Si/Alg 20 nm HZSM-5 275.78 187.34 88.44 0.16 0.05 0.11 38 30 nm HZSM-5 201.45 133.11 68.34 0.15 0.07 0.09 43 40 nm HZSM-5 198.37 159.52 38.85 0.13 0.08 0.05 37 SBETa(surface area calculated by BET), Smicrob (surface area of micropores by t-plot method),Smesoc = SBETa – Smicrob, vmicroe (micropore volume by t-plot method), vtotald (total pore volume), Si/Alg (ICP analysis) 表 2 不同样品的酸性位点含量
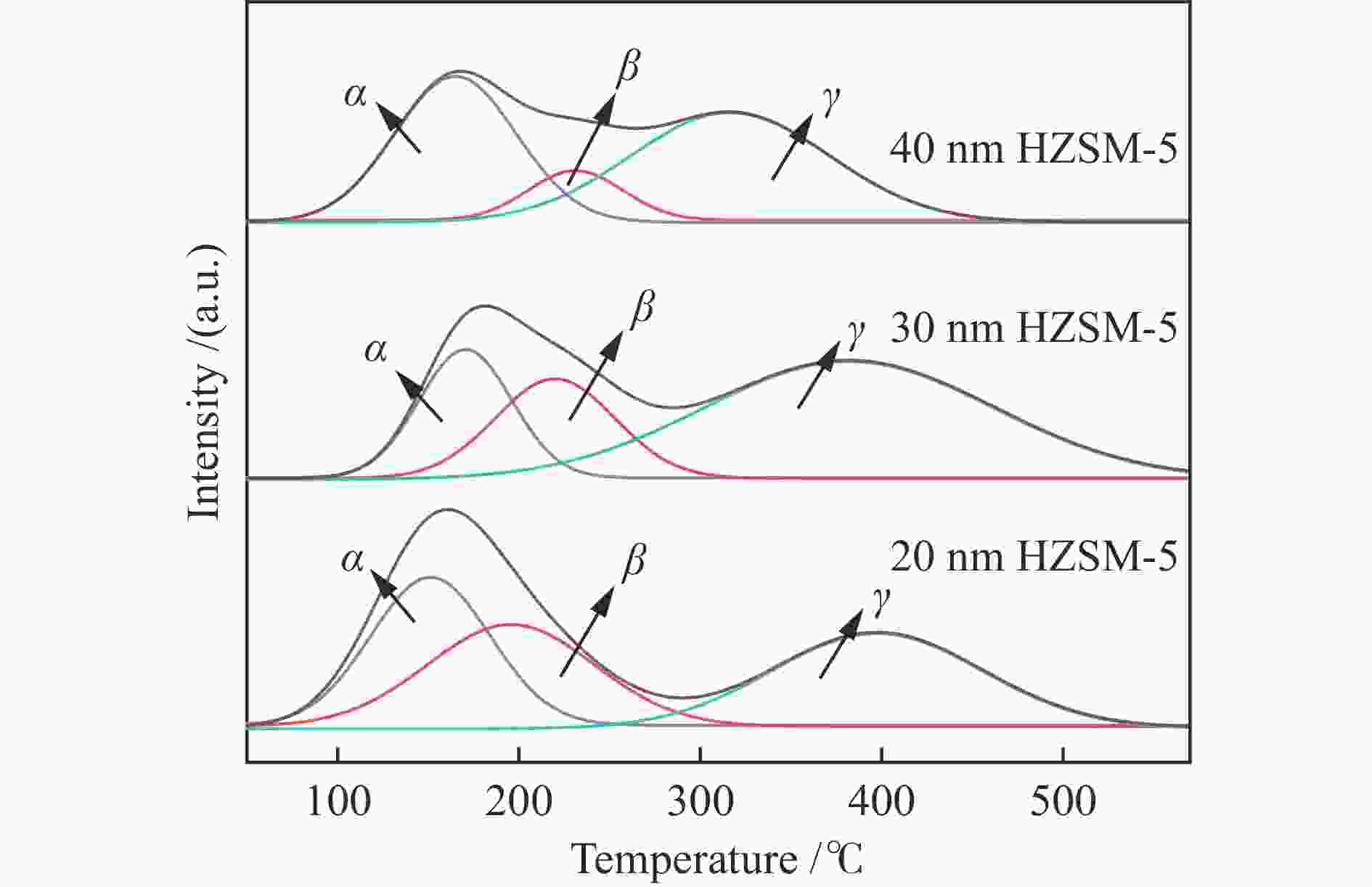
Table 2 Concentrations of acid sites in different samples
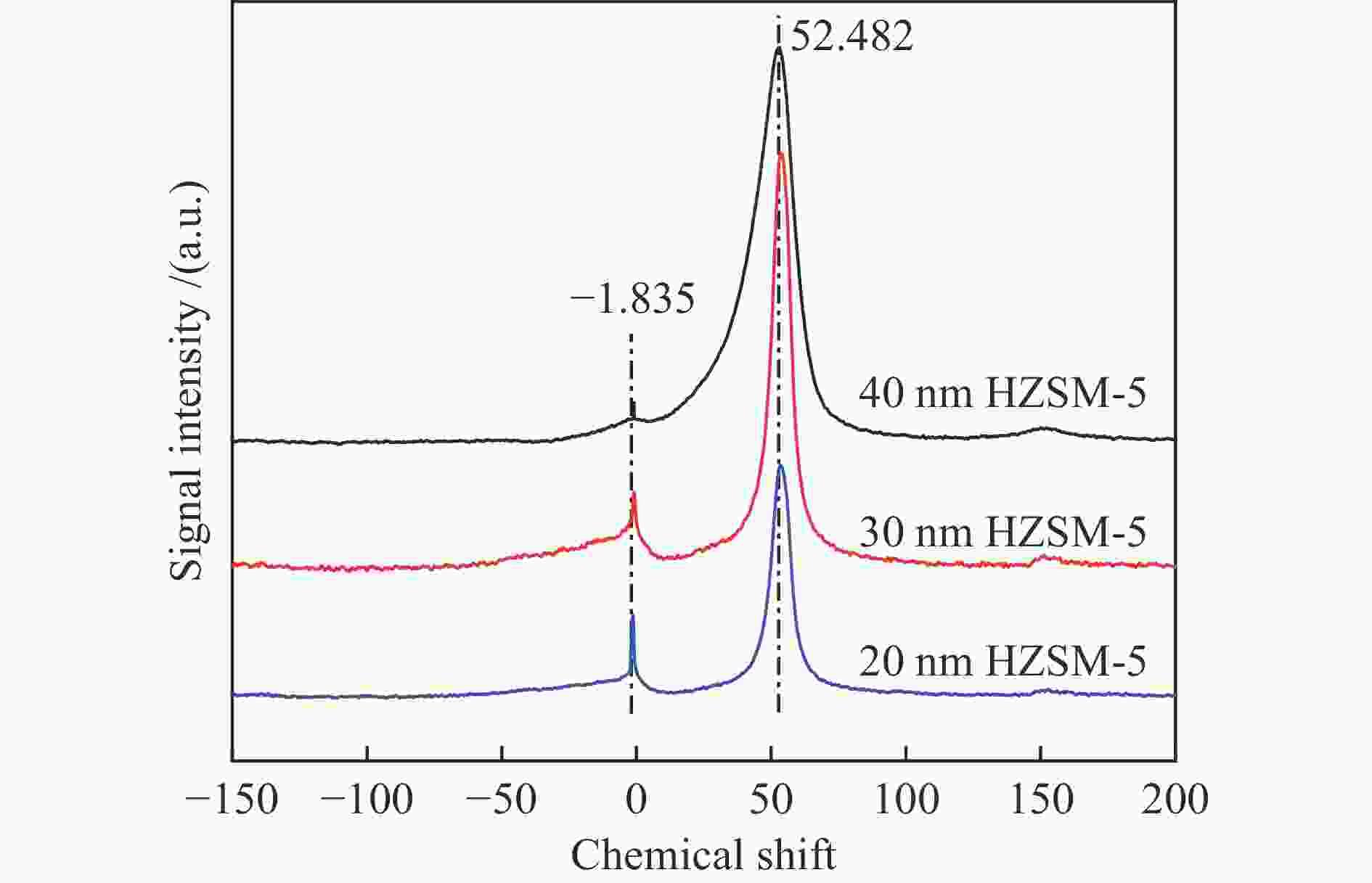
Sample Concentration of acid sites /(a.u.·g–1) weak medium strong total 20 nm HZSM-5 54.31 88.73 141.39 284.43 30 nm HZSM-5 50.64 60.18 166.31 277.13 40 nm HZSM-5 55.92 28.32 139.84 224.08 表 3 不同样品的B酸和L酸性位点分布
Table 3 Distribution of B and L acid sites on different samples
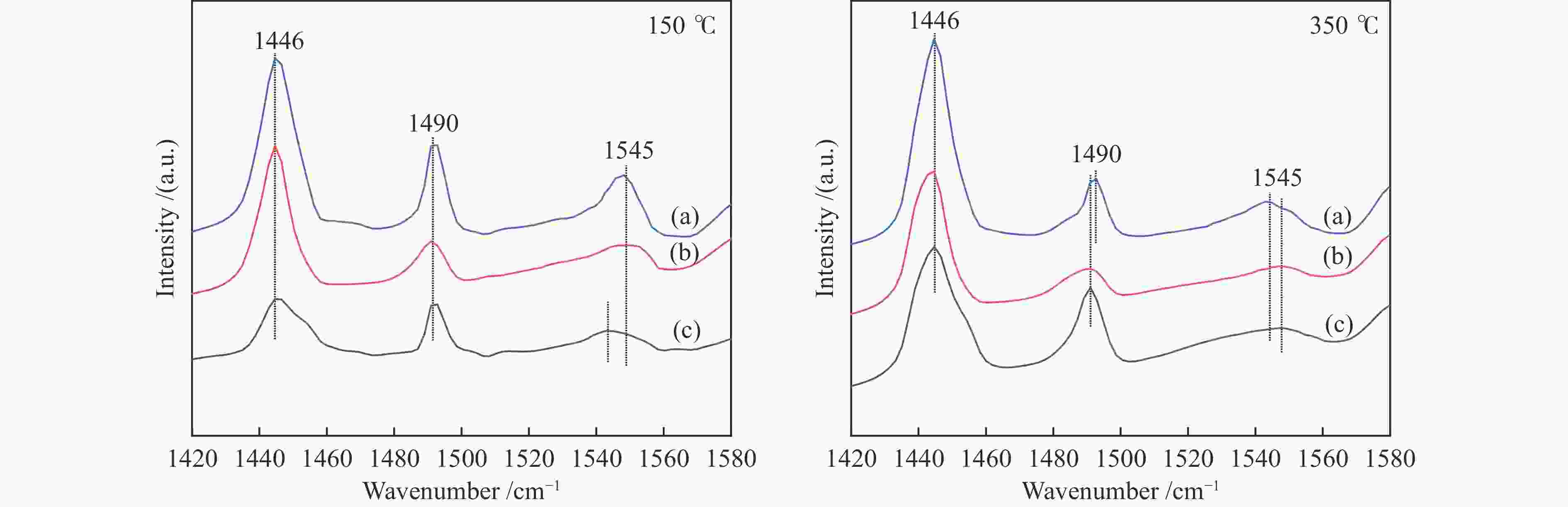
Sample Amount of B /(mmol·g–1) Amount of L /(mmol·g–1) B/L 150 ℃ 350 ℃ total 150 ℃ 350 ℃ total 20 nm HZSM-5 0.056 0.049 0.105 0.068 0.033 0.101 1.04 30 nm HZSM-5 0.040 0.017 0.057 0.075 0.023 0.098 0.58 40 nm HZSM-5 0.025 0.012 0.037 0.046 0.015 0.061 0.61 表 4 不同催化剂用于MTA反应的产物选择性
Table 4 Product selectivity of different catalysts for MTA reaction
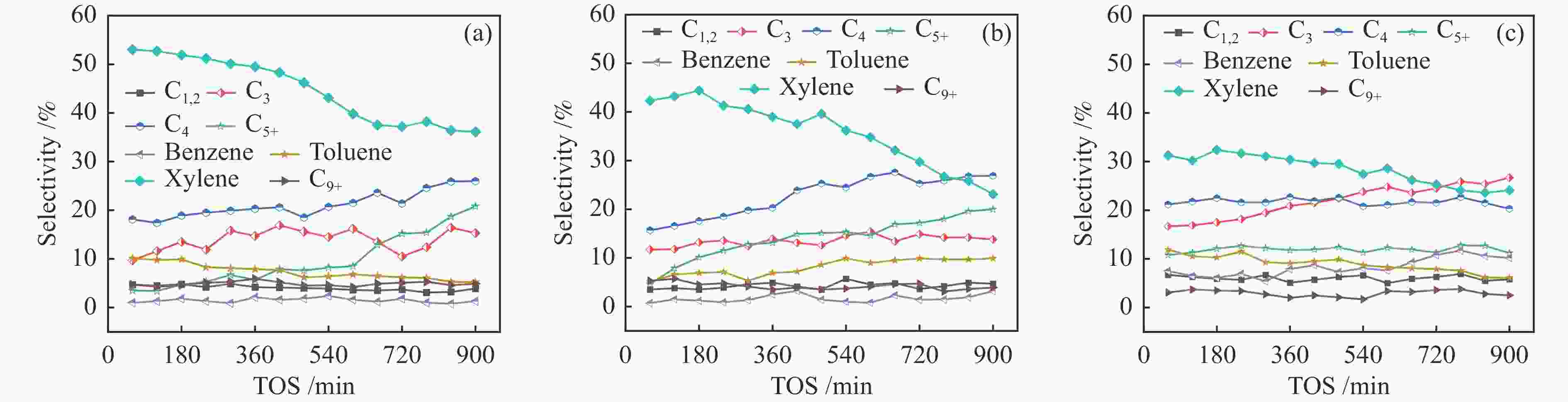
Catalyst Production selectivity /% C1-2 C3 C4 C5 + Ba Tb Xc C9 + arod BTX 20 nm HZSM-5 4.7 9.9 18.1 3.3 1.0 6.1 53.0 4.1 64.1 60.0 30 nm HZSM-5 5.7 11.7 24.7 4.6 0.7 5.1 42.3 5.3 53.4 48.1 40 nm HZSM-5 6.7 16.7 21.2 6.8 6.5 7.9 31.1 3.1 48.6 45.5 Reaction conditions:0.1 MPa,450 ℃,0.5 g catalyst, $p_{{\rm{CH}}_3 {\rm{OH}}} $=20 kPa,WHSV=1.9 h−1,time on stream (TOS)=3 h;Ba=benzene,Tb= toluene,Xc=xylene,arod=B + T + X + C9 + -
[1] TIAN P L, SHEN K, CHEN J Y, FAN T, FANG R Q, LI Y W. Self-templated formation of Pt@ZIF-8/SiO2 composite with 3D-ordered macropores and size-selective catalytic properties[J]. Small Methods,2018,2(12):1800219. doi: 10.1002/smtd.201800219 [2] SHEN K, CAI S, LING R, XIE D, LI X, SUN J, WEI J, SUN X. Flexible, three-dimensional ordered macroporous ZnO electrode with enhanced electrochemical performance in lithium-ion batteries[J]. Microporous Mesoporous Mater,2019,289:109618. doi: 10.1016/j.micromeso.2019.109618 [3] YAN H, BLANFORD C F, LYTLE J C, CARTER C B, SMYRL W H, STEIN A. Influence of processing conditions on structures of 3D ordered macroporous metals prepared by colloidal crystal templating[J]. Chem Mater,2001,13(11):4314−4321. doi: 10.1021/cm0105716 [4] WU Z S, SUN Y, TAN Y Z, YANG S B, FENG X L, MÜLLEN K. Three-dimensional graphene-based macro-and mesoporous frameworks for high-performance electrochemical capacitive energy storage[J]. J Am Chem Soc,2012,134(48):19532−19535. doi: 10.1021/ja308676h [5] YANG W, YANG W, KONG L SONG A L, QIN X J. Synthesis of three-dimensional hierarchical porous carbon for high-performance supercapacitors[J]. Ionics,2018,24(10):3133−3141. doi: 10.1007/s11581-017-2432-z [6] FENG J X, ZHENG D, GAO X L, QUE W B, SHI W H, LIU W X, WU F F, CAO X H. Three-dimensional ordered porous carbon for energy conversion and storage applications[J]. Front Energy Res,2020,8:210. doi: 10.3389/fenrg.2020.00210 [7] CHEN Y, ZHU Y J, CHEN Z G. Three-dimensional ordered macroporous carbon as counter electrodes in dye-sensitized solar cells[J]. Thin Solid Films,2013,539:122−126. doi: 10.1016/j.tsf.2013.05.096 [8] ARANDIYAN H, DAI H X, JI K, SUN H Y, LI J H. Pt nanoparticles embedded in colloidal crystal template derived 3D ordered macroporous Ce0.6Zr0.3Y0.1O2: Highly efficient catalysts for methane combustion[J]. ACS Catal,2015,5(3):1781−1793. doi: 10.1021/cs501773h [9] XUE H, TANG J, GONG H, GUO H, FAN X L, WANG T, HE J P, YAMAUCHI Y. Fabrication of PdCo bimetallic nanoparticles anchored on three-dimensional ordered N-doped porous carbon as an efficient catalyst for oxygen reduction reaction[J]. ACS Appl Mater Interfaces,2016,8(32):20766−20771. doi: 10.1021/acsami.6b05856 [10] DAVIS M E. Ordered porous materials for emerging applications[J]. Nature,2002,417(6891):813−821. doi: 10.1038/nature00785 [11] COLIN S, CUNDY, PAUL A C. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time[J]. Chem Rev,2003,103:663−702. [12] PéREZ-RAMíREZ J, CHRISTENSEN C H, EGEBLAD K, CHRISTENSEND C H. GROENEF J C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design[J]. Chem Soc Rev,2008,37(11):2530−2542. doi: 10.1039/b809030k [13] HARTMANN M. Hierarchical zeolites: A proven strategy to combine shape selectivity with efficient mass transport[J]. Angew Chem Int Ed,2004,43(44):5880−5882. doi: 10.1002/anie.200460644 [14] 陈思姝, 李光兰, 谢洋洋. 胶体晶体模板法制备三维有序多孔碳材料及其电化学性能[J]. 中国科学: 化学,2015,45(6):614−623.CHEN Si-mei, LI Guang-lan, XIE Yang-yang. Preparation of three-dimensional ordered porous carbon materials and their electrochemical properties by colloidal crystal template method[J]. Sci China Chem,2015,45(6):614−623. [15] YU C, TIAN B, FAN J, STUCKY G D, ZHAO D Y. Salt effect in the synthesis of mesoporous silica templated by non-ionic block copolymers[J]. Chem Commun,2001,24:2726−2727. [16] KIM J M, RYOO R. Synthesis of MCM-48 single crystals[J]. Chem Commun,1998,2:259−260. [17] JACOBSEN C J H, MADSEN C, JANSSENS T V W, JJAKOBSENB H, SKIBSTED. Zeolites by confined space synthesis-characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and 27Al/29Si-MAS NMR spectroscopy[J]. Microporous Mesoporous Mater,2000,39(1/2):393−401. [18] SCHMIDT I, MADSEN C, JACOBSEN C J H. Confined space synthesis. A novel route to nanosized zeolites[J]. Inorg Chem,2000,39(11):2279−2283. doi: 10.1021/ic991280q [19] LI Q, CREASER D, STERTE J. The nucleation period for TPA-silicalite-1 crystallization determined by a two-stage varying-temperature synthesis[J]. Microporous Mesoporous Mater,1999,31(1/2):141−150. [20] AGUADO J, SERRANO D P, ESCOLA J M, RODRíGUEZ. Low temperature synthesis and properties of ZSM-5 aggregates formed by ultra-small nanocrystals[J]. Microporous Mesoporous Mater,2004,75(1/2):41−49. [21] NIU X J, GAO J, WANG K, MIAO Q, DONG M, WANG G F, FAN W B, QIN Z F, WANG J G. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics[J]. Fuel Process Technol,2017,157:99−107. doi: 10.1016/j.fuproc.2016.12.006 [22] KIM H S, KANG S K, ZHANG H X, TIKUE E T, LEE J H, LEE P S. Al-ZSM-5 nanocrystal catalysts grown from Silicalite-1 seeds for methane conversion[J]. Energies,2021,14(2):485. doi: 10.3390/en14020485 [23] XUE T, CHEN L, WANG Y M, HE M Y. Seed-induced synthesis of mesoporous ZSM-5 aggregates using tetrapropylammonium hydroxide as single template[J]. Microporous Mesoporous Mater,2012,156:97−105. doi: 10.1016/j.micromeso.2012.02.022 [24] MI X T, HOU Z G, LI X G. Controllable synthesis of nanoscaled ZSM-5 aggregates with multivariate channel under the synergistic effect of silicate-1 and TPABr using dual-silica source[J]. Microporous Mesoporous Mater,2021,323:111224. doi: 10.1016/j.micromeso.2021.111224 [25] REN N, YANG Z J, LV X C, SHI J, ZHANG Y H, TANG Y. A seed surface crystallization approach for rapid synthesis of submicron ZSM-5 zeolite with controllable crystal size and morphology[J]. Microporous Mesoporous Mater,2010,131(1/3):103−114. [26] FERNÁNDEZ-REYES B, MORALES JIMÉNEZ S, SÁNCHEZ-MARRERO G, MUÑOZ-SENMACHE J C, HERNÁNDES-MALDONADO A J. Hierarchical three-dimensionally ordered mesoporous carbon (3DOm) zeolite composites for the adsorption of contaminants of emerging concern[J]. J Hazard Mater Lett,2021,2:100017. doi: 10.1016/j.hazl.2021.100017 [27] WANG J, YANG M F, SHANG W J, SU X P, HAO Q Q, CHEN H Y, MA X X. Synthesis, characterization and catalytic application of hierarchical SAPO-34 zeolite with three-dimensionally ordered mesoporous-imprinted structure[J]. Microporous Mesoporous Mater,2017,252:10−16. doi: 10.1016/j.micromeso.2017.06.012 [28] YOKOI T, SAKAMOTO Y, TERASAKI O, KUBOTA Y, OKUBO T, TATSUMI T. Periodic arrangement of silica nanospheres assisted by amino acids[J]. J Am Chem Soc,2006,128(42):13664−13665. doi: 10.1021/ja065071y [29] FAN W, SNYDER M A, KUMAR S, LEE P S, YOO W C, MCCORMICK A V, LEE PENN R, STEIN A, TSAPATSIS, M. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity[J]. Nat Mater,2008,7(12):984−991. doi: 10.1038/nmat2302 [30] WANG Z, DORNATH P, CHANG C C, CHEN H Y, FAN W. Confined synthesis of three-dimensionally ordered mesoporous-imprinted zeolites with tunable morphology and Si/Al ratio[J]. Microporous Mesoporous Mater,2013,181:8−16. doi: 10.1016/j.micromeso.2013.07.010 [31] GIERSZAL K P, JARONIEC M. Carbons with extremely large volume of uniform mesopores synthesized by carbonization of phenolic resin film formed on colloidal silica template[J]. J Am Chem Soc,2006,128(31):10026−10027. doi: 10.1021/ja0634831 [32] CHEN H Y, WYDRA J, ZHANG X Y, LEE P S, WANG Z P, FAN W, TSAPATSIS M. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure[J]. J Am Chem Soc,2011,133(32):12390−12393. doi: 10.1021/ja2046815 [33] BI Y, WANG Y L, CHEN X, YU Z G, XU L. Methanol aromatization over HZSM-5 catalysts modified with different zinc salts[J]. Chin J Catal,2014,35(10):1740−1751. doi: 10.1016/S1872-2067(14)60145-5 [34] NIU X J, GAO J, MIAO Q, DONG M, WANG G F, FAN W B, QIN Z F, WANG J G. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics[J]. Microporous Mesoporous Mater,2014,197:252−261. doi: 10.1016/j.micromeso.2014.06.027 [35] WEI Z H, XIA T F, LIU M H, CAO Q S, XU Y R, ZHU K, ZHU X D. Alkaline modification of ZSM-5 catalysts for methanol aromatization: The effect of the alkaline concentration[J]. Front Chem Sci Eng,2015,9(4):450−460. doi: 10.1007/s11705-015-1542-2 [36] WANG Z L, CHU W F, ZHAO Z C, LIU Z M, CHEN H Y, XIAO D, GONG K, LI F, LI X J, HOU G J. The role of organic and inorganic structure-directing agents in selective Al substitution of zeolite[J]. J Phys Chem Lett,2021,12(38):9398−9406. doi: 10.1021/acs.jpclett.1c01448 [37] WAN Z J, WU W, LI G, WANG C F, YANG H, ZHANG D K. Effect of SiO2/Al2O3 ratio on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline conversion[J]. Appl Catal A: Gen,2016,523:312−320. doi: 10.1016/j.apcata.2016.05.032 [38] HE Y P, LIU M, DAI C Y, XU S T, WEI Y X, LIU Z M, GUO X W. Modification of nanocrystalline HZSM-5 zeolite with tetrapropylammonium hydroxide and its catalytic performance in methanol to gasoline reaction[J]. Chin J Catal,2013,34(6):1148−1158. doi: 10.1016/S1872-2067(12)60579-8 [39] LIN X L, FAN Y, LIU Z H, SHI G, LIU H Y, BAO X J. A novel method for enhancing on-stream stability of fluid catalytic cracking (FCC) gasoline hydro-upgrading catalyst: Post-treatment of HZSM-5 zeolite by combined steaming and citric acid leaching[J]. Catal Today, 2007, 125 (3/4): 185–191. [40] ZHANG H B, MA Y C, SONG K S, ZHANG Y H, TANG Y. Nano-crystallite oriented self-assembled ZSM-5 zeolite and its LDPE cracking properties: effects of accessibility and strength of acid sites[J]. J Catal,2013,302:115−125. doi: 10.1016/j.jcat.2013.03.019 [41] JIA Y M, SHI Q H, WANG J W, DING C M, ZHANG K. Synthesis, characterization, and catalytic application of hierarchical nano-ZSM-5 zeolite[J]. RSC Adv,2020,10(50):29618−29626. doi: 10.1039/D0RA06040B [42] HU Z J, ZHANG H B, WANG L, ZHANG H X, ZAHNG Y H, XU H L, SHEN W, TANG Y. Highly stable boron-modified hierarchical nanocrystalline ZSM-5 zeolite for the methanol to propylene reaction[J]. Catal Sci Technol,2014,4(9):2891−2895. doi: 10.1039/C4CY00376D -




 下载:
下载: