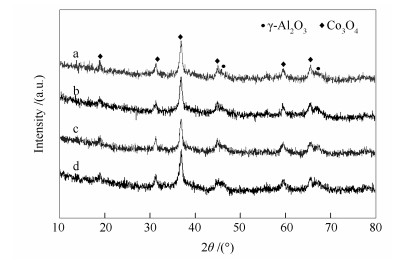
-
摘要: 通过浸渍法制备不同含量SiO2改性的Co/Al2O3催化剂, 结合N2吸附-脱附、XRD、H2-TPR、XPS等表征手段, 研究SiO2助剂对钴基催化剂物相结构、还原行为及F-T合成性能的影响。结果表明, 适量的SiO2改性后, 有效地减弱了载体与活性组分钴之间的相互作用, 显著地提高了催化剂的还原度和催化活性。但继续增加SiO2的含量, 催化剂的还原度继续提高, 分散度同时下降32%, 与未改性之前相比, 催化剂的活性基本没有改变。Abstract: The γ-Al2O3 modified with different content of SiO2 by impregnation method was used as supports to prepare Co/Al2O3 catalyst.The effects of SiO2 additives on phase structure of the cobalt-based catalysts, reduction behavior and the influence on the F-T synthesis performance were studied by using N2 adsorption, XRD, H2-TPR and XPS characterization methods and activity test.The results showed that with the introduction of SiO2, the interaction between support and cobalt was effectively weakened, thus the reducibility and catalytic activity of the catalysts were significantly improved.However, when the amount of SiO2 continued to increase, the reduction degree of the catalyst continued to improve, but the dispersion decreased by 32% at the same time.Compared with unmodified Co/Al2O3 catalyst, the activity of the catalyst remained basically unchanged.
-
Key words:
- Fischer-Tropsch synthesis /
- SiO2 modification /
- reducibility
-
表 1 载体和催化剂的物理化学性质
Table 1 Physico-chemical properties of the supports and the catalysts
Sample ABET/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm Co crystalline a d/nm H2-TPDb dispersion/% H2-TPRc reducibility/% Al2O3 188 0.52 8.3 - - - Al2O3-5SiO2 181 0.49 8.3 - - - Al2O3-10SiO2 178 0.47 8.2 - - - Al2O3-20SiO2 163 0.42 8.0 - - - Co/Al2O3 139 0.34 8.0 7.1 4.99 28.4 Co/Al2O3-5SiO2 138 0.33 7.9 7.7 4.94 38.2 Co/Al2O3-10SiO2 132 0.30 7.8 8.9 4.88 53.5 Co/Al2O3-20SiO2 115 0.27 7.6 10.4 3.36 63.4 a d(Co) =0.75 d(Co3O4); the average particle size of Co3O4 calculated from XRD diffraction peak at 36.8°; b calculated from H2 chemisorption; c calculated by H2-TPR from 373 to 673 K 表 2 催化剂的表面组成
Table 2 Surface composition of the catalysts
Sample Co 2p3/2 EB/eV ICSS/ICo3O4 Surface atom ratiob Co3O4 CSSa ISi/IAl Co/Al2O3 779.275 782.566 0.785 - Co/Al2O3-5SiO2 779.782 782.051 0.640 0.120 Co/Al2O3-10SiO2 779.795 782.076 0.599 0.224 Co/Al2O3-20SiO2 779.834 782.058 0.737 0.319 a:cobalt surface species; b:obtained by XPS measurement 表 3 催化剂的反应性能
Table 3 Catalytic performance of the catalysts for F-T
Catalyst Temperature t/℃ CO conversion x/% Hydrocarbon selectivity s/% CH4 C2-4 C5+ Co/Al2O3 210 23.77 14.99 5.54 79.47 220 45.60 14.00 6.60 79.40 Co/Al2O3-5SiO2 210 24.63 10.33 4.53 85.13 220 48.01 12.88 6.75 80.37 Co/Al2O3-10SiO2 210 38.12 10.66 5.44 83.89 220 62.05 11.49 8.73 79.79 Co/Al2O3-20SiO2 210 21.03 11.49 5.84 82.67 220 44.08 14.71 8.21 77.08 reaction conditions:2 MPa, GHSV=1000 h-1, H2/CO(volume ratio)=2 -
[1] 孙予罕, 陈建刚, 王俊刚, 贾丽涛, 侯博, 李德宝, 张娟.费托合成钴基催化剂的研究进展[J].催化学报, 2010, 31(8):919-927. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201008007SUN Yu-han, CHEN Jian-gang, WANG Jun-gang, JIA Li-tao, HOU Bo, LI De-bao, ZHANG Juan.The development of cobalt-based catalysts for fischer-tropsch synthesis[J].Chin J Catal, 2010, 31(8):919-927. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201008007 [2] XING C, YANG G H, WANG D, ZENG C Y, JIN Y Z, YANG R Q, SUEHIRO Y, TSUBAKI N.Controllable encapsulation of cobalt clusters inside carbon nanotubes as effective catalysts for Fischer-Tropsch synthesis[J].Catal Today, 2013, 215:24-28. doi: 10.1016/j.cattod.2013.02.018 [3] KHODAKOV A Y, CHU W, FONGARLAND P.Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J].Chem Rev, 2007, 107(5):1692-1744. doi: 10.1021/cr050972v [4] 李金林, 完友军, 张煜华, 熊海峰.不同载体负载的钴基费托合成催化剂的还原过程研究[J].中南民族大学学报, 2007, 26(2):1-6.LI Jin-lin, WAN You-jun, ZHANG Yu-hua, XIONG Hai-feng.Studies on the reduction process of the supported cobalt catalysis for Fischer-Tropsch Synthesis[J].J South-Cent Univ Nat(Nat Sci Ed), 2007, 26(2):1-6. [5] ZHANG Y H, XIONG H F, LIEW K Y, LI J L.Effect of magnesia on alumina-supported cobalt Fischer-Tropsch synthesis catalysts[J].J Mol Catal A:Chem, 2005, 237(1/2):172-181. https://www.sciencedirect.com/science/article/pii/S1381116905002980 [6] BAO A, LIEW K Y, LI J L.Fischer-Tropsch synthesis on CaO-promoted Co/Al2O3 catalysts[J].J Mol Catal A:Chem, 2009, 304(1/2):47-51. doi: 10.5339/qfarf.2011.egp1 [7] 李家波, 林泉.费托合成钴催化剂载体改性研究进展[J].洁净煤技术, 2015, 21(1):65-68. doi: 10.13226/j.issn.1006-6772.2015.01.015.htmlLI Jia-bo, LIN Quan.Supporter modification of Fischer-Tropsch cobalt catalyst[J].Clean Coal Technol, 2015, 21(1):65-68. doi: 10.13226/j.issn.1006-6772.2015.01.015.html [8] PATERMARAKIS G, NICOLOPOULOS N.Catalysis ove porous anodic alumina film catalysts with different pore surface concentrations[J].J Catal, 1999, 187(2):311-320. doi: 10.1006/jcat.1999.2627 [9] SUN X Y, ZHANG X J, ZHANG Y, TSUBAKI N.Reversible promotional effect of SiO2 modification to Co/Al2O3 catalyst for Fischer-Tropsch synthesis[J].Appl Cata A:Gen, 2010, 377(1/2):134-139. http://www.chxb.cn/EN/abstract/abstract21517.shtml [10] ARNOLDY P, MOULIJN J A.Temperature-programmed reductio of CoO/Al2O3 catalysts[J].J Catal, 1985(1), 93:38-54. doi: 10.1016/0021-9517(85)90149-6 [11] KOGELBAUER A, GOODWIN J G, QUKACI R.Ruthenium promotion of Co/Al2O3 Fischer-Tropsch catalysts[J].J Catal, 1996, 160(1):125-133. doi: 10.1006/jcat.1996.0130 [12] FAN L, YOKOTA K, FUJIMOTO K.Supercritical phase Fischer-Tropsch synthesis:Catalyst pore-size effect[J].AIChE J, 1992, 38(10):1639-1648. doi: 10.1002/(ISSN)1547-5905 [13] SEXTON B A, HUGHES A E, TURNEY T W.An XPS and TPR study of the reduction of promoted cobalt-kieselguhr Fischer-Tropsch catalysts[J].J Catal, 1986, 97(2):390-406. doi: 10.1016/0021-9517(86)90011-4 [14] GUERRERORUIZ A, SEPULVEDAESCRIBANO A, RODRIGUEZRAMOS I.Carbon monoxide hydrogenation over carbon supported cobalt or ruthenium catalysts:Promoting effects of magnesium, vanadium and cerium oxides[J].Appl Catal A:Gen, 1994, 120(1):71-83. doi: 10.1016/0926-860X(94)80334-X [15] GRASS M E, ZHANG Y, BUTCHER D R, PARK J Y, LI Y, BLUHM H, BRATLIE K M, ZHANG T, SOMORJAI G A.A reactive oxide overlayer on rhodium nanoparticles during CO oxidation and its size dependence studied by in situ ambient-pressure X-ray photoelectron spectroscopy[J].Angew Chem Int Ed Eng, 2008, 47(46):8893-8896. doi: 10.1002/anie.v47:46 [16] PRIETO G, DE M M, CONCEPCION P, MURCIANO R, MURCIANO R, PERGHERS B C, MARTTINEZ A.Cobalt-catalyzed Fischer-Tropsch synthesis:Chemical nature of the oxide support as a performance descriptor[J].ACS Catal, 2015, 5(6):3323-3335. doi: 10.1021/acscatal.5b00057 [17] BEZEMER G L, BITTER J H, KUIPERS H, OOSTERBEEK H, HOLEWIJN J E, XU X D, KAPTEIJN F, VANDILLEN A J, DEJONG K P.Cobalt particle size effects in the Fischer-Tropsch reaction studied with carbon nanofiber supported catalysts[J].J Am Chem Soc, 2005, 128(6):3956-3964. http://s3.amazonaws.com/zanran_storage/www.anorg.chem.uu.nl/ContentPages/16636976.pdf [18] ZHANG Y, KOIKE M, TSUBAKI N.Preparation of alumina-silica bimodal pore catalysts for Fischer-Tropsch synthesis[J].Catal Lett, 2005, 99(3/4):193-198. doi: 10.1007/s10562-005-2118-4.pdf -





 下载:
下载: