Effect of ZSM-5 on hydrocarbon selectivity of corn stalk catalytic pyrolysis at different pyrolysis temperatures
-
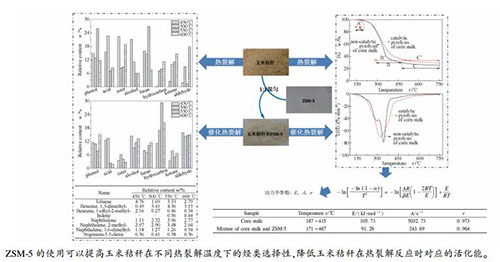
摘要: 为探讨不同热裂解温度下ZSM-5对玉米秸秆催化热裂解特性及烃类选择性的影响,本研究利用TGA对比有无ZSM-5时玉米秸秆的热裂解失重曲线,利用Py-GC/MS对比玉米秸秆在450、500、550和600 ℃下的热裂解和催化热裂解产物分布。结果表明,ZSM-5的使用可以降低玉米秸秆最高分解速率时对应的热裂解温度,降低温度为23 ℃。未使用ZSM-5时,热裂解产物种类以及烃类选择性均随热裂解温度的升高不断增加,在600 ℃时,烃类选择性达到最高,为11.33%;使用ZSM-5后,烃类产率随热裂解温度的升高先增加后减少,在550℃时,烃类选择性达到最高,为29.24%。使用ZSM-5后,玉米秸秆催化热裂解主要产物中出现了甲苯、茚、萘、二甲基萘等烃类,甲苯的最高产率为4.76%,萘的最高产率为3.96%。Abstract: In order to study the effect of ZSM-5 on the catalytic pyrolysis characteristic and hydrocarbon selectivity of corn stalk catalytic pyrolysis at different pyrolysis temperatures, the thermogravimetric analysis (TGA) was used to obtain the TG and DTG profiles of corn stalk pyrolysis with and without ZSM-5, and the pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) tests were conducted to analyze the products distribution of corn stalk pyrolysis with and without ZSM-5 at 450, 500, 550, and 600 ℃. The results show that ZSM-5 can decrease the pyrolysis temperature at the highest pyrolysis rate by 23 ℃. Without ZSM-5, both the categories of pyrolysis compounds and the hydrocarbon yield increase with the increase of pyrolysis temperature, and the selectivity of hydrocarbon reaches to the highest value of 11.33% at 600 ℃. However, with ZSM-5, the hydrocarbon yield increases at first and then decreases with the increase of pyrolysis temperature, and the selectivity of hydrocarbon is up to the highest value of 29.24% at 550 ℃. Toluene, indene, naphthalene and 2-methyl-naphthalene are evolved as the main compounds with ZSM-5. And the maximum yields of toluene and naphthalene reach to 4.76% and 3.96%, respectively.
-
Key words:
- corn stalk /
- ZSM-5 /
- pyrolysis /
- TGA /
- Py-GC/MS /
- hydrocarbon selectivity
-
表 1 玉米秸秆的理化特性
Table 1 Physicochemical characteristics of corn stalk
Item Value Proximate analysis (received basis)w/% M 6.59±0.005 A 5.92±0.07 V 72.83±0.12 FCa 14.66 Ultimate analysis (dry ash-free basis)w/% C 46.35±0.04 H 6.96±0.005 Oa 45.84 N 0.06±0.0002 S 0.79±0.003 Composition analysis (received basis)w/% Cellulose 40.34±0.06 Hemicellulose 25.08±0.38 Lignin 19.23±0.37 QHHV, net/(MJ·kg-1) 16.07±0.73 a: calculated by difference 表 2 基于Coats-Redfern法处理的玉米秸秆热裂解动力学参数
Table 2 Kinetic parameters with Coats-Redfern method for corn stalk
Sample Temperaturet/℃ E/(kJ·mol-1) A/s-1 r Corn stalk 187-415 105.73 5032.73 0.973 Mixture of corn stalk and ZSM-5 171-467 91.28 243.69 0.964 表 3 玉米秸秆在不同温度下的15种主要催化热裂解产物及其相对含量
Table 3 Main compounds and their relative content of corn stalk catalytic pyrolysis at different pyrolysis temperatures
No. Name Molecular formula Relative content /% 450℃ 500℃ 550℃ 600℃ 1 carbon dioxide CO2 18.87 14.15 14.87 12.78 2 methyl glyoxal C3H4O2 7.30 7.34 8.30 7.44 3 2-propanone, 1-hydroxy- C3H6O2 3.11 3.07 2.68 3.36 4 toluene C7H8 4.76 1.69 3.33 2.79 5 furfural C5H4O2 3.92 3.15 3.17 3.31 6 benzene, 1, 3-dimethyl- C8H10 0.45 3.41 4.36 3.15 7 benzene, 1-ethyl-2-methyl- C9H12 2.16 0.27 0.46 0.34 8 valeramide, 5-chloro-N-methyl- C6H12ClNO 4.48 3.13 3.07 2.09 9 indene C9H8 - - 0.56 0.44 10 naphthalene C10H8 1.11 2.32 3.96 2.77 11 benzofuran, 2, 3-dihydro- C8H8O 6.78 6.47 5.48 6.63 12 naphthalene, 2-methyl- C11H10 2.07 2.90 3.48 2.04 13 2-methoxy-4-vinylphenol C9H10O2 4.21 4.45 3.99 3.80 14 naphthalene, 1, 6-dimethyl- C12H12 1.18 1.27 1.26 0.54 15 stigmasta-3, 5-diene C29H48 0.36 0.41 0.38 0.36 -
[1] CHEN D Y, GAO D X, CAPAREDA S C, HUANG S C, WANG Y. Effects of hydrochloric acid washing on the microstructure and pyrolysis bio-oil components of sweet sorghum bagasse[J]. Bioresour Technol, 2019, 277:37-45. doi: 10.1016/j.biortech.2019.01.023 [2] HU J J, LI C, GUO Q H, DANG J T, ZHANG Q G, LEE D J, YANG Y L. Syngas production by chemical-looping gasification of wheat straw with Fe-based oxygen carrier[J]. Bioresour Technol, 2018, 263:273-279. doi: 10.1016/j.biortech.2018.02.064 [3] MEI Y F, CHAI M Y, SHEN C J, LIU B B, LIU R H. Effect of methanol addition on properties and aging reaction mechanism of bio-oil during storage[J]. Fuel, 2019, 244:499-507. doi: 10.1016/j.fuel.2019.02.012 [4] CHEN T J, DENG C J, LIU R H. Effect of selective condensation on the characterization of bio-oil from pine sawdust fast pyrolysis using a fluidized-bed reactor[J]. Energy Fuels, 2010, 24(12):6616-6623. doi: 10.1021/ef1011963 [5] HUBER G W, IBORRA S, CORMA A. Synthesis of Transportation Fuels from Biomass:Chemistry, Catalysts, and Engineering[M]. United States:Chemical Reviews, 2006. [6] WANG S R, DAI G X, YANG H P, LUO Z Y. Lignocellulosic biomass pyrolysis mechanism:A state-of-the-art review[J]. Prog Energy Combust Sci, 2017, 62:33-86. doi: 10.1016/j.pecs.2017.05.004 [7] JAE J, TOMPSETT G A, FOSTER A J, HAMMOND K D, AUERBACH S M, LOBO R F, HUBER G W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion[J]. J Catal, 2011, 279(2):257-268. doi: 10.1016/j.jcat.2011.01.019 [8] DU S C, GAMLIEL D P, VALLA J A, BOLLAS G M. The effect of ZSM-5 catalyst support in catalytic pyrolysis of biomass and compounds abundant in pyrolysis bio-oils[J]. J Anal Appl Pyrolysis, 2016, 122:7-12. doi: 10.1016/j.jaap.2016.11.002 [9] LIU S Y, ZHANG Y N, FAN L L, ZHOU N, TIAN G Y, ZHU X D, CHENG Y L, WANG Y P, LIU Y H, CHEN P, RUAN R. Bio-oil production from sequential two-step catalytic fast microwave-assisted biomass pyrolysis[J]. Fuel, 2017, 196:261-268. doi: 10.1016/j.fuel.2017.01.116 [10] PARK H J, PARK K H, JEON J K, KIM J, RYOO R, JEONG K E, PARK S H, PARK Y K. Production of phenolics and aromatics by pyrolysis of miscanthus[J]. Fuel, 2012, 97:379-384. doi: 10.1016/j.fuel.2012.01.075 [11] TAARNING E, OSMUNDSEN C M, YANG X B, VOSS B, ANDERSEN S I, CHRISTENSEN C H. Zeolite-catalyzed biomass conversion to fuels and chemicals[J]. Energy Environ Sci, 2011, 4(3):793-804. doi: 10.1039/C004518G [12] GAYUBO A G, AGUAYO A T, ATUTXA A, AGUADO R, OLAZAR M, BILBAO J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. II. Aldehydes, ketones, and acids[J]. Ind Eng Chem Res, 2004, 43(11):2619-2626. doi: 10.1021/ie030792g [13] AHO A, KUMAR N, ERÄNEN K, SALMI T, HUPA M, MURZIN D Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor:Influence of the zeolite structure[J]. Fuel, 2008, 87(12):2493-2501. doi: 10.1016/j.fuel.2008.02.015 [14] LU Q, WANG Z, DONG C Q, ZHANG Z F, ZHANG Y, YANG Y P, ZHU X F. Selective fast pyrolysis of biomass impregnated with ZnCl2:Furfural production together with acetic acid and activated carbon as by-products[J]. J Anal Appl Pyrolysis, 2011, 91(1):273-279. doi: 10.1016/j.jaap.2011.03.002 [15] 李志合, 易维明, 高巧春, 李永军.生物质组分及温度对闪速热解挥发分的影响[J].燃料化学学报, 2005, 33(4):502-505. doi: 10.3969/j.issn.0253-2409.2005.04.025LI Zhi-he, YI Wei-ming, Gao Qiao-chun, Li Yong-jun. Effects of chem ical com ponents and tem perature on the volatility of biomass in flash pyrolysis[J]. J Fuel Chem Technol, 2005, 33(4):502-505. doi: 10.3969/j.issn.0253-2409.2005.04.025 [16] 宋春财, 胡浩权.秸秆及其主要组分的催化热解及动力学研究[J].煤炭转化, 2003, 26(3):91-97. doi: 10.3969/j.issn.1004-4248.2003.03.020SONG chun-cai, HU Hao-quan. Catalytic pyrolysis and kinetics of agricultural stalks and their main components[J]. Coal Convers, 2003, 26(3):91-97. doi: 10.3969/j.issn.1004-4248.2003.03.020 [17] 张智博.生物质快速催化热解制备高附加值化学品的研究[D].北京: 华北电力大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-11412-1016268862.htmZHANG Zhi-bo. Research on catalytic fast pyrolysis of biomass to produce value-added chemicals[D]. Beijing: North China Electric Power University, 2016. http://cdmd.cnki.com.cn/Article/CDMD-11412-1016268862.htm [18] CHAI M Y, HE Y F, NISHU, LIU R H. Effect of fractional condensers on characteristics, compounds distribution and phenols selection of bio-oil from pine sawdust fast pyrolysis[J]. J Energy Inst, 2020, 93(2):811-821. doi: 10.1016/j.joei.2019.05.001 [19] 陈登宇, 朱锡锋.生物质热反应机理与活化能确定方法Ⅱ.热解段研究[J].燃料化学学报, 2011, 39(9):670-674. doi: 10.3969/j.issn.0253-2409.2011.09.006CHEN Deng-yu, ZHU Xi-feng. Thermal reaction mechanism of biomass and determination of activation energy II.Pyrolysis section[J]. J Fuel Chem Technol, 2011, 39(9):670-674. doi: 10.3969/j.issn.0253-2409.2011.09.006 [20] GONG X M, WANG Z, DENG S, LI S G, SONG W L, LIN W G. Impact of the temperature, pressure, and particle size on tar composition from pyrolysis of three ranks of Chinese coals[J]. Energy Fuels, 2014, 28:4942-4948. doi: 10.1021/ef500986h [21] CARLSON T R, JAE J, LIN Y C, TOMPSETT G A, HUBER G W. Catalytic fast pyrolysis of glucose with HZSM-5:The combined homogeneous and heterogeneous reactions[J]. J Catal, 2010, 270(1):110-124. doi: 10.1016/j.jcat.2009.12.013 [22] XU X, JIANG E C. "BTX" from guaiacol HDO under atmospheric pressure:Effect of support and carbon deposition[J]. Energy Fuels, 2017, 31(3):2855-2864. doi: 10.1021/acs.energyfuels.6b02700 -





 下载:
下载: