-
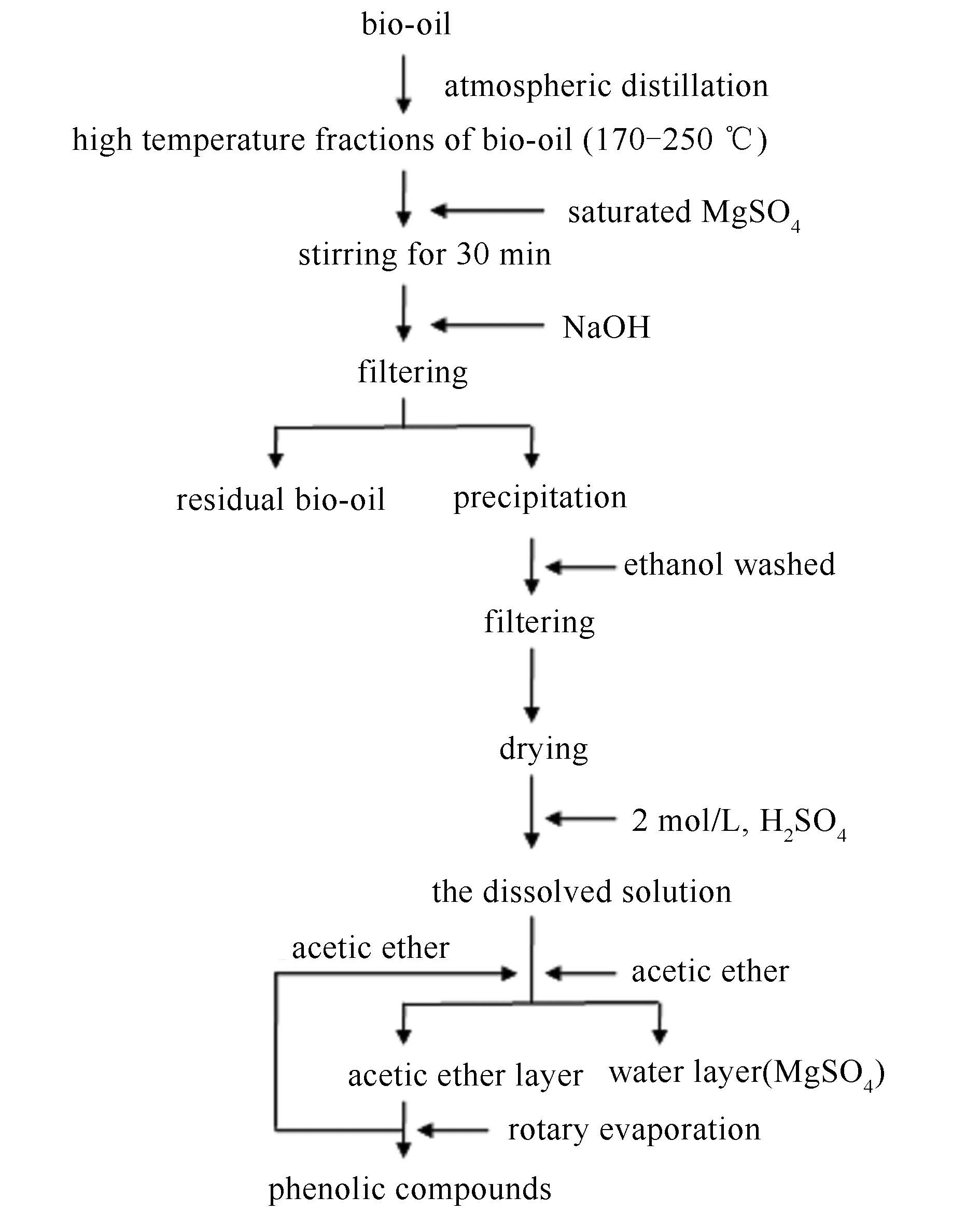
摘要: 为了研究金属离子沉淀法对生物油高温馏分中酚类物质的回收, 提高生物油中化学物质的利用率, 利用气相色谱-质谱联用仪 (GC-MS) 从NaOH试剂浓度、反应温度和反应时间三个方面研究了镁离子对生物油中酚类物质的回收效果。结果表明, 镁离子与酚类物质形成了不溶物, 而且不同浓度的氢氧化钠溶液 (1.0-4.0 mol/L)、不同的反应温度 (25-85℃) 以及不同的反应时间 (5-35 min) 对实验结果有着不同程度的影响。结果表明, 在反应温度为25℃、氢氧化钠浓度为2.5 mol/L, 反应时间在20 min时为最佳反应条件。在此条件下, 对生物油高温馏分中对乙基苯酚的回收率可达34.97%。Abstract: To improve the utilization of chemicals in bio-oil, recovery of phenolic compounds from high temperature bio-oil fractions by metal ion precipitation was studied.The effects of concentration of sodium hydroxide solution, reaction temperature and reaction time on the recovery of phenolic compounds by magnesium ion were examined using gas chromatography-mass spectrometry (GC-MS).The results demonstrate that the precipitation was formed instead of a salt of magnesium phenols.The optimum reaction conditions are reaction temperature of 25℃, 2.5 mol/L of sodium hydroxide solution, and reaction time of 20 min.In this case, the recovery of 4-ethylphenol from high temperature bio-oil fractions reaches 34.97%.
-
Key words:
- bio-oil /
- phenol /
- magnesium ion /
- precipitation /
- recovery
-
表 1 生物油高温馏分中酚类物质的化学成分
Table 1 Chemical compositions of phenolic compounds from high temperature fractions of bio-oil
No. Chemical compound Molecular formula Molecular weight /(g·mol-1) Structure Peak area /% 1 phenol C6H6O 94 
4.47 2 phenol, 2-methyl- C7H8O 108 5.67 3 phenol, 3-methyl- C7H8O 108 6.56 4 phenol, 2-methoxy- C7H8O2 124 3.35 5 2, 5-xylenol C8H10O 122 1.88 6 phenol, 4-ethyl- C8H10O 122 21.5 7 phenol, 2-methoxy-4-methyl- C8H10O2 138 2.86 8 1, 2-benzenediol C6H6O2 110 3.41 9 phenol, 3-(1-methylethyl)- C9H10O 134 3.05 10 phenol, 3-methyl-4-ethyl- C9H10O 134 1.58 11 phenol, 4-isopropyl- C9H12O 136 3.89 12 guaiacol, 4-ethyl- C9H10O2 150 3.75 13 1, 2-benzenediol, 4-methyl- C7H8O2 124 
2.1 14 phenol, 4-sec-butyl- C10H14O 150 2.37 15 phenol, 2, 6-dimethoxy- C8H10O3 154 3.15 16 guaiacol, 5-allyl- C10H12O2 164 2.51 17 phenol, 2-methoxy-4-propyl- C10H14O2 166 2.94 18 catechol, 4-ethyl- C8H10O2 138 2.2 19 guaiacol, 4-propenyl- C10H12O2 164 2.04 表 2 乙酸乙酯层中的酚类化合物
Table 2 Phenolic compounds identified in the ethyl acetate layer
No. Chemical compound Molecular formula Molecular weight /(g·mol-1) Structure Peak area /% 1 phenol C6H6O 94 
5.48 2 phenol, 3-methyl- C7H8O 108 6.47 3 guaiacol C7H8O2 124 4.03 4 phenol, 4-ethyl- C8H10O 122 21.75 5 guaiacol, 4-methyl C8H10O2 138 3.74 6 catechol C6H6O2 110 3.19 7 phenol, 3-(1-methylethyl)- C9H10O 134 3.95 8 phenol, 2-ethyl-5-methyl- C9H12O 136 2.09 9 phenol, 4-propyl- C9H12O 136 4.01 10 guaiacol, 4-ethyl- C9H10O2 150 5.6 11 catechol, 4-methyl- C8H10O2 138 1.77 12 phenol, 4-sec-butyl- C10H14O 150 
1.48 13 phenol, 2, 6-dimethoxy- C8H10O3 154 2.78 14 guaiacol, 5-allyl- C10H12O2 156 1.85 15 phenol, 2-methoxy-4-propyl- C10H14O2 166 3.96 16 catechol, 4-ethyl- C8H10O2 138 1.56 17 guaiacol, 4-propenyl- C9H12O 136 2.1 -
[1] 朱锡锋.生物油制备技术[M].北京:化学工业出版社, 2013.ZHU Xi-feng.Preparation and application of bio-oil[M].Beijing:Chemical Industry Presss, 2013. [2] OASMAA A, CZERNIK S.Fuel oil quality of biomass pyrolysis oils-state of the art for the end user[J].Energy Fuels, 1999, 13:914-921. doi: 10.1021/ef980272b [3] BRIDGWATER A V, PEACOCKE G V C.Fast pyrolysis processes for biomass[J].Renew Sust Energy Rev, 2000, 4:1-73. doi: 10.1016/S1364-0321(99)00007-6 [4] AMEN-CHEN C, PAKDEL H, ROY C.Separation of phenols from Eucalyptus wood tar[J].Biomass Bioenergy, 1997, 13(1/2):25-37. [5] MAHFUD F H, VAN GEEL F P, VENDERBOSCH R H, HEERES H J.Acetic acid recovery from fast pyrolysis oil:An exploratory study on liquid-liquid reactive extraction using aliphatic tertiary amines[J].Sep Sci Technol, 2008, 43(11/12):3056-3074. [6] GANDER M, RAPP K M, SCHIWECK H.Process for preparing 1, 6-β-anhydroglucopyranose (levoglucosan) in high purity:US, 5023330[P].1991-06-11. [7] STRADAL J A, UNDERWOOD G L.Process for producing hydroxyacetaldehyde:US, 5252188[P].1993-10-12. [8] STRADAL J A, UNDERWOOD G L.Process for producing hydroxyacetaldehyde:US, 5393542[P].1995-02-28. [9] DINESH M, CHARLES U P, PHILIP H S.Pyrolysis of wood/biomass for bio-oil:A critical review[J].Energy Fuels, 2006, 20:848-889. doi: 10.1021/ef0502397 [10] EFFENDI A, GERHAUSER H, BRIDGWATER A V.Production of renewable phenolic resins by thermochemical conversion of biomass:A review[J].Renew Sust Energy Rev, 2008, 12:2092-2116. doi: 10.1016/j.rser.2007.04.008 [11] LI J, WANG C, YANG Z.Production and separation of phenols from biomass-derived bio-petroleum[J].J Anal Appl Pyrolysis, 2010, 89(2):218-224. doi: 10.1016/j.jaap.2010.08.004 [12] GE Y Z, JIN H.Recovery process for phenolic compounds from coal-derived oils by ions of solublemetal salts[J].Fuel, 1996, 75:1681-1683. doi: 10.1016/0016-2361(95)00276-6 [13] WANG D, LI D B, LIU Y Q, LV D C, YE Y Y, ZHU S J, ZHANG B B.Study of a new complex method for extraction of phenolic compounds from bio-oils[J].Sep Purif Technol, 2014, 134:132-138. doi: 10.1016/j.seppur.2014.07.033 [14] 葛宜掌, 金红, 李斌.Ba2+离子沉淀法回收酚类化学过程的研究[J].燃料化学学报, 1996, 24(3):266-270. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX603.014.htmGE Yi-zhang, JIN Hong, LI Bin.Study on recovery of phenolic compounds using Ba2+ as precipitant[J].J Fuel Chem Technol, 1996, 24(3):266-270. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX603.014.htm [15] JEWEL A.CAPUNITAN, SERGIO C.CAPAREDA.Characterization and separation of corn stover bio-oil by fractional distillation[J].Fuel, 2013, 112:60-73. doi: 10.1016/j.fuel.2013.04.079 [16] 明珠, 吴云海, 阿依妮尕尔·艾尔肯.茶汤与金属离子络合反应的研究[J].环境科技, 2015, 28(4):5-11. http://youxian.cnki.com.cn/yxdetail.aspx?filename=JSHJ2015072100F&dbname=CAPJ2015MING Zhu, WU Yun-hai, ARKEN·AYINIGAR.Study on complexation reaction of tea infusion with metal ions[J].Environ Sci Technol, 2015, 28(4):5-11. http://youxian.cnki.com.cn/yxdetail.aspx?filename=JSHJ2015072100F&dbname=CAPJ2015 [17] GE Y, JIN H.Recovery of phenols from coal tar and waste water by precipitation[J].J China Coal Soc, 1995, 20:545-548. -





 下载:
下载: