Synthesis of three-dimensionally ordered macroporous LaFe0.7Co0.3O3 perovskites and their performance for chemical-looping steam reforming of methane
-
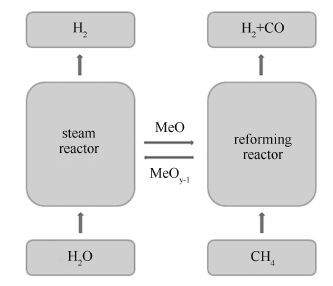
摘要: 采用无皂乳液聚合法制备聚苯乙烯(PS)微球,通过自组装得到排列均匀有序的聚苯乙烯(PS)胶晶模板,然后经过浸渍和煅烧得到三维有序大孔(3DOM)钙钛矿型氧化物LaFe0.7Co0.3O3。通过扫描电镜、透射电镜和X射线衍射等手段对制备的3DOM钙钛矿型氧化物LaFe0.7Co0.3O3的物理化学性能进行表征。在固定床反应器上考察3DOM LaFe0.7Co0.3O3的甲烷化学链水蒸气重整性能。结果表明,聚苯乙烯(PS)微球粒径受苯乙烯单体使用量的影响,随着苯乙烯单体使用量的增加聚苯乙烯(PS)微球粒径呈增大的趋势;煅烧温度对三维有序大孔结构有显著影响,浸渍后模板在500℃煅烧下即能形成三维有序大孔结构比表面积达到19.820 m2/g,随着煅烧温度的升高三维有序大孔结构遭到部分破坏,在900℃煅烧下三维有序大孔结构遭到完全破坏。在氧载体与甲烷的反应前期,气体产物中CO2含量较高,是表面吸附氧将甲烷完全氧化所致,在表面吸附氧消耗完后体相晶格氧将甲烷部分氧化生成H2与CO。在水蒸气氧化阶段,水蒸气与还原态的氧载体发生反应生成氢气,产氢率为4.0-5.0 mmol/g。同时水蒸气氧化阶段气相产物中CO和CO2含量很低,说明3DOM LaFe0.7Co0.3O3具有优秀的抗积炭性能。Abstract: Three-dimensionally ordered macroporous (3DOM) LaFe0.7Co0.3O3 perovskite-type oxides were synthesized using a polystyrene (PS) colloidal crystal templating method. The obtained 3DOM LaFe0.7Co0.3O3 perovskites were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Brunauere-Emmette-Teller (BET) surface area. Its performance as oxygen carriers in chemical looping steam methane reforming (CL-SMR) to produce syngas (H2 + CO) and hydrogen were investigated in a fixed-bed reactor. The size of PS spheres obviously increases as the styrene addition increases. The calcination temperature is the major factor to affect the prepared 3DOM perovskite. SEM and TEM analysis show that the samples calcined at 500, 800 and 850℃ exhibit good 3DOM structures which collapse when the sample is calcined at 900℃. XRD results suggest that the obtained 3DOM LaFe0.7Co0.3O3 perovskites are pure crystalline. Two kinds of oxygen species, bulk lattice oxygen and surface adsorbed oxygen, are found to exist on the 3DOM LaFe0.7Co0.3O3 perovskites. The surface oxygen contributes to the complete oxidization of methane to CO2 and H2O in beginning of the reaction, while the bulk lattice oxygen tends towards partial methane oxidation to H2 and CO. In the methane conversion step, methane is partially oxidized into syngas at a H2/CO mol ratio close to 2:1 by the 3DOM- LaFe0.7Co0.3O3 in a wide range of the reactions, suggesting that the sample exhibits a good resistance to carbon deposition. In the steam oxidation step, the reduced perovskites are oxidized by steam to generate hydrogen with hydrogen productivity about 4 mmol/g oxygen carriers.
-
Key words:
- 3DOM /
- perovskite /
- oxygen carrier /
- syngas /
- chemical-looping steam reforming of methane /
- hydrogen
-
图 3 不同煅烧温度下制备的3DOM LaFe0.7Co0.3O3氧载体的SEM和 TEM照片
Figure 3 SEM and TEM pictures of 3DOM LaFe0.7Co0.3O3
(a1,a2) : SEM of LFC-500; (b1,b2) : TEM of LFC-500; (c1,c2) : SEM of LFC-800; (d1,d2) : TEM of LFC-800;(e1,e2) : SEM of LFC-850; (f1,f2) : TEM of LFC-850; (g1,g2) : SEM of LFC-900; (h1,h2) : TEM of LFC-900
表 1 不同煅烧温度下制备的3DOM-LFC氧载体的BET比表面积及晶粒粒径
Table 1 Specific surface area and crystallite size of 3DOM-LFC with different calcination temperatures
Oxygen carrier LFC-500 LFC-800 LFC-850 LFC-900 Specific surface area A/(m2·g-1) 19.820 17.305 10.192 4.485 Crystallite size d/nm 12.4 68.1 76.1 86.1 表 2 新鲜的LFC-850氧载体以及LFC-850经过循环反应后的BET比表面积及晶粒粒径
Table 2 Specific surface area and crystallite size of LFC-850-fresh and LFC-850 after cyclic redox
Oxygen carrier LFC-850-fresh LFC-850 after cyclic redox Specific surface area A/(m2·g-1) 10.192 8.581 Crystallite size d/nm 76.1 91.8 -
[1] RICHTER H J, KNOCHE K F.Reversibility of combustion process, efficiency and costing, second Law analysis processes[C].Washington DC:ACS Symposium Series, 1983, 235:71-85. [2] 李孔斋, 王华, 魏永刚, 敖先权, 刘明春.晶格氧部分氧化甲烷制合成气[J].化学进展, 2008, 20(9):1306-1314. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200809007.htmLI Kong-zhai, WANG Hua, WEI Yong-gang, AO Xian-quan, LIU Ming-chun.Partial oxidation of methane to synthesis gas using lattice oxygen[J].Prog Chem, 2008, 20(9):1306-1314. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200809007.htm [3] 严前古, 于作龙, 远松月.甲烷部分氧化制合成气研究进展[J].石油与天然气化工, 1997, 26(3):145-151. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG199703004.htmYAN Qian-gu, YU Zuo-long, YUAN Song-yue.Research progresses in methane partial oxidation tosynthesis gas[J].Chem Eng Oil Gas, 1997, 26(3):145-151. http://www.cnki.com.cn/Article/CJFDTOTAL-STQG199703004.htm [4] ZAFAR Q, MATTISSON T, GEVERT B.Integrated hydrogen and power productionwith CO2 capture using chemical-looping reforming-redox reactivity ofparticles of CuO, Mn2O3, NiO, and Fe2O3 using SiO2 as a support[J].Ind Eng Chem Res, 2005, 44(6):3485-3496. [5] DE DIEGO L F, ORTIZ M, ADÁNEZ J, GARCÍA-LABIANO F, ABAD A, GAYÁN P.Synthesis gas generation by chemical-looping reforming in a batch fluidized bed reactor using Ni-based oxygen carriers[J].Chem Eng J, 2008, 144(2):289-298. doi: 10.1016/j.cej.2008.06.004 [6] RYDÉN M, LYNGFELT A, MATTISSON T.Synthesis gas generation by chemicallooping reforming in a continuously operating laboratory reactor[J].Fuel, 2006, 85(12/13):1631-1641. [7] RYDÉN M, LYNGFELT A, MATTISSON T, CHEN D, HOLMEN A, BJØRGUM E.Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming;LaxSr1-xFeyCo1-yO3-δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4[J].Int J Greenh Gas Control, 2008, 2(1):21-36. doi: 10.1016/S1750-5836(07)00107-7 [8] CHENS Q, LIUY.LaFeyNi1-yO3 supported nickel catalysts used for steam reforming of ethanol[J].Int J Hydrogen Energy, 2009, 34(11):4735-4746. doi: 10.1016/j.ijhydene.2009.03.048 [9] 李然家, 余长春, 代小平, 沈师孔.钙钛矿型La0.8Sr0.2FeO3中的晶格氧用于甲烷选择氧化制取合成气[J].催化学报, 2002, 23(6):549-554.LI Ran-jia, YU Chang-chun, DAI Xiao-ping, SHEN Shi-kong.Selective oxidation of methane to synthesis gas using lattice oxygen from perovskite La0.8Sr0.2 FeO3 catalyst[J].Chin J Catal, 2002, 23(6):549-554. [10] HE F, LI X, ZHAO K, HUANG Z, WEI G.The use of La1-xSrxFeO3 perovskite-type oxides as oxygen carriersin chemical-looping reforming of methane[J].Fuel, 2013, 108(11):465-473. [11] JENNIF ER ER, ANJA O, YNGVE L, RICHARD B.La0.8Sr0.2Co0.2Fe0.8O3-δ as a potential oxygen carrier in a chemical looping type reactor, an in-situ powder X-ray diffraction study[J].J Mater Chem, 2005, 15:1931-1937. doi: 10.1039/b416526h [12] SADAKANE M, HORIUCHI T, KATO N, TAKAHASHI C, UEDA W.Facile preparation of three-dimensionally ordered macroporous alumina, iron oxide, chromium oxide, manganese oxide, and their mixed-metal oxides with high porosity[J].Chem Mater, 2007, 19(23):5779-5785. doi: 10.1021/cm071823r [13] 何方, 赵坤, 黄振, 李新爱, 魏国强, 李海滨.三维有序大孔钙钛矿型氧化物LaFeO3的合成及甲烷化学链重整性能[J].催化学报, 2013, 34(6):1242-1249. doi: 10.1016/S1872-2067(12)60563-4HE Fang, ZHAO Kun, HUANG Zhen, LI Xin-ai, WEI Guo-qiang, LI Hai-bin.Synthesis of three-dimensionally ordered macroporous LaFeO3 perovskites and their performance for chemical-looping reforming of methane[J].Chin J Catal, 2013, 34(6):1242-1249. doi: 10.1016/S1872-2067(12)60563-4 [14] ZHAO K, HE F, HUANG Z, ZHENG A, LI H.Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane[J].Int J Hydrogen Energy, 2014, 39(7):3243-3252. doi: 10.1016/j.ijhydene.2013.12.046 [15] SONG Z Q, POEHLEIN G W.Particle nucleation in emulsifier-free aqueous-phase polymerization:Stage 1[J].J ColloidInterf, 1989, 128(2):486-500. [16] SONG Z Q, POEHLEIN G W.Particle formation inemulsifier-freeaqueous-phase polymerization of styrene[J].J ColloidInterf, 1989, 128(2):501-510. [17] ZHENG Y, WEI Y G, LI K Z, ZHU X, WANG H, WANG Y H.Chemical-looping steam methane reforming over macroporous CeO2-ZrO2 solid solution:Effect of calcination temperature[J].Int J Hydrogen Energy, 2014, 39(25):13361-13368. doi: 10.1016/j.ijhydene.2014.04.116 -





 下载:
下载: