Synthesis of Ni-based catalysts supported on nitrogen-incorporated SBA-16 and their catalytic performance in the reforming of methane with carbon dioxide
-
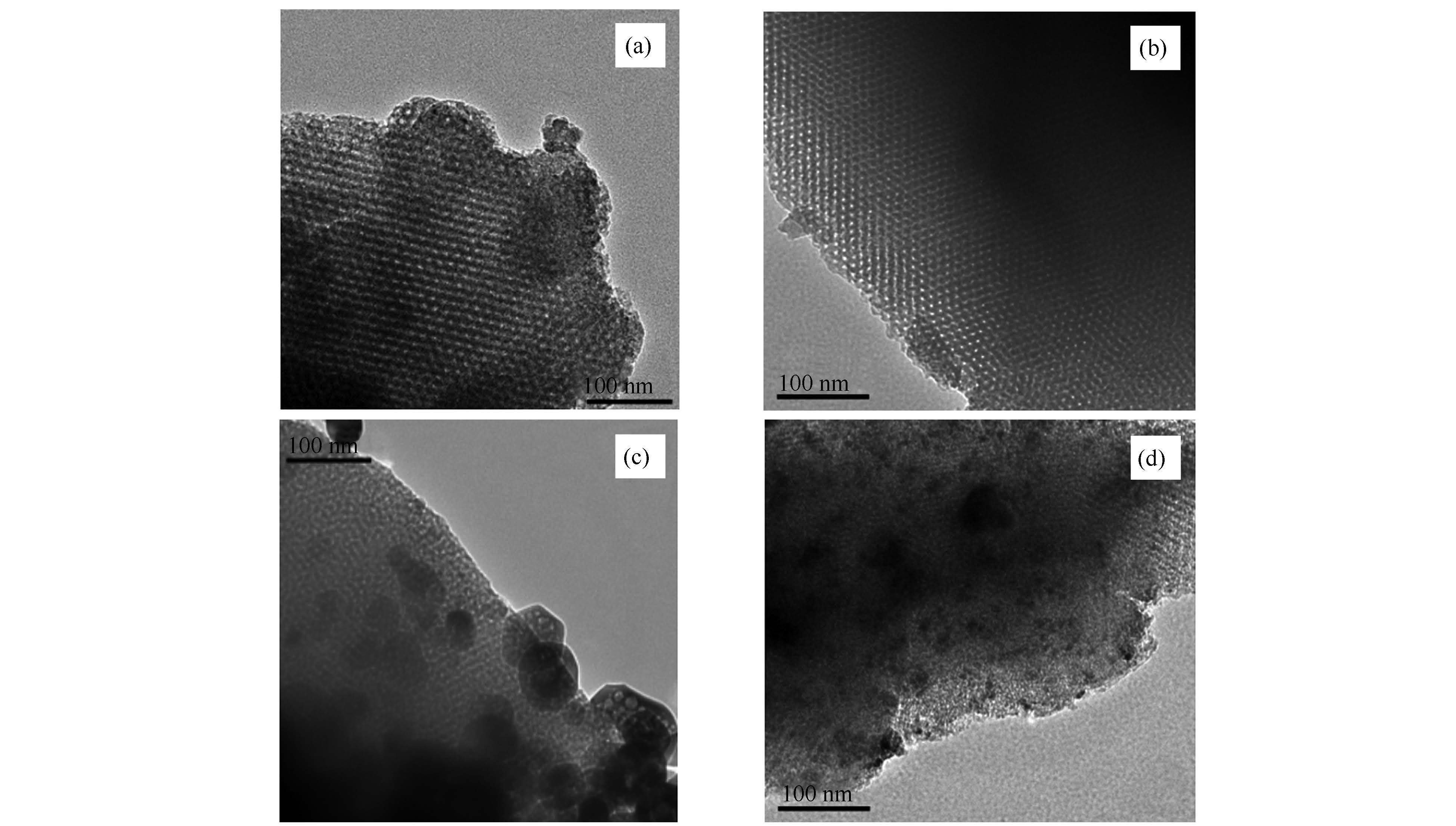
摘要: 通过软模板法合成了SBA-16分子筛,采用高温氨气氮化的方法使有序介孔硅材料中的氧原子部分被氮原子取代,得到氮化的SBA-16载体(SBA-16-N)。采用满孔浸渍法制备了镍基催化剂,并将制得的Ni/SBA-16和Ni/SBA-16-N催化剂用于甲烷二氧化碳重整反应。通过透射电镜、氮气物理吸附、X射线衍射、X射线光电子能谱和二氧化碳程序升温脱附等手段研究了载体和催化剂的结构,并利用热重分析对反应之后回收催化剂进行了表征。结果表明,高温氮化后的分子筛中掺入了氮元素,增加了载体的碱性,改善了载体对反应气体的吸附活化能力,增强了载体与金属之间的相互作用,从而提高了催化剂的活性和抗积炭性能。Abstract: SBA-16 molecular sieve was synthesized by soft template method and modified with ammonia under high temperature to obtain the nitrogen-incorporated SBA-16(SBA-16-N);with SBA-16 and SBA-16-N as the supports, nickel based catalysts (Ni/SBA-16 and Ni/SBA-16-N) were prepared by the incipient impregnation method.The related supports and Ni-based catalysts were characterized by transmission electron microscope, X-ray photoelectron spectroscopy, nitrogen physisorption, carbon dioxide temperature programmed desorption, X-ray diffraction and thermogravimetric analysis;the catalytic performance of Ni/SBA-16 and Ni/SBA-16-N in the reforming of methane with carbon dioxide was comparatively investigated under 500-800℃.The results indicate that after the nitriding modification, the Ni/SBA-16-N catalyst exhibits much higher activity in methane reforming with CO2 and higher selectivity to syngas than the Ni/SBA-16 catalyst without nitridation treatment.During the nitriding modification at high temperature, O atoms in the SBA-16 skeleton are partially replaced by N atoms, which can strengthen the support basicity and the interaction between the support and the nickel nanoparticles.All these may promote the dispersion of nickel nanoparticles on SBA-16-N and the adsorption of CO2 on Ni/SBA-16-N, leading to the improvement of the activity and stability of the Ni/SBA-16-N catalysts in methane reforming with carbon dioxide.
-
Key words:
- methane reforming /
- carbon dioxide /
- nitridation /
- SBA-16 /
- nickel based catalyst
-
表 1 载体及催化剂的孔结构参数
Table 1 Textural properties of various supports and catalysts
Catalyst Specific surface areas A/(m2·g-1) Pore volume v/(cm3·g-1) Pore sized/nm SBA-16 705.0 0.50 3.8 SBA-16-N 203.0 0.16 3.2 Ni/SBA-16 383.1 0.41 3.6 Ni/SBA-16-N 160.2 0.14 3.1 表 2 催化剂Ni/SBA-16和Ni/SBA-16-N的Ni 2p3/2电子结合能
Table 2 Ni 2p3/2 XPS results of the Ni/SBA-16 and Ni/SBA-16-N catalysts
Catalyst Binding energy E/eV Ni/SBA-16 854.5 Ni/SBA-16-N 855.1 -
[1] FAN M S, ABDULLAH A Z, BHATIA S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas[J]. ChemCatChem, 2009, 1(2):192-208. doi: 10.1002/cctc.v1:2 [2] QIAN L, CAI W, ZHANG L, YE L, LI J, TANG M, YUE B, HE H.The promotion effect of hydrogen spillover on CH4 reforming with CO2 over Rh/MCF catalysts[J]. Appl Catal B:Environ, 2015, 164:168-175. doi: 10.1016/j.apcatb.2014.09.006 [3] 王莉, 敖先权, 王诗瀚. 甲烷与二氧化碳催化重整制取合成气催化剂[J]. 化学进展, 2012, 9:1696-1706. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201209010.htmWANG Li, AO Xian-quan,WANG Shi-han.Catalysts for carbon dioxide catalytic reforming of methane to synthesis gas[J]. Prog Chem, 2012, 9:1696-1706. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201209010.htm [4] SHANG R, GUO X, MU S, WANG Y, JIN G, KOSSLICK H, SCHULZ A, GUO X Y. Carbon dioxide reforming of methane to synthesis gas over Ni/Si3N4 catalysts[J]. Int J Hydrogen Energy, 2011, 36(8):4900-4907. doi: 10.1016/j.ijhydene.2011.01.034 [5] 瑙莫汗, 付晓娟, 雷艳秋, 苏海全. 介孔Ni-β, -Mo2C/SBA-16催化剂在CH4/CO2重整制合成气反应中的催化性能[J]. 催化学报, 2013, 34(2):379-384.NAO Mo-han, FU Xiao-juan, LEI Yan-qiu, SU Hai-quan. Catalytic performance of mesoporous material supported bimetallic carbide Ni-β, -Mo2C/SBA-16 catalyst for CH4/CO2 reforming to syngas[J]. Chin J Catal, 2013, 34(2):379-384. [6] BRADFORD M C J, VANNICE M A. CO2 reforming of CH4[J]. Cat Rev Sci Eng, 1999, 41(1):1-42. doi: 10.1081/CR-100101948 [7] WANG N, YU X, WANG Y, CHU W, LIU M.A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier[J]. Catal Today, 2013, 212(1):98-107. [8] LIU H, LI Y, WU H, TAKAYAMA H, MIYAKE T, HE D. Effects of β, -cyclodextrin modification on properties of Ni/SBA-15 and its catalytic performance in carbon dioxide reforming of methane[J]. Catal Commun, 2012, 28(5):168-173. [9] NANDINI A P, KAMAL K P, SUBHASH C D. Deactivation studies over Ni-K/CeO2-Al2O3 catalyst for dry reforming of methane[J]. Ind Eng Chem Res, 2007, 46(6):1731-1736. doi: 10.1021/ie061389n [10] 郭朋飞, 靳国强, 郭聪秀, 王英勇, 童希立, 郭向云. Yb2O3助剂对Ni/SiC催化剂甲烷二氧化碳重整性能的影响[J]. 燃料化学学报, 2014, 42(6):719-726. doi: 10.1016/S1872-5813(14)60033-5GUO Peng-fei, JIN Guo-qiang, GUO Cong-xiu, WANG Ying-yong, TONG Xi-li, GUO Xiang-yun. Effects of Yb2O3 promotor on the performance of Ni/SiC catalysts in CO2 reforming of CH4[J]. J Fuel Chem Technol, 2014, 42(6):719-726. doi: 10.1016/S1872-5813(14)60033-5 [11] AGARWAL V, HUBER G W, CONNER W C J R, AUERBACH S M. DFT study of nitrided zeolites:Mechanism of nitrogen substitution in HYandsilicalite[J]. J Catal, 2010, 269:53-63. doi: 10.1016/j.jcat.2009.10.015 [12] YAMAMOTO K, TATSUMI T. ZOL:A new type of organic-inorganic hybrid zeolites containing organic framework[J]. Chem Mater, 2008, 20:972-980. doi: 10.1021/cm7028646 [13] 武光军, 关乃佳, 李兰冬. 含氮分子筛的研究进展[J]. 催化学报, 2012, 33(1):51-59. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201201008.htmWU Guang-jun, GUAN Nai-jia, LI Lan-dong. Recent development of nitrogen-incorporated molecular sieves[J].Chin J Catal, 2012, 33(1):51-59. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201201008.htm [14] CHINO N, OKUBO T. Nitridationmechanism of mesoporous silica:SBA-15[J]. Microporous Mesoporous Mater, 2005, 87(1):15-22. doi: 10.1016/j.micromeso.2005.07.034 [15] PATIL U, FIHRI A, EMWAS A H, POLSHETTIWAR V. Silicon oxynitrides of KCC-1, SBA-15 and MCM-41 for CO2 capture with excellent stability and regenerability[J]. Chem Sci, 2012, 3(7):2224-2229. doi: 10.1039/c2sc20356a [16] KIM Y, KUSAKABE K, YANG S. Microporous silica membrane synthesized on an ordered mesoporous silica sublayer[J]. Chem Mater, 2003, 15:612-615. doi: 10.1021/cm020136r [17] KLEITZ F, KIM T W, RYOO R. Design of mesoporous silica at low acid concentrations in triblock copolymer-butanol-water systems[J]. Bull Korean Chem Soc, 2005, 26:1653-1668. doi: 10.5012/bkcs.2005.26.11.1653 [18] KIM T W. Characterization of mesoporous carbons synthesized with SBA-16 silica template[J]. J Mater Chem, 2005, 15(15):1560-1571. doi: 10.1039/b417804a [19] ZHAO Y, ZHANG Y, CHEN J, LI J, LIEW K, NORDIN M R B. SBA-16-supported cobalt catalyst with high activity and stability for fischer-tropsch synthesis[J].ChemCatChem, 2012, 4(2):265-272. doi: 10.1002/cctc.201100223 [20] LI L, YANG Z, YUAN Y, ZHANG Y, LI J. Synthesis and characterization of nanosilver catalysts supported on the nitrogen-incorporated-SBA-15 for the low-temperature selective CO oxidation[J]. J Porous Mater, 2015, 22:1473-1482. doi: 10.1007/s10934-015-0028-4 [21] KIM J H, KIM K L.A study of preparation of tungsten nitride catalysts with high surface area[J].Appl Catal A:Gen, 1999, 181(1):103-111. doi: 10.1016/S0926-860X(98)00411-6 [22] ZHANG S, MURATSUGU S, ISHIGURO N, TADA M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane[J]. ACS Catal, 2013, 3(3):1855-1864. [23] XU L, ZHAO H, SONG H, CHOU L. Ordered mesoporous alumina supported nickel based catalysts for carbon dioxide reforming of methane[J]. Int J Hydrogen Energy, 2012, 37(9):7497-7511. doi: 10.1016/j.ijhydene.2012.01.105 [24] PARMALIANA A,ARENA F,FRUSTERI F, GIORDANO N F.Temperature programmed reduction study of NiO-MgO interactions in magnesia supported Ni catalysts and NiO-MgO physical mixture[J]. J Chem Soc, Faraday Trans, 1990, 86(14):2663-2669. doi: 10.1039/FT9908602663 [25] LEDNOR P W, RUITER R D. The preparation of silicon oxynitride, Si2N20, as a high surface area powder by reaction of silica with ammonia at 1 100℃[J]. J Chem Soc, Chem Commun, 1989, 5:320-321. [26] BENDJERIOU-SEDJERARI A, PELLETIER J D, ABOU-HAMAD E, EMSLEY L, BASSET J M. A well-defined mesoporous amine silica surface via a selective treatment of SBA-15 with ammonia[J]. Chem Commun, 2012, 48(25):3067-3069. doi: 10.1039/c2cc00143h [27] ASENCIOSYJ O, ASSAFE M, ASENCIOS Y J O, ASSAF E M. Combination of dry reforming and partial oxidation of methane on NiO-MgO-ZrO2 catalyst:Effect of nickel content[J]. Fuel Process Technol, 2013, 106(2):247-252. [28] GUO J, LOU H, ZHAO H, CHAI, ZHENG X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels[J]. Appl Catal A:Gen, 2004, 273(s1/2):75-82. [29] WANG S, LU G Q. CO2 reforming of methane on Ni catalysts:Effects of the support phase and preparation technique[J]. Appl Catal B:Environ, 1998, 16(3):269-277. doi: 10.1016/S0926-3373(97)00083-0 [30] WANG S AND LU G Q. Effects of promoters on catalytic activity and carbon depositionof Ni/γ, -Al2O3 catalysts in CO2 reforming of CH4[J]. J Chem Technol Biotechnol, 2000, 75(7):589-595. doi: 10.1002/(ISSN)1097-4660 [31] TRIMM D L. Catalysts for the control of coking during steam reforming[J]. Catal Today, 1999, 49(s1/3):3-10. -





 下载:
下载: