Simultaneous removal of SO2, NOx and Hg0 by O3 oxidation integrated with bio-charcoal adsorption
-
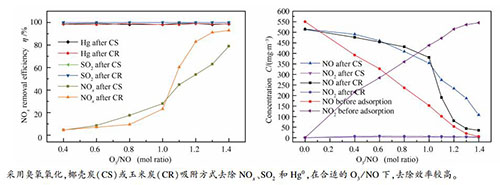
摘要: 以O3为氧化剂,玉米生物炭和椰壳活性炭为吸附剂,开展同时脱硫脱硝除汞研究。研究考察了温度、O3/NO和吸附时间对玉米/椰壳炭脱硫脱硝除汞效率的影响,并对玉米/椰壳炭进行表征分析。结果表明,NO和Hg0氧化率随O3/NO升高而升高,SO2氧化率则先升高后略微降低;温度升高抑制NO氧化但促进Hg0和SO2氧化;在140 ℃下,当O3/NO为1.4时,NO、Hg0和SO2氧化率分别达99%、78.6%和3.5%,随O3/NO从0.4升至1.4,玉米炭对NOx脱除效率从4.6%提升至93%,椰壳炭从4.5%提升至79%。玉米/椰壳炭会还原部分NO2造成出口NO浓度上升,但玉米炭的NOx吸附性能较椰壳炭强,而椰壳炭对Hg0和SO2的吸附性能较强。椰壳炭较玉米炭具有更强的物理吸附能力;玉米炭表面含氧官能团C-O和C=O的相对含量较椰壳炭高,而COOH和O=C-O相对含量较椰壳炭低。Abstract: Simultaneous removal of SO2, NOx and Hg0 by using O3 and corn bio-charcoal/coconut shell activated carbon was compared in a fixed bed. The effects of temperature, adsorption time and ratio of O3/NO on the removal efficiency of NO, Hg0 and SO2 by corn/coconut shell charcoal were studied, and the corn/coconut shell charcoal was characterized and analyzed. The results indicated that the oxidation rate of NO and Hg0 increases with the increase of O3/NO ratio, while the oxidation rate of SO2 first increases and then decreases slightly. The increase of temperature inhibits the oxidation of NO but promotes the oxidation of Hg0 and SO2. At 140 ℃ and O3/NO ratio of 1.4, the oxidation rate of NO, Hg0 and SO2 is 99%, 78.6% and 3.5%, respectively. As O3/NO ratio increases from 0.4 to 1.4, the removal efficiency of corn charcoal for NOx increases from 4.6% to 93%, and coconut shell charcoal increases from 4.5% to 79%. Corn/coconut shell charcoal can reduce part of NO2 and increase NO concentration at the outlet. NOx adsorption performance on corn charcoal is relatively better, while those of Hg0 and SO2 on coconut charcoal are relatively stronger. Coconut shell charcoal has stronger physical adsorption capacity than corn charcoal. The relative content of oxygen-containing functional groups C-O and C=O on the surface of corn charcoal is higher than that of coconut shell charcoal, while its relative content of COOH and O=C-O is lower.1) 本文的英文电子版由Elsevier出版社在ScienceDirect上出版(http://www.sciencedirect.com/science/journal/18725813).
-
表 1 样品的元素分析
Table 1 Elemental composition of the sample
Sample Uitimate analysis w/% O/C N/C (O+N)/C C N H S O CS 79.984 0.249 1.258 0.667 17.842 0.223 0.003 0.226 CR 63.254 1.582 1.698 0.268 33.198 0.525 0.025 0.549 表 2 C 1s、O 1s和N 1s含量及比例
Table 2 Content and proportion of C 1s, O 1s and N 1s obtained from XPS
Sample C 1s O 1s N 1s O/C N/C CS 91.38 7.44 0.58 0.081 0.006 CR 81.71 14.53 1.61 0.178 0.019 表 3 样品的C 1s分峰
Table 3 C 1s peak fitting results of samples
Sample Peak label Peak position /eV Functional group Content CS C1 284.8 C-C 0.554 C2 286.1 C-O 0.228 C3 288.5 C=O 0.067 C4 290.6 COOH 0.151 CR C1 284.8 C-C 0.599 C2 286.1 C-O 0.267 C3 288.5 C=O 0.115 C4 290.6 COOH 0.100 表 4 样品的O 1s分峰
Table 4 O 1s peak fitting results of samples
Sample Peak label Peak position /eV Functional group Content CS O1 531.5 C=O 0.171 O2 532.5 C-O 0.532 O3 533.3 O=C-O 0.098 O4 534.2 COOH 0.199 CR O1 531.5 C=O 0.284 O2 532.5 C-O 0.621 O3 533.3 O=C-O 0.039 O4 534.2 COOH 0.066 -
[1] SARBASSOV Y, DUAN L, JEREMIAS M, MANOVIC V, EDWARD J. SO3 formation and the effect of fly ash in a bubbling fluidised bed under oxy-fuel combustion conditions[J]. Fuel Process Technol, 2017, 167(1): 314-21. [2] ZHENG Y, JENSEN A D, WINDELIN C, JENSEN F. Review of technologies for mercury removal from flue gas from cement production processes[J]. Prog Energy Combust Sci, 2012, 38 (5): 599-629. [3] JIN D S, DESHWAL B R, PARK Y S, LEE H K. Simultaneous removal of SO2 and NO by wet scrubbing using aqueous chlorine dioxide solution[J]. J Hazaed Mater, 2006, 135(1/3): 412-417. [4] 周国民, 唐建成, 胡振广, 赵海军, 龚家猷.燃煤锅炉SNCR脱硝技术应用研究[J].电站系统工程, 2010, 26(1): 18-21.ZHOU Guo-min, TANG Jian-cheng, HU Zhen-guang, ZHAO Hai-jun, GONG Jia-you. Application eesearch of SNCR technology in the pulverized-coal fired boiler[J]. Pwr Sy Eng, 2010, 26(1): 18-21. [5] 顾卫荣, 周明吉, 马薇.燃煤烟气脱硝技术的研究进展[J].化工进展, 2012, 31(9): 2084-2092.GU Wei-rong, ZHOU Ming-ji, MA Wei. Technology status and analysis on coal-fired flue gas denitrification[J]. Chem Ind Eng Prog, 2012, 31(9): 2084-2092. [6] 张杰儒, 罗津晶, 牛强.垃圾焚烧烟气汞的治理技术与评价[J].环境卫生工程, 2012, 20(5): 34-36.ZHANG Jie-ru, LUO Jin-jing, NIU Qiang. Control technologies and evaluation of mercury in flue gas of waste incineration[J]. Environ Sanitation Eng, 2012, 20(5): 34-36. [7] ZHANG B, XU P, QIU Y, YU Q, MA J J, WU H, LUO G Q, XU M H, YAO H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases[J]. Chem Eng J, 2015, 263: 1-8. [8] WANG L, ZHAO W R, WU Z B. Simultaneous absorption of NO and SO2 by FeⅡEDTA combined with Na2SO3 solution[J]. Chem Eng J, 2007, 132(1/3): 227-232. [9] ZHAO Y, XU P Y, FU D, HUANG J J, YU H H. Experimental study on simultaneous desulfurization and denitrification based on highly active absorbent[J]. J Environ Sci, 2006, 18(2): 281-286. [10] CHIU C H, HSI H C, LIN H P. Multipollutant control of Hg/SO2/NO from coal-combustion flue gases using transition metal oxide-impregnated SCR catalysts[J]. Catal Today, 2015, 245: 2-9. [11] ZHANG S, ZHAO Y, WANG Z, ZHANG J Y, WANG L L, ZHENG C G. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2[J]. J Environ Sci, 2016, 53 (3): 141-150. [12] ZHANG S B, ZHANG Q Z, ZHAO Y C, YANG J P, XU Y, ZHANG J Y. Enhancement of CeO2 modified commercial SCR catalyst for synergistic mercury removal from coal combustion flue gas[J]. Rsc Adv, 2020, 10: 25325-25338. [13] 温正城, 王智化, 杨卫娟, 周俊虎, 岑可法.臭氧在烟气中氧化零价汞的机理研究[J].浙江大学学报(工学版), 2009, 43(9): 1625-1631.WEN Zheng-cheng, WANG Zhi-hua, YANG Wei-juan, ZHOU Jun-hu, CEN Ke-fa. Mechanism investigation on oxidization of Hg0 by ozone in flue gas[J]. J Zhejiang Univ (Eng Sci), 2009, 43(9): 1625-1631. [14] 王智化, 周俊虎, 温正城, 张彦威, 岑可法.利用臭氧同时脱硫脱硝过程中NO的氧化机理研究[J].浙江大学学报(工学版), 2007, 41(5): 765-769.WANG Zhi-hua, ZHOU Jun-hu, WEN Zheng-cheng, ZHANG Yan-wei, CEN Ke-fa. Mechanism investigation on NO oxidization during NOx and SO2 simultaneous removal process by Ozone[J]. J Zhejiang Univ (Eng Sci), 2007, 41(5): 765-769. [15] 张明慧, 马强, 徐超群, 朱燕群, 周俊虎.臭氧氧化结合湿法喷淋对玻璃窑炉烟气同时脱硫脱硝实验研究[J].燃料化学学报, 2015, 43(1): 88-93.ZHANG Ming-hui, MA Qiang, XU Chao-qun, ZHU Yan-qun, ZHOU Jun-hu. Simultaneous removal of NOx and SO2 from glass furnace flue gas by ozone oxidation and spray tower[J]. J Fuel Chem Technol, 2015, 43(1): 88-93. [16] 张瑞, 张佳, 郭少鹏, 刘勇弟, 鲁军.臭氧氧化同时脱除烟气中NO和SO2的研究[J].化学世界, 2015, (3): 158-161.ZHANG Rui, ZHANG Jia, GUO Shao-peng, LIU Yong-di, LU Jun. Study on simultaneous removal of NO and SO2 from flue gas by ozone oxidation[J]. Chem World, 2015, (3): 158-161. [17] 王智化, 周俊虎, 魏林生, 温正城, 岑可法.用臭氧氧化技术同时脱除锅炉烟气中NOx及SO2的试验研究[J].中国电机工程学报, 2007, 27(11): 1-5.WANG Zhi-hua, ZHOU Jun-hu, WEI Lin-sheng, WEN Zheng-cheng, CEN Ke-fa. Experimental research for the simultaneous removal of NOx and SO2 in flue gas by O3[J]. Proc CSEE, 2007, 27(11): 1-5. [18] 牛强, 罗津晶, 郑锦森, 王巍, 汪可涛.臭氧氧化技术同时脱除烟气中NO和SO2的试验研究[J].广东化工, 2020, 12(47): 159-161.NIU Qiang, LUO Jin-jing, ZHENG Jin-sen, WANG Wei, WANG Ke-tao. Experimental research for the simultaneous removal of NO and SO2 in flue gas[J]. Guangdong Chem Ind, 2020, 12(47): 159-161. [19] 李兵, 张立强, 蒋海涛, 王志强, 马春元.活性炭孔隙结构和表面化学性质对吸附氧化NO的影响[J].煤炭学报, 2011, 36(11): 1906-1910.LI Bing, ZHANG Li-qiang, JIANG Hai-tao, WANG Zhi-qiang, MA Chun-yuan. Influence of activated carbon pore structure and surface chemical properties on the adsorption and oxidation of NO[J]. J China Coal Soc, 2011, 36(11): 1906-1910. [20] ZHANG W J, RABIEI S, BAGREEV A, ZHUANG M S, RASOULI F. Study of NO adsorption on activated carbons[J]. Appl Catal B: Environ, 2008, 83(1/2): 63-71. [21] 张波文, 唐晓龙, 易红宏, 赵顺征, 左嫣然, 王志祥, 高凤雨.改性活性炭吸附去除NO实验研究[J].化工新型材料, 2015, (7): 111-113.ZHANG Bo-wen, TANG Xiao-long, YI Hong-hong, ZHAO Shun-zheng, ZUO Yan-ran, WANG Zhi-xiang, GAO Feng-yu. Study on NO adsorptive removal on modified activated carbon[J]. New Chem Mater, 2015, (7): 111-113. [22] 张鹏宇, 杨巧云, 许绿丝, 曾汉才, 张柳.活性炭纤维低温吸附氧化NO的试验研究[J].电力科技与环保, 2004, 20(2): 25-28.ZHANG Peng-yu, YANG Qiao-yun, XU Lv-si, ZENG Han-cai, ZAHNG Liu. Experimental study on adsorption and oxidatiou of activated carbon fiber to NO at low temperature[J]. Electric Power Environ Protect, 2004, 20(2): 25-28. [23] SOUSA J P S, PEREIRA M F R, FIGUEIREDO J L. Catalytic oxidation of NO to NO2 on N-doped activated carbons[J]. Catal Today, 2011, 176(1): 383-387. [24] JEGUIRIM M, TSCHAMBER V, BRILHAC J F, EHRBURGER P. Interaction mechanism of NO2 with carbon black: Effect of surface oxygen complexes[J]. J Anal Appl Pyrolysis, 2004, 72(1): 171-181. [25] 卢静静.苯甲酸改性活性炭对燃煤烟气中元素汞的吸附机理研究[D].福建: 厦门大学, 2015.LU Jing-jing. Study on removal of elemental mercury from coal-fired flue gas by benzoic acid-modified activated carbon[J]. Fujian: Xiamen University, 2015. [26] 李兵.粉末活性炭循环流化床吸附脱除烟气中SO2的实验研究[D].山东: 山东大学, 2012.LI Bing. Experimental study on the adsorption removal of SO2 from flue gas by powder activated carbon in circulating fluidized bed[J]. Shandong: Shandong University, 2012. [27] GUO Z, XIE Y, HONG I, KIM J. Catalytic oxidation of NO to NO2 on activated carbon[J]. Energ Convers Manage, 2001, 42(15): 2005-2018. [28] ZHANG P, SUN H W, YU L, SUN T H. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars[J]. J Hazard Mater, 2013, 244-245(3): 217-224. [29] CHEN B L, JOHNSON E J, BENNY C, ZHU L Z, XING B S. Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: role of polarity and accessibility[J]. Environ Sci Technol, 2005, 39(16): 6138-6146. [30] 苏青青, 杨嘉谟.活性炭固定床吸附SO2的机理及动力学研究[J].三峡大学学报(自然科学版), 2010, 32(5): 97-101.SU Qing-qing, YANG Jia-mo. Mechanism and kinetic characteristics of adsorbing sulfur dioxide by activated carbon on fixed bed[J]. J Chin Three Gorges Univ (Nat Sci), 2010, 32(5): 97-101. [31] GUO Y, LI Y, ZHU T, YE M. Effects of concentration and adsorption product on the adsorption of SO2 and NO on activated carbon[J]. Energy Fuels, 2013, 27(1): 360-366. [32] 李雪飞.改性活性炭脱除烟气中NOx研究[D].北京: 煤炭科学研究总院, 2006.LI Xue-fei. Reduction of NOx from flue gas with modified activated carbon[J]. Beijing: China Coal Research Institute, 2006. [33] JIA F R, LI Z, WANG E G, HE J C, DONG H, LIU G X, JIAN W W. Preparation and SO2 adsorption behavior of coconut shell-based activated carbon via microwave-assisted oxidant activation[J]. China Pet Process Petrochen Technol, 2018, 20(1): 67-74. [34] 华坚, 刘宁, 尹华强, 汪南方.脱硫对活性碳表面结构的影响[J].四川大学学报(工程科学版), 2007, 39(1): 98-103.HUA Jian, LIU Ning, YI Hua-qiang, WANG Nan-fang. Influence of desulfuration on the surface structure of activated carbon[J]. J Chin Coal Soc, 2007, 39(1): 98-103. -





 下载:
下载: