Study on ex-situ presulfurization technologies with different presulfiding agents for CoMo-based sulfur-tolerant shift catalysts
-
摘要: 从多种硫化剂中筛选出(NH4)2S和Na2S,分别采用不同的预硫化技术对工业级CoMo型耐硫变换催化剂进行器外预硫化,采用XRD、XPS和HRTEM等表征手段对催化剂的晶相结构、表面特性和微观形貌进行表征。(NH4)2S和Na2S作为预硫化剂对催化剂的晶相结构没有明显影响;用(NH4)2S进行预硫化时活性组分发生部分O-S交换,而用Na2S进行预硫化时活性组分仍保持为氧化态,两种预硫化型催化剂在反应器内随着反应温度升高,硫化深度为S-Na2S > S-(NH4)2S >常规器内预硫化;器内预硫化和(NH4)2S作为预硫化剂MoS2片层结构堆叠层数集中在2-3层,而用Na2S进行预硫化时MoS2片层堆叠层数明显变多,集中在3-5层。采用微型固定床评价装置,选择285、350、450 ℃三个反应温度,催化剂的活性顺序为S-Na2S > S-(NH4)2S >常规器内预硫化。Abstract: (NH4)2S and Na2S were selected from several presulfiding agents. Industrial Co-Mo based sulfur-tolerant shift catalysts were ex-situ presulfided with different presulfurization technologies. The crystal structures, surface characteristics and micro appearance were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (HRTEM). (NH4)2S and Na2S as presulfiding agents did not show obvious effects on the crystal structures of the catalysts. Partial O-S exchange of the active components was observed when (NH4)2S was used for presulfirization. And when presulfided with Na2S, the active components still remained oxidation state. As the ex-situ presulfurization temperature increased, the extent of vulcanization was:S-Na2S > S-(NH4)2S > the general in situ pre-vulcanization. When presulfided with (NH4)2S or the general in situ pre-vulcanization, the stacking number of MoS2 layers was mainly two to three. And when presulfided with Na2S, the stacking number of MoS2 layers increased obviously, mostly three to five. The activity sequence of the catalysts was S-Na2S > S-(NH4)2S > the general in situ pre-vulcanization when the catalysts were tested in a micro-fixed reactor at temperatures of 285, 350 and 450℃ respectively.
-
Key words:
- sulfur-tolerant shift catalyst /
- ex-situ presulfurization technology /
- (NH4)2S /
- Na2S
-
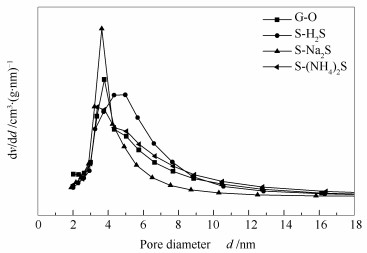
表 1 不同硫化剂制备预硫化型催化剂的孔结构性质
Table 1 Physical properties of the catalysts treated by different sulfiding agents
Sample ABET/(m2·g-1) Dpore/nm vpore/(cm3·g-1) G-O 182 5.69 0.26 S-(NH4)2S 153 5.65 0.28 S-Na2S 142 4.80 0.20 S-H2S 147 6.20 0.28 表 2 不同硫化剂制备预硫化型催化剂Mo 3d、Co 2p和S 2p的结合能
Table 2 Mo 3d, Co 2p and S 2p binding energy(BE) of the catalysts treated by different sulfiding agents
Sample Binding energy E/eV Mo 3d5/2 Mo 3d3/2 Co 2p3/2 Co 2p1/2 S 2p3/2 S 2p1/2 G-O 232.6 235.7 788.2 804.2 - - 781.2 797.2 S-H2S 232.5 235.6 788.2 804.2 168.6 169.8 230.9 234.0 781.2 797.2 162.0 163.2 229.1 232.2 779.1 795.1 S-(NH4)2S 232.6 235.7 788.2 804.2 168.6 169.8 229.2 232.3 781.2 797.2 162.2 163.4 779.1 795.1 R-(NH4)2S 232.6 235.7 788.2 804.2 168.7 169.9 231.0 234.1 781.2 797.2 162.1 163.3 229.1 232.2 779.1 795.1 S-Na2S 232.7 235.8 788.2 804.2 168.0 169.2 781.2 797.2 161.9 163.1 R-Na2S 232.5 235.6 788.2 804.2 168.6 169.8 230.9 234.0 781.2 797.2 161.8 163.0 229.2 232.3 779.1 795.1 表 3 不同硫化剂制备预硫化型催化剂的原子比
Table 3 Atomic ratio of the catalysts treated by different sulfiding agents
Sample Mo4+/Mo /% Mo5+/Mo /% Mo6+/Mo /% S/Mo S2-/ Mo G-O 0 0 100 0 0 S-H2S 27.2 2.6 70.17 1.95 0.74 S-(NH4)2S 8.84 0 91.16 2.06 1.72 R-(NH4)2S 52.2 1.2 46.60 1.91 0.93 S-Na2S 0 0 100 2.14 0.82 R-Na2S 54.9 0.8 44.3 2.37 1.40 -
[1] RATNASAMY C, WAGNER J P. Water gas shift catalysis[J]. Cat Rev, 2009, 51(3):325-440. doi: 10.1080/01614940903048661 [2] GE H, LI X K, Wang J G, LU Z J, QIN Z F, ZHOU L G. Study on hydrodesulfurization of thiophene over Mo/Al2O3 catalyst presulfided by thiosulfate ammonium[J]. J Fuel Chem Technol, 2009, 37(2):199-204. doi: 10.1016/S1872-5813(09)60016-5 [3] LIU B, CHAI Y M, WANG Y J, ZHANG T T, LIU Y Q, LIU C G. A simple technique for preparation of presulfided eggshell MoS2/Al2O3 catalysts and kinetics approach for highly selective hydrodesulfurization of FCC gasoline[J]. Appl Catal, A, 2010, 388(1/2):248-255. http://www.sciencedirect.com/science/article/pii/S0926860X1000640X [4] CHOUZIER S, VRINAT M, CSREI T, ROY-AUBERGER M, AFANASIEV P. HDS and HDN activity of (Ni, Co)Mo binary and ternary nitrides prepared by decomposition of hexamethylenetetramine complexes[J]. Appl Catal, A, 2011, 400(1/2):82-90. doi: 10.1021/jp002133c [5] LI Y P, LIU D P, LIU C G. Hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) performance of an ex situ presulfided MoNiP/Al2O3 catalyst:Model compounds study and pilot test for fluidized catalytic cracking (FCC) diesel oil[J]. Energy Fuels, 2010, 24(2):820-829. doi: 10.1021/ef901084f [6] 许世业. 加氢催化预硫化剂的研究与开发[D]. 西安: 西安石油大学, 2015.XU Shi-ye. Research and development on presulfurizing agent for catalytic hydrogenation[D]. Xi`an: Xi`an Shiyou University, 2015. [7] GUILLAUME D, LOPEZ S, CSERI T. Process for sulfurization of catalysts for hydrotreatment: US, US7513990[P]. 2009. [8] GAO Y L, FANG X C, CHENG Z M. Development and application of ex-situ presulfurization technology for hydrotreating catalysts in China[J]. Front Chem Sci Eng, 2011, 5(3):287-296. doi: 10.1007/s11705-010-0529-2 [9] GAO Y L, FANG X C, CHENG Z M. A comparative study on the ex situ and in situ presulfurization of hydrotreating catalysts[J]. Catal Today, 2010, 158(3):496-503. https://www.sciencedirect.com/science/article/pii/S0920586110004815 [10] LIAN Y X, WANG H F, FANG W P, YANG Y Q. Water gas shift activity of Co-Mo/MgO-Al2O3 catalysts presulfided with ammonium sulfide[J]. Energy Chem, 2010, 19(1):61-66. http://dl.acm.org/citation.cfm?id=2355575.2356857 [11] SEAMANS J D, WELCH J G, GASSER N G. Method of presulfiding a hydrotreating catalyst: US, US4943547[P]. 1990. [12] RATNASAMY P, RODRIQUE L, LEONARD A J. Structural and textural studies in molybdenum sulfide systems[J]. J Phys Chem, 1973, 77(18):2242-2245. doi: 10.1021/j100637a017 [13] CHIANELLI R R, PRESTRIDGE E B, PECORARO T A, DENEUFVILLE J P. Molybdenum disulfide in the poorly crystalline "rag" structure[J]. Science, 1979, 203(4385):1105-1107. doi: 10.1126/science.203.4385.1105 [14] MOSES P G, HINNEMANN B, TOPSØE H, NØRSKOV J K. The hydrogenation and direct desulfurization reaction pathway in thiophene hydrodesulfurization over MoS2 catalysts at realistic conditions:A density functional study[J]. J Catal, 2007, 248(2):188-203. doi: 10.1016/j.jcat.2007.02.028 [15] ABART J, DELGADO E, ERTL G, JEZIOROWSKI H, KNÖZINGER H, THIELE N, WANG X Z H, TAGLAUER E. Surface structure and reduction behaviour of Nio-MoO3/Al2O3 catalysts[J]. Appl Catal, 1982, 2(3):155-176. doi: 10.1016/0166-9834(82)80198-X [16] WANG C M, TSAI T C, WANG I. Deep hydrodesulfurization over Co/Mo catalysts supported on oxides containing vanadium[J]. J Catal, 2009, 262(2):206-214. doi: 10.1016/j.jcat.2008.12.012 [17] QIU L, XU G. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts[J]. Appl Surf Sci, 2010, 256(11):3413-3417. doi: 10.1016/j.apsusc.2009.12.043 [18] 户安鹏, 聂红, 陈文斌, 龙湘云.柠檬酸对CoMo/Al2O3催化剂中助剂作用的影响[J].石油炼制与化工, 2015, 46(9):1-6. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201509001.htmHU An-peng, NIE Hong, CHEN Wen-bin, LONG Xiang-yun. Influence of citric acid on promoter role in CoMo/Al2O3 catalyst[J]. Pet Process Petroc, 2015, 46(9):1-6. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201509001.htm [19] GRIGOR V V V, GEL MAN V N, SOBOLEVSKⅡ V S, KREINDEL A I, GOLOSMAN E Z, SALOMATIN G I, DANTSIG G A, ABDULLAEV T R, LAFER L I, TAKERSON V I. Study of reaction mechanism for conversion of carbon monoxide and steam on copper catalysts employing mass spectrometry and IR spectroscopy[J]. Russ Chem Bull, 1978, 27(5):1015-1017. doi: 10.1007/BF00929015 [20] 柴永明, 南军, 相春娥, 柳云骐, 刘晨光.以硫化态前驱物制备的NiMoS/γ-Al2O3催化剂表面活性相HRTEM研究[J].石油学报(石油加工), 2007, 23(3):20-26. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200703003.htmCHAI Yong-ming, NAN Jun, XIANG Chun-e, LIU Yun-qi, LIU Chen-guang. Investigation of active phase of NiMoS/γ-Al2O3 prepared by thiosalt as precursor through HRTEM[J]. Acta Petrol Sinica(Pet. Process Sec., 2007, 23(3):20-26. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200703003.htm [21] 贺胜如, 袁赞根, 刘建平.加氢催化剂器外预硫化技术的工业应用[J].石油炼制与化工, 2004, 35(8):34-36. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH200507000.htmHE Sheng-ru, YUAN Zan-gen, LIU Jian-ping. Commercial application of hydrogenation catalyst with external presulfurizing technology[J]. Pet Process Petroc, 2004, 35(8):34-36. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH200507000.htm -





 下载:
下载: