Methanol oxidation in acidic and alkaline electrolytes using PtRuIn/C electrocatalysts prepared by borohydride reduction process
-
Abstract: PtRuIn/C electrocatalysts (20% metal loading by weight) were prepared by sodium borohydride reduction process using H2PtCl6·6H2O, RuCl3·xH2O and InCl3·xH2O as metal sources, borohydride as reducing agent and Carbon Vulcan XC72 as support. The synthetized PtRuIn/C electrocatalysts were characterized by X-ray diffraction (XRD), energy dispersive analysis (EDX), transmission electron microscopy(TEM), cyclic voltammetry (CV), chronoamperommetry (CA) and polarization curves in alkaline and acidic electrolytes (single cell experiments). The XRD patterns show Pt peaks are attributed to the face-centered cubic (fcc) structure, and a shift of Pt (fcc) peaks indicates that Ru or In is incorporated into Pt lattice. TEM micrographs show metal nanoparticles with an average nanoparticle size between 2.7 and 3.5 nm. Methanol oxidation in acidic and alkaline electrolytes was investigated at room temperature, by CV and CA. PtRu/C (50:50) shows the highest activity among all electrocatalysts in study considering methanol oxidation for acidic and alkaline electrolyte. Polarization curves at 80℃ show PtRuIn/C (50:25:25) with superior performance for methanol oxidation, when compared to Pt/C, PtIn/C and PtRu/C for both electrolytes. The best performance obtained by PtRuIn/C (50:25:25) in real conditions could be associated with the increased kinetics reaction and/or with the occurrence simultaneously of the bifunctional mechanism and electronic effect resulting from the presence of Pt alloy.
-
Key words:
- borohydride reduction process /
- PtRuIn/C electrocatalysts /
- methanol oxidation /
- acidic and alkaline electrolytes /
- polarization curves
-
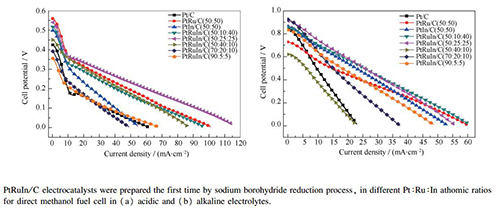
Figure 8 Polarization curves (a) and power density curves (b) in a 5 cm2 DMFC at 80 ℃ using Pt/C, PtRu/C, PtIn/C and PtRuIn/C electrocatalysts as anode catalysts (1 mg (Pt) /cm2) and Pt/C BASF as the cathode catalyst (1 mg (Pt) /cm2), Nafion 117 was used as the membrane. Methanol 2 mol/L with 1.0 mL/min flux and oxygen pressure (0.2 MPa). (c)I-V polarization curves and the (d) power density curves at 80 ℃ of a 5 cm2 DAMFC using Pt/C, PtRu/C, PtIn/C and PRuIn/C electrocatalysts anodes (1 mg (Pt) /cm2 catalyst loading) and Pt/C BASF electrocatalyst cathode (1 mg (Pt) /cm2 catalyst loading with 20% Pt loading on carbon), Nafion 117 membrane treated with KOH, 1.0 mol/L KOH + 1.0 mol/L methanol was used as fuel
■:Pt/C; ●:PtRu/C(50:50); ▲:PtIn/C(50:50); ▼:PtRuIn/C(50:10:40); ◀:PtRuIn/C(50:25:25); ▶:PtRuIn/C(50:40:10); ◆:PtRuIn/C(70:20:10); PtRuIn/C(90:5:5)
Table 1 Pt:Ru, Pt:In and Pt:Ru:In atomic ratios of the prepared electrocatalysts
Eletrocatalyst EDX results (molar ratio) Pt/C PtRu/C (50:50) 62:38 PtIn/C (50:50) 58:42 PtRuIn/C (50:40:10) 63:31:06 PtRuIn/C (50:10:40) 71:13:16 PtRuIn/C (50:25:25) 61:23:16 PtRuIn/C (70:20:10) 80:16:04 PtRuIn/C (90:5:5) 93:5:2 Table 2 The average crystallite size and lattice parameter values for Pt/C, PtRu/C, PtIn/C and PtRuIn/C prepared with different atomic ratios
Material Average crystallite size d/nm Lattice parameter values /nm Pt/C 5 0.392 PtRu/C (50:50) 6 0.388 PtIn/C (50:50) 3 0.398 PtRuIn/C (50:10:40) 3 0.396 PtRuIn/C (50:25:25) 2 0.397 PtRuIn/C (50:40:10) 3 0.390 PtRuIn/C (70:20:10) 4 0.392 PtRuIn/C (90:5:5) 5 0.393 -
[1] GERALDES A N, DA SILVA D F, DA SILVA J C M, DE SA O A, SPINACE E V, NETO A O, DOS SANTOS M C. Palladium and palladium-tin supported on multi wall carbon nanotubes or carbon for alkaline direct ethanol fuel cell[J]. J Power Sources, 2015, 275(2):189-199. http://d.old.wanfangdata.com.cn/Periodical/hxxb201804006 [2] BROUZGOU A, SONG S Q, TSIAKARAS P. Low and non-platinum electrocatalysts for PEMFCs:Current status, challenges and prospects[J]. Appl Catal B:Environ, 2012, 127(1):371-388. http://www.sciencedirect.com/science/article/pii/S092633731200389X [3] SHEN S Y, ZHAO T S, XU J B, LI S Y. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells[J]. J Power Sources, 2010, 195(4):1001-1006. doi: 10.1016/j.jpowsour.2009.08.079 [4] SANTOS M C L, DUTRA R M, RIBEIRO V A, SPINACÉ E V, NETO A O. Preparation of PtRu/C electrocatalysts by borohydride reduction for methanol oxidation in acidic and alkaline medium[J]. Int J Electrochem Sci, 2017, 12(5):3549-3560. [5] VEIZAGA N S, PAGANIN V A, ROCHA T A, SCELZA O A, DE MIGUEL S R, GONZALEZ E R. Development of PtGe and PtIn anodic catalysts supported on carbonaceous materials for DMFC[J]. Int J Hydrogen Energy, 2014, 39(16):8728-8737. doi: 10.1016/j.ijhydene.2013.12.041 [6] IWASITA T. Handbook of Fuel Cells (Vol. 2)[M]. Chichester UK:Wiley, 2003. [7] MULLER J, FRANK G, COLBOW K, WILKINSON D. Handbook of Fuel Cells (Vol. 4)[M]. Chichester UK:Wiley, 2003. [8] NETO A O, BRANDALISE M, DIAS R R, AYOUB J M S, SILVA A C, PENTEADO J C, LINARDI M, SPINACé E V. The performance of Pt nanoparticles supported on Sb2O5SnO2, on carbon and on physical mixtures of Sb2O5.SnO2 and carbon for ethanol electro-oxidation[J]. Int J Hydrogen Energy, 2010, 35(17):9177-9181. doi: 10.1016/j.ijhydene.2010.06.028 [9] ARICÒ A S, SRINIVASAN S, ANTONUCCI V. DMFCs:From fundamental aspects to technology development[J]. Fuel Cells, 2001, 1(2):133-161. doi: 10.1002/(ISSN)1615-6854 [10] SANTOS M L C, OTTONI C A, DE SOUZA R F B, DA SILVA S G, ASSUMPÇÃO M H M T, SPINACé E V, NETO A O. Methanol oxidation in alkaline medium using PtIn/C electrocatalysts[J]. Electrocatal, 2016, 7(6):445-450. doi: 10.1007/s12678-016-0324-z [11] FRELINK T, VISSCHER W, VANVEEN J A R. The effect of Sn on Pt/C catalysts for the methanol electro-oxidation[J]. Electrochim Acta, 1994, 39(11/12):1871-1875. http://www.sciencedirect.com/science/article/pii/0013468694851778 [12] FRELINK T, VISSCHER W, COX A P, VANVEEN J A R. Ellipsometry and dems study of the electrooxidation of methanol at Pt and Ru-and Sn-promoted Pt[J]. Electrochim Acta, 1995, 40(10):1537-1543. doi: 10.1016/0013-4686(95)00034-C [13] GURAU B, VISWANATHAN R, LIU R X, LAFRENZ T J, LEY K L, SMOTKIN E S, REDDINGTON E, SAPIENZA A, CHAN B C, MALLOUK T S, SARANGAPANI S. Structural and electrochemical characterization of binary, ternary, and quaternary platinum alloy catalysts for methanol electro-oxidation[J]. J Phys Chem B, 1998, 102(49):9997-10003. doi: 10.1021/jp982887f [14] XIE J, ZHANG Q, GU L, XU S, WANG P, LIU J, DING Y, YAO Y F, NAN C, ZHAO M, YOU Y, ZOU Z. Ruthenium-platinum core-shell nanocatalysts with substantially enhanced activity and durability towards methanol oxidation[J]. Nano Energy, 2016, 21(3):247-257. http://www.sciencedirect.com/science/article/pii/S2211285516000240 [15] HUANG L, ZHANG X, WANG Q, HAN Y, FANG Y, DONG S. Shape-control of Pt-Ru nanocrystals:Tuning surface structure for enhanced electrocatalytic methanol oxidation[J]. J Am Chem Soc, 2018, 140(3):1142-1147. doi: 10.1021/jacs.7b12353 [16] DEMIRCI U B. Theoretical means for searching bimetallic alloys as anode electrocatalysts for direct liquid-feed fuel cells[J]. J Power Sources 2007, 173(1):11-18. doi: 10.1016/j.jpowsour.2007.04.069 [17] ZHU M, SUN G, YAN S, LI H, XIN Q. Preparation, structural characterization, and activity for ethanol oxidation of carbon-supported PtSnIn catalyst[J]. Energy Fuels, 2009, 23(1):403-407. doi: 10.1021/ef800726b [18] SANTASALO-AARNIO A, TUOMI S, JALKANEN K, KONTTURI K, KALLIO T. The correlation of electrochemical and fuel cell results for alcohol oxidation in acidic and alkaline media[J]. Electrochim Acta, 2013, 87(1):730-738. http://www.sciencedirect.com/science/article/pii/S0013468612015782 [19] HOU H, WANG S, JIN Q W, JIANG L, SUN L, JIANG G. KOH modified Nafion112 membrane for high performance alkaline direct ethanol fuel cell[J]. Int J Hydrogen Energy, 2011, 36(8):5104-5109. doi: 10.1016/j.ijhydene.2010.12.093 [20] FONTES E H, DA SILVA S G, SPINACE E V, NETO A O, DE SOUZA R F B. In situ ATR-FTIR studies of ethanol electro-oxidation in alkaline medium on PtRh/C electrocatalyst prepared by an alcohol reduction process[J]. Electrocatal, 2016, 7(4):297-304. doi: 10.1007/s12678-016-0308-z [21] ROYCHOWDHURY C, MATSUMOTO F, ZELDOVICH V B, WARREN S C, MUTOLO P F, BALLESTEROS M J, WIESNER J, ABRUÑA H D, DISALVO F J. Synthesis, characterization, and electrocatalytic activity of PtBi and PtPb nanoparticles prepared by borohydride reduction in methanol[J]. Chem Mater, 2006, 18(14):3365-3372. doi: 10.1021/cm060480e [22] DA SILVA S G, SILVA J C M, BUZZO G S, DE SOUZA R F B, SPINACé E V, NETO A O, ASSUMPÇÃO M H M T. Electrochemical and fuel cell evaluation of PtAu/C electrocatalysts for ethanol electro-oxidation in alkaline media[J]. Int J Hydrogen Energy, 2014, 39(19):10121-10127. doi: 10.1016/j.ijhydene.2014.04.169 -





 下载:
下载: