Occurrence and transformation behavior of AAEMs in the flotation fraction of a typical Xinjiang coal
-
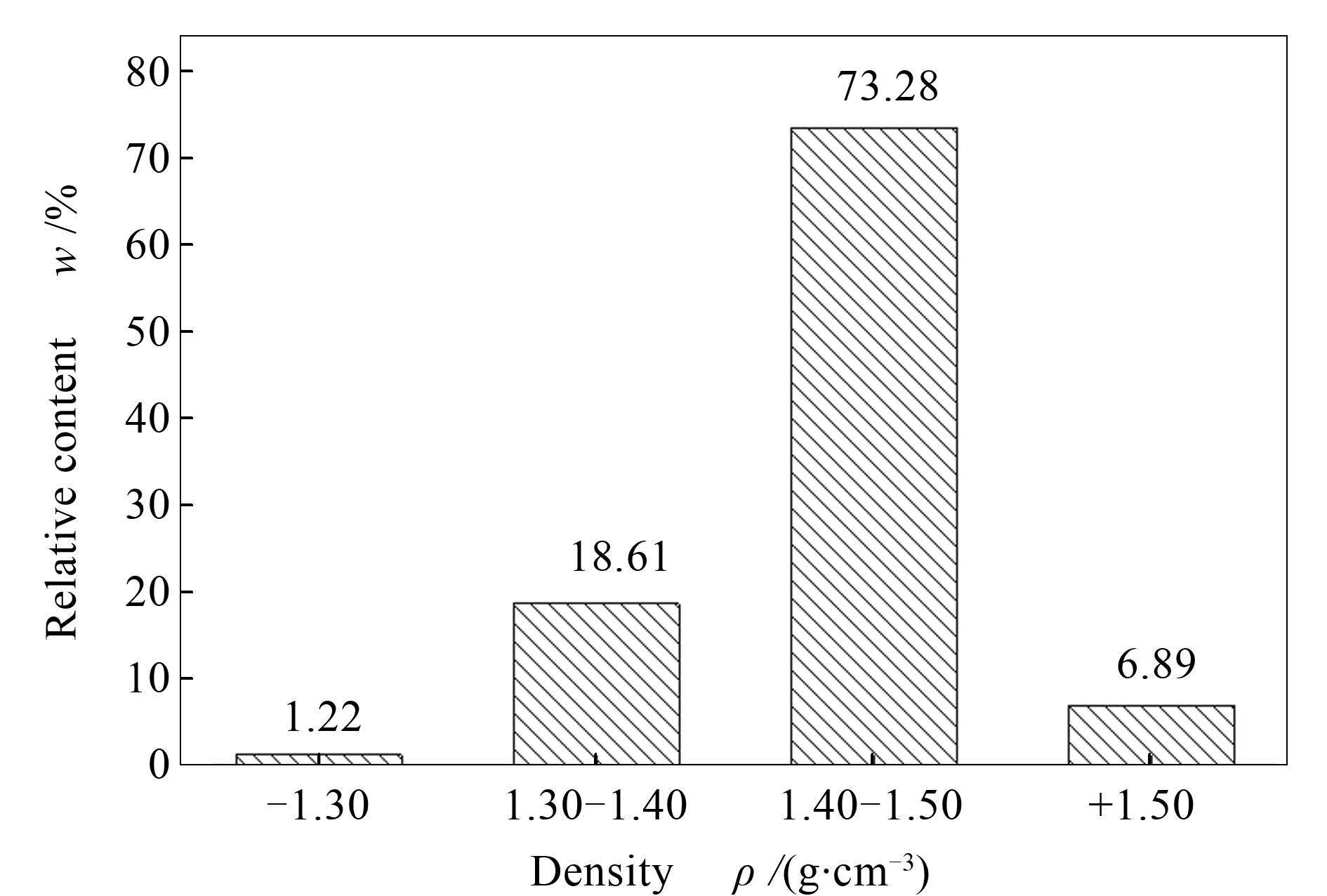
摘要: 以新疆五彩湾高碱煤为原料,通过有机重液浮选和理化分析及热力学模拟解析活性矿物质热演化特性。结果表明,五彩湾煤以密度在1.40-1.50 g/cm3的组分为主(质量分数超过70%)。可溶性Na和K等主要存在于较低密度组分(-1.40 g/cm3)中;在高密度(+1.50 g/cm3)组分中发现了以硅铝酸盐形式存在的碱及碱土金属(AAEMs)。Ca主要以方解石形式存在,伴随高岭土和石英富集在高密度组分中。在低温(500℃)灰化时少量Na释放,大量可溶钠盐的残留导致各分选组分中Na的扩散量相差不大。在815℃热处理温度下,低密度组分中大部分Na挥发到气相中;高密度组分中的部分碱金属可被黏土(高岭土)和石英等矿物质捕获,抑制其释放。FactSage热力学模拟发现,温度低于550℃时Na盐仍稳定存在于灰中;温度高于550℃时Na盐开始挥发,同时部分Na向NaAlSiO4转化;在约620℃时,Na盐消失,同时NaAlSiO4的含量逐渐趋于稳定。SiO2含量的差异显著影响含Na等矿物质的演变行为。在500-815℃,Ca以CaSO4和硅铝酸钙的形式稳定存在,而Mg则在不同形式的硅酸盐之间发生转变。Abstract: The occurrence and thermal transformation behaviors of AAEMs (alkali and alkaline earth metal species) in a typical Xinjiang coal (Wucaiwan) were systematically investigated.The raw coal sample was primarily separated to four fractions based on different densities by float-sink experiments using organic heavy solutions.Subsequently, the BSE-EDX (backscattered electron-energy dispersive X-ray detector) and XRD were employed to analyze the mineral matters in coal fractions.Moreover, the thermal transformation behaviors of minerals in coal were simulated using FactSage.The results show that the active minerals (i.e., AAEMs) in coal vary with the flotation density and exhibit quite different transformation characterizations.Samples with density of 1.40-1.50 g/cm3 are the major fraction (70%) for Wucaiwan coal.The Na and K exist principally in the lower density (-1.40 g/cm3) fraction;in contrast, the AAEMs are combined with the alumina-silicate in the high density (+1.50 g/cm3) fraction.Additionally, Ca is mainly in the form of calcite;and kaolinite and quartz are enriched in the high density fraction.Less Na could release at lower heat treatment temperature (500℃);thus the release amounts of Na in four fractions present less difference.The majority of Na is volatilized from the low density coal sample at 815℃;but for the high density coal samples the clay (mainly kaolinite) and quartz could capture Na and restrain its release.According to the FactSage calculation, the NaCl is found to be still stable in the coal ash at 550℃;it would start evaporate over 550℃ and generate NaAlSiO4.The NaCl would vanish at 620℃ and the NaAlSiO4 gradually becomes constant.Moreover, at the temperature range of 500-815℃, Ca is mainly in the form of CaSO4 and Ca-Si-Al, while Mg varies in several forms.
-
Key words:
- flotation /
- occurrence /
- transformation distinction /
- Xinjiang coal
-
表 1 原煤及分选组分的工业分析、元素分析和灰成分分析
Table 1 Proximate,ultimate and ash composition analyses of coal samples
Sample Proximate analysis wad/% Ultimate analysis wdaf/% M A V FC C H N Std W 11.81 6.24 27.01 54.94 79.07 4.05 0.79 0.51 W1 5.18 2.80 32.78 59.24 74.62 4.33 0.57 2.74 W2 7.88 2.98 34.27 54.87 73.05 4.79 0.83 0.52 W3 8.13 3.17 35.98 52.72 73.02 4.00 0.90 0.48 W4 5.85 31.63 29.83 32.69 74.44 4.64 1.14 3.25 Compositions of W ash w/% SiO2 Al2O3 Fe2O3 CaO K2O Na2O MgO SO3 SrO TiO2 BaO P2O5 MnO Cr2O3 17.77 10.30 4.21 29.55 0.32 2.40 9.45 22.84 1.24 1.23 0.35 0.06 0.10 0.06 表 2 五彩湾煤样的X射线能谱分析
Table 2 EDX analyses of Wucaiwan coal samples
Testing point Atom w/% Si Al O Ca Fe Na K Mg S P Cl Ti Cr a 20.42 24.51 55.07 - - - - - - - - - - b - - 71.96 28.04 - - - - - - - - - c - - 47.81 - - - - - - - - - 52.19 d 20.93 21.41 57.66 - - - - - - - - - - e - 4.63 82.55 7.20 - - - 2.61 3.01 - - - - f 1.33 - 44.33 - 49.91 - - - - - - 4.44 - g 42.77 2.39 51.34 - 2.04 0.78 0.69 - - - - - - 表 3 样品煤岩组分分析
Table 3 Maceral distribution of the flotation fractions
Sample W2 W3 W4 Vitrinite(de mineral basis) 62.27 34.51 40.21 Inertiniete(de mineral basis) 37.73 65.49 59.79 表 4 五彩湾煤在500和815 ℃下灰的化学组成
Table 4 Ash compositions of Wucaiwan coal prepared at 500 and 815℃
Sample Chemical composition w/% SiO2 Al2O3 Fe2O3 CaO K2O Na2O MgO SO3 SrO TiO2 BaO P2O5 MnO Cr2O3 500 ℃ W2 6.18 14.26 2.07 39.50 0.07 3.51 9.44 22.25 1.59 0.70 - 0.19 0.12 0.11 W3 7.78 12.03 2.47 41.72 0.06 3.57 9.98 19.92 1.59 0.40 - 0.27 0.12 0.08 W4 63.18 13.16 8.53 4.38 0.89 0.67 1.52 4.34 0.30 2.39 0.32 0.07 0.04 0.10 815 ℃ W2 7.54 15.74 2.85 31.17 - 0.52 16.25 22.87 1.80 0.88 - 0.09 0.14 0.08 W3 10.26 12.32 3.66 32.05 - 0.29 19.05 19.66 1.86 0.47 - 0.12 0.13 0.03 W4 64.61 12.59 9.31 4.12 0.83 0.49 1.49 2.94 0.30 2.62 0.38 0.03 0.04 0.09 -
[1] 刘敬, 王智化, 项飞鹏, 黄镇宇, 刘建忠, 周俊虎, 岑可法. 准东煤中碱金属的赋存形式及其在燃烧过程中的迁移规律实验研究[J]. 燃料化学学报, 2014, 42(3):316-322. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18373.shtmlLIU Jing, WANG Zhi-hua, XIANG Fei-peng, HUANG Zhen-yu, LIU Jian-zhong, ZHOU Jun-hu, CEN Ke-fa. Modes of occurrence and transformation of alkali metals in Zhundong coal during combustion[J]. J Fuel Chem Technol, 2014, 42(3):316-322. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18373.shtml [2] 邱朋华, 赵岩, 陈希叶, 徐健健, 杜亚文, 方来煕, 孙绍增. 碱及碱土金属对准东煤热解特性及动力学影响分析[J]. 燃料化学学报, 2014, 42(10):1178-1189. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18501.shtmlQIU Peng-hua, ZHAO Yan, CHEN Xi-ye, XU Jian-jian, DU Ya-wen, FANG Lai-xi, SUN Shao-zeng. Effects of alkali and alkaline earth metallic species on pyrolysis characteristics and kinetics of Zhundong coal[J]. J Fuel Chem Technol, 2014, 42(10):1178-1189. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18501.shtml [3] KOICHI M, TORU Y, KOJI K, YOSHIZO S, AKIRA T. Transformation of alkali and alkaline earth metals in low rank coal during gasification[J]. Fuel, 2008, 87:885-893. doi: 10.1016/j.fuel.2007.05.031 [4] BENSON S A, HOLM P L. Comparison of inorganic constituents in three low-rank coals[J]. Ind Eng Chem Prod Res Dev, 1985, 24(1):145-149. doi: 10.1021/i300017a027 [5] 张军, 汉春利, 颜峥, 余刚, 刘坤磊, 徐益谦. 煤中钠在燃烧初期行为的研究[J]. 燃料化学学报, 2001, 29(1):49-53.ZHANG Jun, HAN Chun-li, YAN Zheng, YU Gang, LIU Kun-lei, XU Yi-qian. Experimental studies on the behavior of sodium of coal in the initial state of combustion[J]. J Fuel Chem Technol, 2001, 29(1):49-53. [6] 翁青松, 王长安, 车得福, 付子文. 准东煤碱金属赋存形态及对燃烧特性的影响[J]. 燃烧科学与技术, 2014, 20(3):216-221. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201403004.htmWENG Qing-song, WANG Chang-an, CHE De-fu, FU Zi-wen. Alkali metal occurrence mode and its influence on combustion characteristics in Zhundong coals[J]. J Combust Sci Technol, 2014, 20(3):216-221. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201403004.htm [7] 陈川, 张守玉, 刘大海, 郭熙, 董爱霞, 熊绍武, 施大钟, 吕俊复. 新疆高钠煤中钠的赋存形态及其对燃烧过程的影响[J]. 燃料化学学报, 2013, 41(7):832-838. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18218.shtmlCHEN Chuan, ZHANG Shou-yu, LIU Da-hai, GUO Xi, DONG Ai-xia, XIONG Shao-wu, SHI Da-zhong, LV Jun-fu. Existence form of sodium in high sodium coals from XinJiang and its effect on combustion process[J]. J Fuel Chem Technol, 2013, 41(7):832-838. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18218.shtml [8] 刘大海, 张守玉, 涂圣康, 金涛, 施登宇, 裴育峰. 五彩湾煤中钠在热解过程中的形态变迁[J]. 燃料化学学报, 2014, 42(10):1190-1196. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18502.shtmlLIU Da-hai, ZHANG Shou-yu, TU Sheng-kang, JIN Tao, SHI Deng-yu, PEI Yu-feng. Transformation of sodium during Wucaiwan coal pyrolysis[J]. J Fuel Chem Technol, 2014, 42(10):1190-1196. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18502.shtml [9] 卫小芳, 刘铁锋, 黄戒介, 房倚天, 王洋. 澳大利亚高盐煤中钠在热解过程中的形态变迁[J]. 燃料化学学报, 2010, 38(2):144-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17553.shtmlWEI Xiao-fang, LIU Tie-feng, HUANG Jie-jie, FANG Yi-tian, WANG Yang. Transformation of Na in an Australian high-sodium coal during pyrolysis[J]. J Fuel Chem Technol, 2010, 38(2):144-148. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17553.shtml [10] LIN X C, YANG Y P, YANG S S, LI S Y, TIAN Y J, WANG Y G. Initial deposition feature during high-temperature pressurized pyrolysis of a typical Zhundong coal[J].Energy Fuels, 2016, 30(8):6330-6341. doi: 10.1021/acs.energyfuels.6b01074 [11] DIMPLE M Q, WU H W, SANKAR P B, LI C Z. Volatilization and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part Ⅱ. Effects of chemical form and valence[J]. Fuel, 2002, 81(8):151-158. [12] DIMPLE M Q, WU H W, LI C Z. Volatilization and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. PartⅠ. Volatilization of Na and Cl from a set of NaCl-loaded samples[J]. Fuel, 2002, 81(8):143-149. [13] ULRICH G, PENELOPE M, BERNBARD B. Dependence of alkali emission in PFB combustion on coal composition[J]. Fuel, 2001, 80(13):1893-1899. doi: 10.1016/S0016-2361(01)00045-X [14] LI G Y, WANG C A,YAN Y, JIN X, LIU Y H, CHE D F. Release and transformation of sodium during combustion of Zhundong coals[J]. J Energy Inst, 2016, 89(1):48-56. doi: 10.1016/j.joei.2015.01.011 [15] TAY H L, KAJITANI S, ZHANG S, LI C Z. Effects of gasifying agent on the evolution of char structure during the gasification of Victorian brown coal brown coal[J]. Fuel, 2013, 103(1):22-28. [16] ZHANG S, HAYASHI J I, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. Part IX. Effects of volatile-char interactions on char-H2O and char-O2 reactivities[J]. Fuel, 2011, 90(4):1655-1661. doi: 10.1016/j.fuel.2010.11.008 [17] 张双全. 煤化学[M]. 第二版. 徐州:中国矿业大学出版社, 2009.ZHANG Shuang-quan. Coal Chemistry[M]. 2nd ed. Xuzhou:China University of Mining and Technology Press, 2009. [18] QI X B, SONG G L, SONG W J, YANG S B, LU Q G. Effects of wall temperature on slagging and ash deposition of Zhundong coal during circulating fluidized bed gasification[J]. Appl Therm Eng, 2016, 106:1127-1135. doi: 10.1016/j.applthermaleng.2016.06.092 [19] 沈铭科, 邱坤赞, 黄镇宇, 王智化, 刘建忠. 准东煤掺烧高岭土对固钠率及灰熔融特性影响研究[J]. 燃料化学学报, 2015, 43(9):1044-1050. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18688.shtmlSHEN Ming-ke, QIU Kun-zan, HUANG Zhen-yu, WANG Zhi-hua, LIU Jian-zhong. Influence of kaolin on sodium retention and ash fusion characteristic during combustion of Zhundong coal[J]. J Fuel Chem Technol, 2015, 43(9):1044-1050. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18688.shtml [20] 杨涛, 李文广, 吴莎, 张兰, 王学斌, 谭厚章. 新疆高钙钠煤燃烧设备结焦机理研究[J]. 燃料化学学报, 2015, 43(11):1320-1326. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18725.shtmlYANG Tao, LI Wen-guang, WU Sha, ZHANG Lan, WANG Xue-bin, TAN Hou-zhang. Study on fouling mechanism in a boiler burning Xinjiang coal with high content of calcium and sodium[J]. J Fuel Chem Technol, 2015, 43(11):1320-1326. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18725.shtml [21] 杨燕梅, 张海, 吴玉新, 吕俊复. 不同灰化温度下准东煤碱/碱土金属的析出特性[J]. 燃烧科学与技术, 2015, 21(4):297-300. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201504003.htmYANG Yan-mei, ZHANG Hai, WU Yu-xin, LV Jun-fun. Release of alkali/alkaline earth metal species in Zhundong coal at different ashing temperature[J]. J Combust Sci Technol, 2015, 21(4):297-300. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201504003.htm [22] WANG Y, WANG D, DONG C, YANG Y. The behaviour and reactions of sodium containing minerals in ash melting process[J]. J Energy Inst, 2016, 2:1-7. -





 下载:
下载: