Effect of antimony doped vanadium-titanium catalyst on low-temperature NH3-SCR activity
-
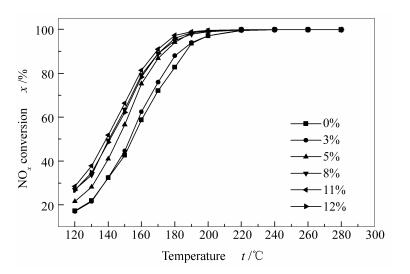
摘要: 用浸渍法制备Sb-V2O5-TiO2催化剂,在质量分数3%V2O5-TiO2催化剂基础上,研究锑的负载量、焙烧温度对催化剂活性的影响。结果表明,锑的负载量为11%(质量分数),500 ℃焙烧的催化剂具有最佳的SCR活性,在进口浓度为0.07% NOx、O2体积分数5%、空速27 000 h-1的条件下,170 ℃时脱硝活性可达92%。对催化剂进行H2-TPR表征,发现锑修饰后的催化剂氧化能力增强,使催化剂效率上升。通过XPS和NH3-TPD表征测试,催化剂表面的锑主要以五价的形式存在且随着锑负载量的增加催化剂表面酸性增强。考察SO2和H2O对催化剂的影响发现,加锑催化剂具有一定的抗硫抗水性能。通过FT-IR、TG、孔隙结构测试表明,锑的加入可以有效地抑制硫酸铵盐在催化剂表面的聚集,从而延长催化剂的寿命。Abstract: Sb-V2O5-TiO2 catalysts were prepared by wet impregnation method. The effect of antimony loadings and calcination temperatures on the activity of catalysts were investigated on the basis of 3%V2O5-TiO2. The results indicate that the catalyst with 11% Sb loading, calcined at 500℃, has the best activity of SCR. The NOx conversion could reach 92% at 170℃ with the inlet NOx concentration of 0.07%, the O2 volume fraction of 5%, and the space velocity of 27 000 h-1. The H2-TPR data reveal that the increase of activity can be attributed to the promotion of the catalyst oxidation ability by the modifying of antimony. Sb is mainly in the pentavalent antimony form on the surface of the catalyst, and the increase in surface acidity of the catalyst is identified by means of XPS and NH3-TPD. The effects of SO2 and H2O on the catalyst is also studied, showing that the Sb-V2O5-TiO2 has an excellent catalytic activity in the presence of H2O and SO2. FT-IR, TG and pore structure test results suggest that the addition of Sb can effectively inhibit the aggregation of ammonium sulfate on the catalyst surface, thereby improving the service life of the catalyst.
-
Key words:
- low-temperature /
- SCR /
- sulfur resistance /
- ammonium sulfate
-
表 1 催化剂的孔隙结构和H2-TPR表征
Table 1 Pore structure parameters and H2-TPR results

表 2 不同焙烧温度下催化剂的孔隙结构
Table 2 Catalyst pore structure parameters at different calcination temperature

表 3 中毒前后催化剂的孔隙结构
Table 3 Pore structure parameters of catalyst before and after poisoning

-
[1] FORZATTI P. Present status and perspectives in deNOxSCR catalysis[J]. Appl Catal A:Gen, 2001, 222(1/2):221-236. https://www.researchgate.net/publication/222548668_Present_Status_and_Perspectives_in_de-NOx_SCR_Analysis [2] BUSCA G, LIETTI L, RAMIS G, BERTI F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts:A review[J]. Appl Catal B:Environ, 1998, 18(1/2):1-36. doi: 10.1007/s11706-016-0331-2 [3] PARVULESCU V I, GRANGE P, DELMON B. Catalytic removal of NO[J]. Catal Today, 1998, 46(4):233-316. doi: 10.1016/S0920-5861(98)00399-X [4] PUTLURU S S R, SCHILL L, JENSEN A D, SIRET B, TABARIES F, FEHRMANN R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B:Environ, 2015, 165:628-635. doi: 10.1016/j.apcatb.2014.10.060 [5] THIRUPATHI B, SMIRNIOTIS P G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures[J]. Appl Catal B:Environ, 2011, 110:195-206. doi: 10.1016/j.apcatb.2011.09.001 [6] TANG X F, LI J H., SUN L, HAO J M. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3[J]. Appl Catal B:Environ, 2010, 99(1/2):156-162. https://www.researchgate.net/publication/257370561_Origination_of_N2O_from_NO_reduction_by_NH3_over_beta-MnO2_and_alpha-Mn2O3 [7] CHEN Z H, YANG Q, LI H, LI X H, WANG L F, TSANG S C. Cr-MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3at low temperature[J]. J Catal, 2010, 276(1):56-65. doi: 10.1016/j.jcat.2010.08.016 [8] JIN R B, LIU Y, WU Z B, WANG H Q, GU T T. Relationship between SO2 poisoning effects and reaction temperature for selective catalytic reduction of NO over Mn-Ce/TiO2 catalyst[J]. Catal Today, 2010, 153(3/4):84-89. https://www.researchgate.net/publication/257324519_Relationship_between_SO2_poisoning_effects_and_reaction_temperature_for_selective_catalytic_reduction_of_NO_over_Mn-CeTiO2_catalyst [9] 黄增斌, 李翠清, 王振, 徐胜美, 冯凌波, 王虹, 宋永吉, 张伟.不同分子筛负载锰铈催化剂的低温NH3-SCR脱硝性能[J].燃料化学学报, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016HUANG Zeng-bin, LI Cui-qing, WANG Zhen, XU Sheng-mei, FENG Ling-bo, WANG Hong, SONG Yong-ji, ZHANG Wei. Performance of Mn-Ce catalysts supported on different zeolites in the NH3-SCR of NOx[J]. J Fuel Chem Technol, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016 [10] 叶飞, 刘荣, 贡湘君, 管昊. ZrO2晶相对锰铈系SCR催化剂脱硝活性的影响[J].环境科学研究, 2015, 28(11):1720-1727. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hjkx201511010&dbname=CJFD&dbcode=CJFQYE Fei, LIU Rong, GONG Xiang-jun, GUAN Hao. Effects of ZrO2 crystallite phases on MnOx-CeO2 SCR DeNOx catalysts[J]. Res Environ Sci-China, 2015, 28(11):1720-1727. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hjkx201511010&dbname=CJFD&dbcode=CJFQ [11] QI G, YANG R T, CHANG R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3at low temperatures[J]. Appl Catal B:Environ, 2004, 51(2):93-106. doi: 10.1016/j.apcatb.2004.01.023 [12] PHIL H H, REDDY M P, KUMAR P A, JU K L, HYO J S. SO2resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOx at low temperatures[J]. Appl Catal B:Environ, 2008, 78(3/4):301-308. https://www.researchgate.net/publication/244278830_Low_temperature_propylene_SCR_of_NO_by_copper_alumina_catalyst [13] 曹政, 黄妍, 彭莉莉, 李建光. V2O5-Sb2O3-TiO2催化剂低温NH3还原NO及其抗H2O和SO2毒化性能[J].燃料化学学报, 2012, 40(4):456-462. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17927.shtmlCAO Zheng, HUANG Yan, PENG Li-li, LI Jian-guang. Selective catalytic reduction of NO with ammonia over V2O5-Sb2O3-TiO2 at low temperature and resistance to H2O and SO2 poisoning[J]. J Fuel Chem Technol, 2012, 40(4):456-462. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17927.shtml [14] XU T F, WU X D, GAO Y X, LIN Q W, HU J F, WENG D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR[J]. Catal Commun, 2017, 93:33-36. doi: 10.1016/j.catcom.2017.01.021 [15] BESSELMANN S, FREITAG C, HINRICHSEN O, MUHLER M. Temperature-programmed reduction and oxidation experiments with V2O5/TiO2 catalysts[J]. Phys Chem Chem Phys, 2001, 3(21):4633-4638. doi: 10.1039/b105466j [16] DANH H T, KUMAR P A, JESONG Y E, HA H P. Enhanced NH3-SCR activity of Sb-V/CeO2-TiO2 catalyst at low temperatures by synthesis modification[J]. Res Chem Intermed, 2016, 42(1):155-169. doi: 10.1007/s11164-015-2329-2 [17] XU C, LIU J, ZHAO Z, YU F, CHENG K, WEI Y C, DUAN A J, JIANG G Y. NH3-SCR denitration catalyst performance over vanadium-titanium with the addition of Ce and Sb[J]. J Environ Sci China, 2015, 31:74-80. doi: 10.1016/j.jes.2014.09.040 [18] 杨睿, 黄海凤, 陈一杰, 张细雄, 卢晗锋. Cr改性钒基催化剂对NH3低温选择性催化还原NOx的影响[J].催化学报, 2015, 36(8):1256-1262. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201508015YANG Rui, HUANG Hai-feng, CHEN Yi-jie, ZHANG Xi-xiong, LU Han-feng. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3[J]. Chin J Catal, 2015, 36(8):1256-1262. http://d.wanfangdata.com.cn/Periodical/cuihuaxb201508015 [19] AMIRIDIS M D, WACHS I E, DEO G, JEHNG J M, KIM D S. Reactivity of V2O5 catalysts for the selective catalytic reduction of NO by NH3:Influence of vanadia loading, H2O, and SO2[J]. J Catal, 1996, 161:247-257. doi: 10.1006/jcat.1996.0182 [20] WAGNER C D, RIGGERS W M, DAVIS L E, MOULDER J F, MUILENBERG G E. Handbook of X-ray Photoelectron Spectroscopy[M].Minnesota:In Perkin-Elmer Corporation, 1979. [21] MONTILLA F, MORALLON E, DE BATTISTI A, BARISON S, DAOLIO S, VAZQUEZ J L. Preparation and characterization of antimony-doped tin dioxide electrodes.3.XPS and SIMS characterization[J]. J Phys Chem B, 2004, 108(41):15976-15981. doi: 10.1021/jp048674+ [22] DU X S, GAO X, FU Y C, GAO F, LUO Z Y, CEN K F. The co-effect of Sb and Nb on the SCR performance of the V2O5/TiO2 catalyst[J]. J Colloid Interf Sci, 2012, 368:406-412. doi: 10.1016/j.jcis.2011.11.026 [23] NOVA I, LIETTI L, ACQUA L D, GIAMELLO E, FORZATTI P. Study of thermal deactivation of a de-NOx commercial catalyst[J]. Appl Catal B:Environ, 2001, 35(1):31-42. doi: 10.1016/S0926-3373(01)00229-6 [24] 沈岳松, 祝杜民, 丘泰, 沈树宝. Ti-Zr-V-O复合材料的制备及其选择性催化还原NO[J].无机材料学报, 2009, 24(3):458-462. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL200903009.htmSHEN Yue-song, ZHU Du-min, QIU Tai, SHEN Shu-bao. Preparation of Ti-Zr-V-O catalytic composite material and its selective catalytic reduction of NO[J]. J Inorg Mater, 2009, 24(3):458-462. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL200903009.htm [25] WAQIF M, PIEPLU A, SAUR O, LAVALLEY JC, BLANCHARD G. Use of CeO2-Al2O3 as a SO2 sorbent[J].Solid State Ionics, 1997, 95(1/2):163-167. https://www.researchgate.net/publication/256711928_Hydrogen_production_by_supercritical_water_gasification_of_glucose_with_NiCeO2Al2O3_Effect_of_Ce_loading [26] LI L D, SHEN Q, CHENG J, HAO Z P. Catalytic oxidation of NO over TiO2 supported platinum clusters. Ⅱ:Mechanism study by in situ FTIR spectra[J]. Catal Today, 2010, 158(3/4):361-369. http://journal.hep.com.cn/fese/EN/abstract/abstract19654.shtml [27] 范云珠, 曹发海.硫酸铵热分解反应动力学研究[J].高校化学工程学报, 2011, 25(2):342-346. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHX201102029.htmFAN Yun-zhu, CAO Fa-hai. Thermal decomposition kinetics of ammonium sulfate[J]. J Chem Eng Chin Univ, 2011, 25(2):342-346. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHX201102029.htm -





 下载:
下载: