Effect of Cr doping on hydrogen production via methanol steam reforming over Cu-Ce composite catalysts
-
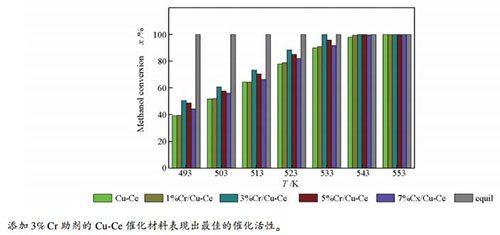
摘要: 采用浸渍法制备了不同Cr含量的Cr/Cu-Ce催化剂,通过N2O滴定、H2-TPR和XPS等表征,对其结构、性质和催化性能进行了探究。结果表明,Cr助剂会改变Cu-Ce催化剂的铜比表面积、CuO还原温度以及氧空穴含量。其中,添加3%Cr的催化剂的Cu比表面积较大,CuO还原温度较低,氧空穴较多,进而表现出优良的催化性能。当反应温度为533 K,n(水):n(甲醇)=1.2:1,甲醇和水的进料量为0.072 mL/min时,其催化效率达100%,重整气体组分中的CO体积分数为0.15%。与未掺杂Cr助剂的催化剂相比,其催化效率提高了10%,重整气体组分中的CO体积分数降低了0.34%。Abstract: Cr/Cu-Ce catalyts with different Cr contents were prepared by impregnation method, and their structures, properties and catalytic performance were investigated using N2O titration, H2-TPR and XPS techniques. It is found that the Cr doping changs the specific surface area of copper, the reduction temperature of CuO and oxygen vacancies of the Cu-Ce catalysts. In addition, the catalyst with 3% Cr addition has larger Cu specific surface area, lower CuO reduction temperature and more oxygen vacancies, thus exhibits excellent catalytic performance. The catalytic efficiency reaches 100% and CO volume fraction in outlet gas is 0.15% when the reaction temperature is 533 K, n(water):n(methanol) is 1.2:1 and the feeding rate of methanol and water is 0.072 mL/min. Compared with the un-doped Cr catalyst, the catalytic efficiency increases by 10% and the volume fraction of CO in the outlet gas decreases by 0.34%.
-
Key words:
- Cr content /
- oxygen vacancy /
- Cu-Ce catalyst /
- reduction temperature
-
表 1 不同Cr含量的催化剂中元素含量
Table 1 Elemental content of the catalysts with different Cr contents
Catalyst Elemental content w/% Cu Ce O Cra Crb Cu-Ce 7.9 73.4 18.7 - - 1%Cr/Cu-Ce 7.8 72.5 18.9 0.8 1.0 3%Cr/Cu-Ce 7.3 70.7 19.3 2.7 2.9 5%Cr/Cu-Ce 7.0 69.2 19.5 4.3 4.8 7%Cr/Cu-Ce 6.9 67.5 19.8 5.8 6.5 a: test value; b: designed value 表 2 催化剂的H2产率和物化性质
Table 2 H2 production rate and physicochemical properties of the catalysts
Catalyst Surface areaA/(m2·g-1) Pore volumev/(cm3·g-1) dCuO /nm Cu dispersion /% Cu surface areaaA/(m2·g-1) H2 production rateb /(cm3·g-1·min-1) CeO2 37.4 0.10 - - - - Cu-Ce 21.9 0.09 29.9 15.3 8.8 20.4 1%Cr/Cu-Ce 20.3 0.06 28.1 15.4 8.9 24.6 3%Cr/Cu-Ce 18.6 0.08 20.2 16.8 9.7 35.5 5%Cr/Cu-Ce 18.2 0.06 21.6 16.6 9.6 32.7 7%Cr/Cu-Ce 14.3 0.05 24.8 15.8 9.1 30.4 a: measured by N2O titration; b: reaction conducted at 533 K 表 3 CuO还原温度
Table 3 CuO reduction temperature
Catalyst Peak positionT/K α β γ Cu-Ce 451 495 522 1%Cr/Cu-Ce 445 492 523 3%Cr/Cu-Ce 423 478 508 5%Cr/Cu-Ce 436 490 524 7%Cr/Cu-Ce 441 479 503 表 4 催化剂的Ce 3d拟合
Table 4 Ce 3d fitting results of the catalysts
Catalyst Ce(III)/(Ce(III)+Ce(IV))/% Cu-Ce 18.5 1%Cr/Cu-Ce 20.5 3%Cr/Cu-Ce 34.4 5%Cr/Cu-Ce 33.9 7%Cr/Cu-Ce 27.4 表 5 催化剂的CO、CO2以及H2选择性
Table 5 Selectivity of CO, CO2 and H2
Catalyst Selectivityas/% CO CO2 H2 Cu-Ce 0.59 25.04 74.37 1%Cr/Cu-Ce 0.14 25.43 74.43 3%Cr/Cu-Ce 0.15 25.46 74.39 5%Cr/Cu-Ce 0.23 25.48 74.29 7%Cr/Cu-Ce 0.33 25.23 74.44 a: reaction conducted at 533 K -
[1] EPPINGER J, HUANG K W. Formic acid as a hydrogen energy carrier[J]. ACS Energy Lett, 2017, 2(1):188-195. doi: 10.1021/acsenergylett.6b00574 [2] HONG X L, REN S Z. Selective hydrogen production from methanol oxidative steam reforming over Zn-Cr catalysts with or without Cu loading[J]. Int J Hydrogen Energy, 2008, 33(2):700-708. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ028188648/ [3] VAZQUEZ F V, SIMELL P, PENNANEN J, LEHTONENB J. Reactor design and catalysts testing for hydrogen production by methanol steam reforming for fuel cells applications[J]. Int J Hydrogen Energy, 2016, 41(2):924-935. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3b7d70a6719244202923f6d8545987b7 [4] MIROSLAV S, ALEKSANDRA M N. Nickel catalysts on porous ceramic supports for the reaction of partial oxidation of propane to CO and H2[J]. J Fluorine Chem, 2017, 155(23):132-142. doi: 10.2991/978-94-6239-213-7_28 [5] RICHARDS N O, ERICKSON P A. An investigation of a stratified catalyst bed for small-scale hydrogen production from methanol autothermal reforming[J]. Int J Hydrogen Energy, 2014, 39(31):18077-18083. doi: 10.1016/j.ijhydene.2014.03.131 [6] LIU Y, HAYAKAWA T, TSUNODA T, SUZUKI K, HAMAKAWA S, MURATA K, SHIOZAKI R, ISHII T, KUMAGAI M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts[J]. Top Catal, 2003, 22(3/4):205-213. doi: 10.1023/A:1023519802373 [7] 覃志强, 高文桂, 王华, 韩冲, 郭伟.稀土助剂Pr改性Cu/Zn/ZrO2合成甲醇催化剂的催化性能[J].化工进展, 2013, 32(4):820-823. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201304016QIN Zhi-qiang, GAO Wen-gui, WANG Hua, HAN Chong, GUO Wei. Research on rare-earth promoter Pr-modified Cu/Zn/ZrO2 catalyst for methanol synthesis[J]. Chem Ind Eng Prog, 2013, 32(4):820-823. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201304016 [8] LI J, HAN Y X, ZHU Y H, ZHOU R X. Purification of hydrogen from carbon monoxide for fuel cell application over modified mesoporous CuO-CeO2 catalysts[J]. Appl Catal B:Environ, 2011, 108(1/2):72-80. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=708b574e41ec2c4a7d15b260fd3c5561 [9] YANG S Q, ZHOU F, LIU Y J, ZHANG L, CHEN Y, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(14):7252-7261. doi: 10.1016/j.ijhydene.2019.01.254 [10] AMIN N A S, TAN E F, MANAN Z A. Selective reduction of NOx with C3H6 over Cu and Cr promoted CeO2 catalysts[J]. Appl Catal B:Environ, 2003, 43(1):57-69. doi: 10.1016/S0926-3373(02)00275-8 [11] 李吉刚, 孙杰, 张立功, 程玉龙, 邱新平, 陈立泉.花状微球NiO/CeO2催化剂上乙醇水蒸气重整制氢研究[J].燃料化学学报, 2010, 38(3):332-336. doi: 10.3969/j.issn.0253-2409.2010.03.014LI Ji-gang, SUN Jie, ZHANG Li-gong, CHENG Yu-long, QIU Xin-ping, CHEN Li-quan. Hydrogen production by steam reforming of ethanol over flowerlike micro spheres NiO/CeO2 catalyst[J]. J Fuel Chem Technol, 2010, 38(3):332-336. doi: 10.3969/j.issn.0253-2409.2010.03.014 [12] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学学报, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of support calcination atmospheres on the activity of CuO/CeO2 catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011 [13] 贺建平, 张磊, 陈琳, 杨占旭, 佟宇飞. CeO2改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].高等学校化学学报, 2017, 38(10):1822-1828. doi: 10.7503/cjcu20170158HE Jian-ping, ZHANG Lei, CHEN Lin, YANG Zhan-xu, TONG Yu-fei. Effect of CeO2 on Cu/Zn-Al catalysts derived from hydrotalcite precursor for methanol steam reforming[J]. Chem J Chin Univ, 2017, 38(10):1822-1828. doi: 10.7503/cjcu20170158 [14] 张磊, 潘立卫, 倪辉, 孙天军, 王树东, 胡永康, 王安杰, 赵生生.陈化时间对CuO/ZnO/CeO2/ZrO2甲醇水蒸气重整制氢催化剂性能的研究[J].燃料化学学报, 2013, 41(7):883-888. doi: 10.3969/j.issn.0253-2409.2013.07.016ZHANG Lei, PAN Li-wei, NI Hui, SUN Tian-jun, WANG Shu-dong, HU Yong-kang, WANG Aa-jie, ZHAO Sheng-sheng. Effects of precipitation aging time on the performance of CuO/ZnO/CeO2-ZrO2 for methanol steam reforming[J]. J Fuel Chem Technol, 2013, 41(7):883-888. doi: 10.3969/j.issn.0253-2409.2013.07.016 [15] 杨淑倩, 张娜, 贺建平, 张磊, 王宏浩, 白金, 张健, 刘道胜, 杨占旭. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014YANG Shu-qian, ZHANG Na, HE Jian-ping, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Jian, LIU Dao-sheng, YANG Zhan-xu. Effect of impregnation sequence of Ce on the performance of Cu/Zn-Al catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014 [16] 王保伟, 孙启梅, 李艳平, 刘思含.简单浸渍法制备纳米CuO/TiO2及其光催化剂活性[J].燃料化学学报, 2013, 41(6):741-747. doi: 10.3969/j.issn.0253-2409.2013.06.016WANG Bao-wei, SUN Qi-mei, LI Yan-ping, LIU Si-nian. Photocatalytic activity of nano-CuO/TiO2 composites prepared by a simple impregnated method[J]. J Fuel Chem Technol, 2013, 41(6):741-747. doi: 10.3969/j.issn.0253-2409.2013.06.016 [17] 邓双, 李会泉, 张懿.纳米Cr2O3的制备、表征及催化性能[J].无机化学学报, 2003, 19(8):825-830. doi: 10.3321/j.issn:1001-4861.2003.08.006DENG Shuang, LI Hui-quan, ZHANG Yi. Preparation, characterization and catalytic activity of nanosized Chromium oxide[J]. Chinese J Inorg Chem, 2003, 19(8):825-830. doi: 10.3321/j.issn:1001-4861.2003.08.006 [18] 王东哲, 冯旭, 张健, 陈琳, 张磊, 王宏浩, 白金, 张财顺.助剂M(M=Cr, Zn, Y, La)对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J], 燃料化学学报, 2019, 47(10):1251-1257. doi: 10.3969/j.issn.0253-2409.2019.10.012WANG Dong-zhe, FENG Xu, ZHANG Jian, CHEN Lin, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Cai-shun. Effect of promoter M (M=Cr, Zn, Y, La) on CuO/CeO2 catalysts for hydrogen production from steam reforming of methanol[J]. J Fuel Chem Technol, 2019, 47(10):1251-1257. doi: 10.3969/j.issn.0253-2409.2019.10.012 [19] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11):4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [20] BARBATO P S, COLUSSI S, BENEDETTO A D, LANDI G, LISI L, LIORCA J, TROVARELLI A. On the origin of high activity and selectivity of CuO/CeO2 catalysts prepared by solution combustion synthesis in CO-PROX reaction[J]. J Phys Chem C, 2016, 120(24):13039-13048. doi: 10.1021/acs.jpcc.6b02433 [21] SOLEIMANI E, MOGHADDAMI R. Synthesis, characterization and thermal properties of PMMA/CuO polymeric nanocomposites[J]. J Mater Sci-Mater El, 2018, 29(6):4842-4854. doi: 10.1007/s10854-017-8440-y [22] 刘玉娟, 王东哲, 张磊, 白金, 陈琳, 刘道胜. CeO2形貌对甲醇水蒸汽重整CuO/CeO2催化剂的影响[J].精细化工, 2018, 35(12):2045-2051. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, BAI-Jin, CHEN Lin, LIU Dao-sheng. Effect of CeO2 morphology on the performance of CuO/CeO2 catalysts for methanol steam reforming[J]. Fine Chem, 2018, 35(12):2045-2051. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011 [23] GROHMANN I, KEMNITZ E, LIPPITZ A, UNGER W E S. Curve fitting of Cr 2p photoelectron spectra of Cr2O3 and CrF3[J]. Surf Interface Anal, 1995, 23(13):887-891. doi: 10.1002/sia.740231306 [24] 荆国华, 李俊华, 郝吉明. Cr对In/WO3/ZrO2催化剂上甲烷选择性催化还原NO的促进作用[J].催化学报, 2009, 30(10):973-975. doi: 10.3321/j.issn:0253-9837.2009.10.001JING Guo-hua, LI Jun-hua, HAO Ji-ming. Promotional effect of Cr on the activity of In/WO3/ZrO2 for selective reduction of NO with methane[J]. Chin J Catal, 2009, 30(10):973-975. doi: 10.3321/j.issn:0253-9837.2009.10.001 -





 下载:
下载: