Effect of Ce/Co ratio on the catalytic performance of Ag/CeO2-Co3O4 in the low-temperature oxidation of formaldehyde
-
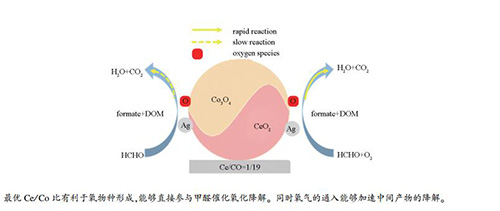
摘要: 采用共沉淀法制备了一系列具有不同Ce/Co比的Ag/CeO2-Co3O4催化剂,对其在甲醛低温氧化降解中的催化性能进行了研究。结果发现,Ag/CeO2-Co3O4催化剂具有较好的甲醛低温降解活性,而Ce/Co比是影响其催化性能的一个重要因素。XRD、氮吸附-脱附、Raman光谱、H2-TPR和in-situ DRIFTS等表征结果表明,随着Co含量的增加,Ag/CeO2-Co3O4催化剂的孔体积随之增大,而比表面积减小。CeO2有利于Ag/CeO2-Co3O4催化剂的氧化还原性能提高,促进氧空位增加,提升Co2+的含量,从而有利于氧分子的活化,促进甲醛降解。同时,in-situ DRIFTS结果表明,甲酸盐物种的分解是甲醛在Ag/CeO2-Co3O4催化剂表面催化氧化降解的速控步骤。
-
关键词:
- 共沉淀法 /
- CeO2-Co3O4 /
- 甲醛催化氧化 /
- 氧空位 /
- 甲酸盐
Abstract: A series of Ag/CeO2-Co3O4 catalysts with various Ce/Co ratios were prepared by co-precipitation method and employed in the low-temperature oxidation of formaldehyde. The results demonstrate that the Ag/CeO2-Co3O4 catalysts exhibit excellent performance in the oxidation of formaldehyde and the Ce/Co ratio has a significant impact on the catalytic performance. The XRD, nitrogen adsorption-desorption, Raman spectroscopy, H2-TPR and in-situ DRIFTS results illustrate that with the increase of Co content in Ag/CeO2-Co3O4, the pore volume increases whereas the surface area decreases. The presence of CeO2 is beneficial to enhancing the redox ability and increasing the oxygen vacancies and Co2+ species, which can promote the activation of oxygen species and then the degradation of formaldehyde. According to the in-situ DRIFTS results, the decomposition of formates on the catalyst surface is probably the rate-determining step for the degradation of formaldehyde at low temperature.-
Key words:
- co-precipitation /
- CeO2-Co3O4 /
- formaldehyde oxidation /
- oxygen vacancy /
- formate
-
图 6 70 ℃下HCHO吸附(a)随后通氧反应(b)期间在Ag/Ce5Co15上及HCHO吸附(c)随后通氧反应(d)期间在Ag/Ce1Co19上获得的原位漫反射傅里叶红外光谱谱图
Figure 6 In situ DRIFTS spectra obtained during HCHO adsorption (a) and then HCHO oxidation (b) on Ag/Ce5Co15 catalyst, and those during HCHO adsorption (c) and then HCHO oxidation (d) on Ag/Ce1Co19 catalyst under 70 ℃
表 1 不同催化剂的孔结构参数
Table 1 Textural properties of various Ag/CeO2-Co3O4 catalysts
Sample ABET /(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm Ag/Co 33.63 0.206 17.1 Ag/Ce1Co19 35.88 0.148 16.7 Ag/Ce5Co15 40.05 0.123 12.0 Ag/Ce10Co10 53.67 0.117 8.4 Ag/Ce15Co5 73.94 0.116 6.9 Ag/Ce19Co1 99.78 0.109 5.9 -
[1] MIAO L, WANG J L, ZHANG P Y. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde[J]. Appli Sur Sci, 2019, 466:441-453. doi: 10.1016/j.apsusc.2018.10.031 [2] World Health Organization (WHO): Air Quality Guidelines-2nd edition[S]. WHO Regional Office for Europe, 2001. [3] 彭家喜, 王树东.甲醛低温催化氧化动力学研究[J].燃料化学学报, 2006, 34(2):252-256. doi: 10.3969/j.issn.0253-2409.2006.02.026PENG Jia-xi, WANG Shu-dong. Kinetic study on catalytic oxidation of formaldehyde at low temperature[J]. J Fuel Chem Technol, 2006, 34(2):252-256. doi: 10.3969/j.issn.0253-2409.2006.02.026 [4] QUIROZ J, GIRAUDON J, GERVASINI A, DUJARDIN C, LANCELOT C, TRENTESAUX M, LAMONIER J. Total oxidation of formaldehyde over MnOx-CeO2 catalysts:Effect of acid treatment[J]. ACS Catal, 2015, 5:2260-2269. doi: 10.1021/cs501879j [5] BAI B Y, ARANDIYAN H, LI J H. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts[J]. Appl Catal B:Environ, 2013, 142-143:677-683. doi: 10.1016/j.apcatb.2013.05.056 [6] CHEN Y X, GAO J Y, HUANG Z W, ZHOU M J, CHEN J X, LI C, MA Z, CHEN J M, TANG X F. Sodium rivals silver as single-atom active centers for catalyzing abatement of formaldehyde[J]. Environ Sci Technol, 2017, 51(12):7084-7090. doi: 10.1021/acs.est.7b00499 [7] ZHANG C B, LIU F D, ZHAI Y P, ARIGA H, YI N, LIU Y C, ASAKURA K, FLYTZANI-STEPHANOPOULOS M, HE H. Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures[J]. Angew Chem Int Ed, 2012, 51:9628-9632. doi: 10.1002/anie.v51.38 [8] YANG T F, HUO Y, LIU Y, RUI Z B, JI H B. Efficient formaldehyde oxidation over nickel hydroxide promoted Pt λ-Al2O3 with a low Pt content[J]. Appl Catal B:Environ, 2017, 200:543-551. doi: 10.1016/j.apcatb.2016.07.041 [9] LI Y B, ZHANG C B, MA J Z, CHEN M, DENG H, HE H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation[J]. Appl Catal B:Environ, 2017, 217:560-569. doi: 10.1016/j.apcatb.2017.06.023 [10] LI Y B, ZHANG C B, HE H. Significant enhancement in activity of Pd/TiO2 catalyst for formaldehyde oxidation by Na addition[J]. Catal Today, 2017, 281:412-417. doi: 10.1016/j.cattod.2016.05.037 [11] CHEN B B, ZHU X B, CROCHER M, WANG Y, SHI C. Complete oxidation of formaldehyde at ambient temperature over λ-Al2O3 supported Au catalyst[J]. Catal Commun, 2013, 42:93-97. doi: 10.1016/j.catcom.2013.08.008 [12] CHEN B B, ZHU X B, WANG Y D, YU L M, LU J Q, SHI C. Nano-sized gold particles dispersed on HZSM-5 and SiO2 substrates for catalytic oxidation of HCHO[J]. Catal Today, 2017, 281:512-519. doi: 10.1016/j.cattod.2016.06.023 [13] BAI B Y, QIAO Q, ARANDIYAN HAMIDREZA, LI J H, HAO J M. Three-dimensional ordered mesoporous MnO2-supported Ag nanoparticles for catalytic removal of formaldehyde[J]. Environ Sci Technol, 2016, 50:2635-2640. doi: 10.1021/acs.est.5b03342 [14] QU Z P, CHEN D, SUN Y H, WANG Y. High catalytic activity for formaldehyde oxidation of AgCo/APTES@MCM-41 prepared by two steps method[J]. Appl Catal A:Gen, 2014, 487:100-109. doi: 10.1016/j.apcata.2014.08.044 [15] MA L, SEO C Y, CHEN X Y, LI J H, W.SCHWANK J. Sodium-promoted Ag/CeO2 nanospheres for catalytic oxidation of formaldehyde[J]. Chem Eng J, 2018, 350:419-428. doi: 10.1016/j.cej.2018.05.179 [16] 段言康, 宋忠贤, 张秋林, 刘启宪, 张金辉, 张腾飞, 孙二波.酸性质对磷钨酸改性CeO2上NH3选择性催化还原NO性能影响[J].燃料化学学报, 2016, 44(10):1259-1265. doi: 10.3969/j.issn.0253-2409.2016.10.014DUAN Yan-kang, SONG Zhong-xian, ZHANG Qiu-lin, LIU Qi-xian, ZHANG Jin-hui, ZHANG Teng-fei, SUN Er-bo. Effect of acid property on selective catalytic reduction of NO with ammonia over photungstic acid modified CeO2 catalyst[J]. J Fuel Chem Technol, 2016, 44(10):1259-1265. doi: 10.3969/j.issn.0253-2409.2016.10.014 [17] YU F L, QU Z P, ZHANG X D, FU Q, WANG Y. Investigation of CO and formaldehyde oxidation over mesoporous Ag/Co3O4 catalysts[J]. J Energy Chem, 2013, 22:845-862. doi: 10.1016/S2095-4956(14)60263-1 [18] YAN Z X, XU Z H, YANG Z H, YUE L, HUANG L Y. Graphene oxide/Fe2O3 nanoplates supported Pt for enhanced room temperature oxidation of formaldehyde[J]. Appl Sur Sci, 2019, 457/468:277-285. [19] ZHU L, WANG J L, RONG S P, WANG H Y, ZHANG P Y. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature[J]. Appl Catal B:Environ, 2017, 211:212-221. doi: 10.1016/j.apcatb.2017.04.025 [20] LIU B C, LIU Y, LI C Y, HU W T, JING P, WANG Q, ZHANG J. Three-dimensionally ordered macroporous Au/CeO2-Co3O4 catalysts with nanoporous walls for enhanced catalytic oxidation of formaldehyde[J]. Appl Catal B:Environ, 2012, 127:47-58. doi: 10.1016/j.apcatb.2012.08.005 [21] TANG X F, CHEN J L, LI Y G, LI Y, XU Y D, SHEN W J. Complete oxidation of formaldehyde over Ag/MnOx-CeO2 catalysts[J]. Chem Eng J, 2006, 118:119-125. doi: 10.1016/j.cej.2006.02.002 [22] DZIEMBAJ R, CHOJNACKA A, PIWOWARSKA Z, GAJEWSKA M, SWIETOSŁAWSKIA M, GÓRECKAA S, MOLENDA M. Comparative study of Co-rich and Ce-rich oxide nanocatalysts (CoxCe1-xOy) for low-temperature total oxidation of methanol[J]. Catal Today, 2018. [23] REN Q M, FENG Z T, MO S P, HUANG C L, LI S J, ZHANG W X, CHEN L M, FU M L, WU J L, YE D Q. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene[J]. Catal Today, 2018. [24] ZHENG Y L, WANG W Z, JIANG D, ZHANG L. Amorphous MnOx modified Co3O4 for formaldehyde oxidation:improved low-temperature catalytic and photothermocatalytic activity[J]. Chem Eng J, 2016, 284:21-27. doi: 10.1016/j.cej.2015.08.137 [25] GOMEZ L, MUNERA J, SOLLIER B, MIRO E, BOIX A. Raman in situ characterization of the species present in Co/CeO2 and Co/ZrO2 catalysts during the COPrOx reaction[J]. Int J Hydrogen Energy, 2016, 41:4993-5002. doi: 10.1016/j.ijhydene.2016.01.099 [26] ZHAO S, HU F Y, LI J H. Hierarchical core-shell Al2O3@Pd-CoAlO microspheres for low temperature toluene combustion[J]. ACS Catal, 2016, 6:3433-3441. doi: 10.1021/acscatal.6b00144 [27] LOU Y, WANG L, ZHAO Z Y, ZHANG Y H, ZHANG Z G, LU G Z, GUO Y, GUO Y L. Low-temperature CO oxidation over Co3O4-based catalysts:Significant promoting effect of Bi2O3 on Co3O4 catalyst[J]. Appli Catal B:Environ, 2014, 146:43-49. doi: 10.1016/j.apcatb.2013.06.007 [28] WANG C, ZHANG C H, HUA W C, GUO Y L, LU G Z, GILB SONIA, GIROIR-FENDLER NNE. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts[J]. Chem Eng J, 2017, 315:392-402. doi: 10.1016/j.cej.2017.01.007 [29] 邵建军, 朱锡, 申文杰. Co3O4/CeO2的氧化还原性能及反应条件对其CO氧化活性的影响[J].燃料化学学报, 2012, 40(1):75-79. doi: 10.3969/j.issn.0253-2409.2012.01.012SHAO Jian-jun, ZHU Xi, SHEN Wen-jie.Redox property of Co3O4/CeO2 and the effect of reaction conditions on its performance in CO oxidation[J]. J Fuel Chem Technol, 2012, 40(1):75-79. doi: 10.3969/j.issn.0253-2409.2012.01.012 [30] MA L, WANG D S, LI J H, BAI B Y, FU L X, LI Y D. Ag/CeO2 nanospheres:Efficient catalysts for formaldehyde oxidation[J]. Appli Catal B:Environ, 2014, 148/149:36-43. doi: 10.1016/j.apcatb.2013.10.039 [31] FAN Z Y, ZHANG Z X, FANG W J, YAO X, ZOU G C, SHANGGUAN W F. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants[J]. Chinese J Catal, 2016, 37:947-954. doi: 10.1016/S1872-2067(15)61086-5 [32] CHEN B B, SHI C, CROCHER MARK, WANG Y, ZHU A M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts[J]. Appli Catal B:Environ, 2013, 132/133:245-255. doi: 10.1016/j.apcatb.2012.11.028 [33] 拜冰阳.介孔猛、钴氧化物的研制及其催化氧化乙醇和甲醛的研究[D].北京: 清华大学, 2014.BAI Bing-yang. The synthesis of mesoporous manganese and cobalt oxide and its catalytic oxidation of ethanol and formaldehyde[D]. Beijing: Tsinghua University, 2014. [34] SHI C, CHEN B B, LI X S, CROCHER M, WANG Y, ZHU A M. Catalytic formaldehyde removal by "storage-oxidation" cycling process over supported silver catalysts[J]. Chem Eng J, 2012, 200/202:729-737. doi: 10.1016/j.cej.2012.06.103 [35] SHI C, WANG Y, ZHU A M, CHEN B B, AU C T. MnxCo3-xO4 solid solution as high-efficient catalysts for low-temperature oxidation of formaldehyde[J]. Catal Commun, 2012, 28:18-22. doi: 10.1016/j.catcom.2012.08.003 [36] YAN Z X, XU Z H, CHENG B, JIANG C J. Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature[J]. Appli Sur Sci, 2017, 404:426-434. doi: 10.1016/j.apsusc.2017.02.010 [37] MA C Y, WANG D H, XUE W J, DOU B J, WANG H L, HAO Z P. Investigation of formaldehyde oxidation over Co3O4-CeO2 and Au/Co3O4-CeO2 catalysts at room temperature:Effective removal and determination of reaction mechanism[J]. Environ Sci Technol, 2011, 45:3628-3634. doi: 10.1021/es104146v -





 下载:
下载: