-
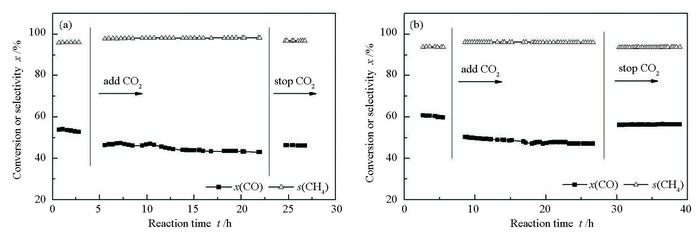
摘要: 在反应温度550℃、空速5000h-1和1.2% H2S浓度下,考察了反应气中添加CO2对负载型Mo基催化剂甲烷化活性的影响。结果表明,添加CO2会促进逆水煤气变换反应,从而降低MoO3/Al2O3催化剂的耐硫甲烷化活性。与MoO3/Al2O3催化剂相比,添加CO2对铈铝复合载体负载的Co-Mo双组分催化剂的影响较小。通过表征发现,添加CO2引起催化剂活性下降的主要原因是由于其增强了逆水煤气变换反应过程,使甲烷化过程可用氢气量减小。另外,逆水煤气变换反应生成的水会影响催化剂表面结构和组成。在连续加入10% CO2 20h后停止加入CO2,催化剂的耐硫甲烷化活性可以得到恢复,因此,认为CO2加入量低于10%时,对催化剂及甲烷化反应的影响是可逆的;但CO2加入量大于10%后由于生成的水量增大会破坏催化剂的结构并减少活性位,从而造成催化剂的不可逆失活。Abstract: The methanation of synthesis gas is the key process of coal to natural gas. Considering the existence of CO2 in the synthesis gas, it is important to investigate the influence of CO2 on the sulfur-resistant methanation. In this paper, the effect of CO2 on methanation activity of Mo-based catalysts was investigated at the reaction temperature of 550℃ and the gas space velocity of 5000h-1 with the syngas containing 1.2% H2S (volume ratio). The results show that the promoter Co and the cerium-aluminum composite support can improve the stability of the catalyst and reduce the deactivation. The CO2 is proved to promote the reverse water gas shift reaction, which would inhibit the activity of MoO3/Al2O3 catalyst more heavily than MoO3-CoO/CeO2-Al2O3 catalyst. When the CO2 adding to the inlet gas is less than 10% for 20h, the catalyst activity could be restored to its original activity after stopping the addition of CO2. However, as the added CO2 in inlet gas is over 10%, more H2O will be generated through reverse water gas shift reaction to damage the catalyst structure and decrease the active component, resulting in an irreversible loss of catalyst activity.
-
Key words:
- sulfur-resistant methanation /
- Mo-based catalyst /
- carbon dioxide /
- deactivation /
- water-gas shift
-
表 1 Lurgi炉、GE (Texaco) 炉、Shell炉气化产品对比[9]
Table 1 Comparion of gas composition from Lurgi, GE (Texaco) and Shell gasifier
Gasifier type Composition of gas φ/% H2 CO CO2 CH4 Lurgi 38-41 17-21 28-32 10-12 GE (Texaco) 36-39 43-46 16-18 <0.01 Shell 26-30 65-69 2-4 <0.01 表 2 甲烷化反应前后Mo基催化剂的织构性质
Table 2 Textural properties of Mo-based catalysts before and after reaction
Mo-based catalyst BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm BR ARN AR BR ARN AR BR ARN AR Mo/Al 204 154 156 0.25 0.26 0.14 6.0 6.0 4.4 Co-Mo/Al 165 133 135 0.27 0.20 0.19 5.0 6.0 5.3 Mo/CeAl 118 102 88 0.28 0.35 0.16 7.8 13.0 5.8 Co-Mo/CeAl 125 115 111 0.18 0.19 0.19 5.4 5.9 6.0 BR: before reaciton; ARN: after reaction without CO2; AR: after reaction at 10% CO2 concentration 表 3 添加不同含量CO2经甲烷化反应前后Co-Mo/CeAl催化剂的织构性质
Table 3 Textural properties of Co-Mo/CeAl catalyst before and after reaction with CO2 added
Co-Mo/CeAl catalyst BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm ARN 115 0.19 5.9 AR10 111 0.19 6.0 AR20 100 0.26 8.2 AR30 103 0.28 8.3 ARN:after reaction without CO2; AR10:after reaction at 10% CO2 concentration; AR20:after reaction at 20% CO2 concentration; AR30:after reaction at 30% CO2 concentration -
[1] 张东柯.面对人类社会可持续发展的能源选择[J].燃料化学学报, 2005, 33(4):399-406. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16583.shtmlZHANG Dong-ke.Energy options in sustainable development[J].J Fuel Chem Technol, 2005, 33(4):399-406. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16583.shtml [2] 胡亮华, 冯再南, 姚泽龙, 刘维, 申屠梁, 王俊峰.焦炉煤气甲烷化工艺过程的Aspen Plus模拟[J].天然气化工, 2013, 38(3):53-57. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201303013.htmHU Liang-hua, FENG Zai-nan, YAO Ze-long, LIU Wei, SHEN Tu-liang, WANG Jun-feng.Aspen Plus simulation of coke oven gas methanation process[J].Nat Gas Ind, 2013, 38(3):53-57. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201303013.htm [3] SABATIER P, SENDERENS J B.New synthesis of methane[J].CR Acad Sci Paris, 1902, 134:514-516. [4] SABATIER P, SENDERENS J B.Hydrogenation of CO over nickel to produce methane[J].J Soc Chim Ind, 1902, 21:504-506. [5] 黄国宝, 王志青, 李庆峰, 黄戒介, 房倚天, 液相中镍催化剂催化合成气甲烷化的初步研究[J].燃料化学学报, 2014, 42(8):952-957. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18470.shtmlHUANG Guo-bao, WANG Zhi-qing, LI Qing-feng, HUANG Jie-jie, FANG Yi-tian.Syngas methanation over nickel catalyst in liquid-phase[J].J Fuel Chem Technol, 2014, 42(8):952-957. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18470.shtml [6] MINCHENER A J.Coal gasification for advanced power generation[J].Fuel, 2005, 84(17):2222-2235. doi: 10.1016/j.fuel.2005.08.035 [7] SCHILDHAUER T J, SEEMANN M C, BIOLLAZ S M A.Fluidized bed methanation of wood-derived producer gas for the production of synthetic natural gas[J].Ind Eng Chem Res, 2010, 49(15):7034-7038. doi: 10.1021/ie100510m [8] 吴且毅, 卿涛, 颜智.一种利用焦炉气合成甲烷的方法:中国, 101391935 A[P].2009-03-25.WU Qie-yi, QIN Tao, YAN Zhi.Method for synthesizing methane by using coke-oven gas:CN, 101391935 A[P].2009-03-25. [9] 黄明金, 郭成宇, 尹明大.一种合成氨原料气甲烷化工艺:中国, 1313241 A[P].2001-09-19.HUANG Ming-jin, GUO Cheng-yu, YIN Ming-da.Methanation process for raw gas of synthetic ammonia:CN, 1313241 A[P].2001-09-19. [10] GALLETTI C, SPECCHIAS, SARACCO G, SPECCHIA V.CO-selective methanation over Ru/γ-Al2O3 catalysts in H2-rich gas for PEMFC applications[J].Chem Eng Sci, 2010, 65(1):590-596. doi: 10.1016/j.ces.2009.06.052 [11] 徐超, 王兴军, 胡贤辉, 陈雪莉, 王辅臣.镍基催化剂用于合成气甲烷化的实验研究[J].燃料化学学报, 2012, 40(2):216-220. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17886.shtmlXU Chao, WANG Xing-jun, HU Xian-hui, CHEN Xue-li, WANG Fu-chen.Study on the syngas methanation of nickel-based catalyst[J].J Fuel Chem Technol, 2012, 40(2):216-220. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17886.shtml [12] GAO J J, LIU Q, GU F N, LIU B, ZHONG Z Y, SU F B.Recent advances in methanation catalysts for the production of synthetic natural gas[J].RSC Adv, 2015, 5(29):22759-22776. doi: 10.1039/C4RA16114A [13] 高聚忠.煤气化技术的应用与发展[J].洁净煤技术, 2013, (1):65-71. http://www.cnki.com.cn/Article/CJFDTOTAL-JJMS201301018.htmGAO Ju-zhong.Application and development of coal gasification technologies[J].Clean Coal Technol, 2013, (1):65-71. http://www.cnki.com.cn/Article/CJFDTOTAL-JJMS201301018.htm [14] 袁勇天, 尹燕华, 周旭, 周军成.CO、CO2及其共存体系的甲烷化反应[J].化工进展, 2014, 33(1):173-180.YUAN Yong-tian, YIN Yan-hua, ZHOU Xu, ZHOU Jun-cheng.Methanation of thoree diffenent reaction systems of carbon oxides[J].Chem Ind Eng Process, 2014, 33(1):173-180. [15] 易丽丽, 马磊, 卢春山, 李小年.CO2催化加氢甲烷化研究进展[J].化工生产与技术, 2004, 11(5):33-35, 43.YI Li-li, MA Lei, LU Chun-shan, LI Xiao-nian.Study on the catalytic hydrogenation of carbon dioxide for methanation[J].Chem Prod Technol, 2004, 11(5):33-35, 43. [16] JIMÉNEZ V, SANCHEZ P, PANAGIOTOPOULOU P, VALVERDE J, ROMERO A.Methanation of CO, CO2 and selective methanation of CO, in mixtures of CO and CO2, over ruthenium carbon nanofibers catalysts[J].Appl Catal A:Gen, 2010, 390(1/2):35-44. [17] PANAGIOTOPOULOU P, KONDARIDES D, VERKIOS X.Selective methanation of CO over supported noble metal catalysts:Effects of the nature of the metallic phase on catalytic performance[J].Appl Catal A:Gen, 2008, 344(1/2):45-54. [18] ECKLE S, ANFANG H, BEHM R.What drives the selectivity for CO methanation in the methanation of CO2-rich reformate gases on supported Ru catalysts[J].Appl Catal A:Gen, 2011, 391(1):325-333. [19] 王承学, 龚杰.二氧化碳加氢甲烷化镍锰基催化剂的研究[J].天然气化工, 2011, 36(1):4-15. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201101001.htmWANG Cheng-xue, GONG Jie.Study on Ni-Mn-based catalysts for methanation of carbon dioxide[J].Nat Gas Ind, 2011, 36(1):4-15. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201101001.htm [20] 王二东, 王海洋, 丁国忠, 尚玉光, 李振花, 王保伟, 马新宾, 秦绍东, 孙琦.不同工艺条件下耐硫甲烷化催化剂的反应活性研究[J].化学反应工程与工艺, 2012, 28(1):75-81. http://www.cnki.com.cn/Article/CJFDTOTAL-HXFY201201014.htmWANG Er-dong, WANG Hai-yang, DING Guo-zhong, SHANG Yu-guang, LI Zhen-hua, WANG Bao-wei, MA Xin-bin, QIN Shao-dong, SUN Qi.Effect of reaction parameters on the activity of sulfur-resistant methanation catalyst[J].Chem React Eng Technol, 2012, 28(1):75-81. http://www.cnki.com.cn/Article/CJFDTOTAL-HXFY201201014.htm [21] LI Z H, WANG H Y, WANG E D, LV J, SHANG Y G, DING G Z, WANG B W, MA X B, QIN S D, SU Q.The main factors controlling generation of synthetic natural gas by methanation of synthesis gas in the presence of sulphur-resistant Mo-based catalysts[J].Kinet Catal, 2013, 54(3):338-343. doi: 10.1134/S0023158413030117 [22] WANG B W, DING G Z, SHANG Y G, LV J, WANG H Y, WANG E D, LI Z H, MA X B, QIN S D, SUN Q.Effects of MoO3 loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3[J].Appl Catal A:Gen, 2012, 431-432(1):144-150. [23] LIN C, WANG H Y, LI Z H, WANG B W, MA X B, QIN S D, SUN Q.Effect of a promoter on the methanation activity of Mo-based sulfur-resistant catalyst[J].Front Chem Sci Eng, 2013, 7(1):88-94. doi: 10.1007/s11705-013-1301-1 [24] 王保伟, 尚玉光, 丁国忠, 王海洋, 王二东, 李振花, 马新宾, 秦绍东, 孙琦.铈铝复合载体对钼基催化剂耐硫甲烷化催化性能的研究[J].燃料化学学报, 2012, 40(11):1390-1396. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18073.shtmlWANG Bao-wei, SHANG Yu-guang, DING Guo-zhong, WANG Hai-yang, WANG Er-dong, LI Zhen-hua, MA Xin-bin, QIN Shao-dong, SUN Qi.Ceria-alumina composite support on the sulfur-resistant methanation activity of Mo-based catalyst[J].J Fuel Chem Technol, 2012, 40(11):1390-1396. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18073.shtml [25] 崔晓曦, 曹会博, 孟凡会, 李忠.合成气甲烷化热力学计算分析[J].天然气化工, 2012, 37(5):15-19. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201205003.htmCUI Xiao-xi, CAO Hui-bo, MENG Fan-hui, LI Zhong.Thermodynamic analysis for methanation of syngas[J].Nat Gas Ind, 2012, 37(5):15-19. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201205003.htm [26] 陈宏刚, 王腾达, 张锴, 牛玉广, 杨勇平.合成气甲烷化反应积碳过程的热力学分析[J].燃料化学学报, 2013, 41(8):978-984. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18239.shtmlCHENG Hong-gang, WANG Teng-da, ZHANG Kai, NIU Yu-guang, YANG Yong-ping.Thermodynamic analysis of carbon deposition on catalyst for the production of substitute natural gas[J].J Fuel Chem Technol, 2013, 41(8):978-984. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18239.shtml [27] 窦伯生, 谢筱帆, 王玉杰, 石新.Co-Mo-K/Al2O3水煤气变换催化剂的失硫与活性[J].石油化工, 1990, (6):387-389.DOU Bo-sheng, XIE Xiao-fan, WANG Yu-jie, SHI Xin.Sulfer loss and activity of Co-Mo-K/Al2O3 water gas shift catalyst[J].Petrochem Technol, 1990, (6):387-389. [28] LAURENT E, DELMON B.Influence of water in the deactivation of a sulfided NiMo/γ-Al2O3 catalyst during hydrodeoxygenation[J].J Catal, 1994, 146(1):281-285, 288-291. doi: 10.1016/0021-9517(94)90032-9 -





 下载:
下载: