Hydrogen production from methane/steam by dielectric barrier discharge plasma reforming
-
摘要: 采用自制的介质阻挡放电实验系统,进行了甲烷/水蒸气大气压下重整制氢实验研究。考察了水碳比(水蒸气/甲烷物质的量比)、气体总流量、放电电压和放电频率对甲烷转化率及氢气等主要产物产率的影响。结果表明,甲烷转化率和氢气产率随着水碳比和放电电压的增加而增大,随着气体总流量和放电频率的增加呈现先增大后减小的变化规律。在放电电压18.6 kV、放电频率9.8 kHz、水碳比3.4、反应气体总流量79 mL/min时,获最大氢气产率(14.38%)。此外,利用发射光谱对放电过程中的活性基团进行了原位诊断,得到了CH·、OH·、H2及Hα活性粒子的光谱信号强度随实验参数的变化规律,并结合放电机理推测了氢气的生成路径。Abstract: The hydrogen production by methane/steam reforming at atmospheric pressure was investigated by using a self-made dielectric barrier discharge experimental system.The effect of water/carbon ratio (steam/methane molar ratio), total gas flow, discharge voltage and discharge frequency on the methane conversion, hydrogen and other major product yields was examined.The experimental results show that the methane conversion rate and hydrogen yield increase with the increase of water carbon ratio and discharge voltage, and the methane conversion rate and hydrogen yield increase first and then decrease with the increase of total gas flow rate and discharge frequency. The maximum hydrogen yield (14.38%) can be obtained at the discharge voltage of 18.6 kV, discharge frequency of 9.8 kHz, water/carbon ratio of 3.4, and total reaction gas flow rate of 79 mL/min. In addition, The active group in the discharge process was diagnosed by in-situ emission spectroscopy, and the changing trend in the spectral signal intensity of CH·, OH·, H2 and Hα active particles with the experimental parameters was obtained. The possible generation path of hydrogen is predicted by combining with the discharge mechanism.
-
Key words:
- methane /
- steam /
- dielectric barrier discharge /
- hydrogen /
- water/carbon ratio
-
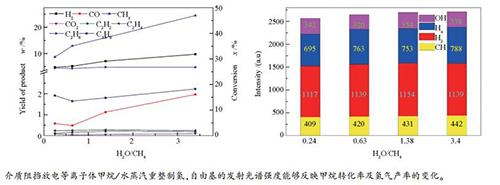
图 2 水/碳比对甲烷转化率及主要产物产率的影响(a)和CH·、OH·、Hα及H2活性粒子的光谱信号强度随水/碳比的变化(b)
Figure 2 Effect of water/carbon ratio on the methane conversion and main product yield (a)and the intensity change of the spectral signal of CH·, OH·, Hα and H2 active particles with the H2O/CH4 ratio (b)
■: H2; ●: CO; ▲: CH4; ▼: CO2; ◆: C2H2; ◀: C2H4; ▶: C2H6; ◆: C3H8
图 3 气体总流量对甲烷转化率及主要产物产率的影响(a)和CH·、OH·、Hα及H2活性粒子的光谱信号强度随气体总流量的变化(b)
Figure 3 Effect of total gas flow on the methane conversion and main product yield (a) and the intensity change of the spectral signal of CH·, OH·, Hα and H2 active particles with the total gas flow (b)
■: H2; ●: CO; ▲: CH4; ▼: CO2; ◆: C2H2; ◀: C2H4; ▶: C2H6; ◆: C3H8
图 4 放电电压对甲烷转化率及主要产物产率的影响(a)和CH·、OH·、Hα及H2活性粒子的光谱信号强度随放电电压的变化(b)
Figure 4 Effect of discharge voltage on the methane conversion and main product yield (a)and the intensity change of the spectral signal of CH·, OH·, Hα and H2 active particles with the discharge voltage (b)
■: H2; ●: CO; ▲: CH4; ▼: CO2; ◆: C2H2; ◀: C2H4; ▶: C2H6; ◆: C3H8
图 5 放电频率对甲烷转化率及主要产物产率的影响(a)和CH·,OH·、Hα及H2活性粒子的光谱信号强度随放电频率的变化(b)
Figure 5 Effect of discharge frequency on the methane conversion and main product yield (a)and the intensity change of the spectral signal of CH·, OH·, Hα and H2 active particles with the Discharge frequency (b)
■: H2; ●: CO; ▲: CH4; ▼: CO2; ◆: C2H2; ◀: C2H4; ▶: C2H6; ◆: C3H8
表 1 气体总流量对能量密度的影响
Table 1 Effect of total gas flow on energy density
Total flow
/(mL·min-1)40 79 118 158 Energy density
/(kJ·mol-1)5813 2943 1970 1472 表 2 放电频率对放电功率的影响
Table 2 Effect of discharge frequency on the discharge power
Discharge frequency
f/kHz8.5 9.0 9.5 9.8 10.25 Discharge power
/W147.8 156.5 169.6 173 88 表 3 回归分析模型常数
Table 3 Calculated values of model parameters
α α1 α2 α11 α22 α12 348.1 -0.75 0.24 0.0003 -0.0001 0 表 4 回归分析模型常数
Table 4 Calculated values of model parameters
A B C D E F G H I J K L M N O 407.64 -87.29 15.19 -135 119.83 -0.31 -0.0006 -0.33 -6.24 1.09 27.96 -61.22 -0.86 -0.28 12.18 -
[1] WANG B W, LIU Y J, ZHANG X, XU S H. Hydrogen production from methanol through dielectric barrier discharge[J]. J Nat Gas Chem, 2011, 5(2):209-214. http://d.old.wanfangdata.com.cn/Periodical/zggdxxxswz-hxgc201102008 [2] PHILIP G R, VADIM A K, VICTOR E P, SERGEY D P, ALEXANDER V S, DMITRY I S, ALEXANDER N B. Conversion of methane by CO2+H2O+CH4 plasma[J]. Appl Energy, 2015, 148(C):159-168. [3] SANCHES S G, HUERTAS FLORES J, PAIS DA SILVA M I. Influence of aging time on the microstructural characteristics of a Cu/ZnO based catalyst prepared by homogeneous precipitation for use in methanol steam reforming[J]. React Kinet Mech Catal, 2017, 121(2):473-485. doi: 10.1007/s11144-017-1161-7 [4] 庆绍军, 侯晓宁, 刘雅杰, 王磊, 李林东, 高志贤. Cu-Ni-Al尖晶石催化甲醇水蒸气重整制氢性能的研究[J].燃料化学学报, 2018, 46(10):1210-1217. doi: 10.3969/j.issn.0253-2409.2018.10.008QING Shao-jun, HOU Xiao-ning, LIU Ya-jie, WANG Lei, LI Lin-dong, GAO Zhi-xian. Catalytic performance of Cu-Ni-Al spinel for methanol steam reforming to hydrogen[J]. J Fuel Chem Technol, 2018, 46(10):1210-1217. doi: 10.3969/j.issn.0253-2409.2018.10.008 [5] 谢欣烁, 杨卫娟, 施伟, 张圣胜, 王智化, 周俊虎.制氢技术的生命周期评价研究进展[J].化工进展, 2018, 37(6):2147-2158. http://d.old.wanfangdata.com.cn/Periodical/hgjz201806015XIE Xin-shuo, YANG Wei-juan, SHI Wei, ZHANG Sheng-sheng, WANG Zhi-hua, ZHOU Jun-hu. Life cycle assessment of technologies for hydrogen productiona review[J]. Chem Ind Eng Prog, 2018, 37(6):2147-2158. http://d.old.wanfangdata.com.cn/Periodical/hgjz201806015 [6] NAIR S A, NOZAKI T O, OKAZAKI K. Methane oxidative conversion pathways in a dielectric barrier discharge reactor-Investigation of gas phase mechanism[J]. Chem Eng J, 2007, 132(1/3):85-95. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=61b89539932d1274b542420c474db7f1 [7] INDARTO A, COOWANITWONG N, CHOI JAE-WOOK, LEE H, SONG H K. Kinetic modeling of plasma methane conversion in a dielectric[J]. Fuel Process Technol, 2008, 89(2):214-219. doi: 10.1016/j.fuproc.2007.09.006 [8] 邵涛, 严萍.大气压气体放电及其等离子体应用[M].北京:科学出版社, 2015.SHAO Tao, YAN Ping. Atmospheric Gas Discharge and its Plasma Application[M]. Beijing:Science Press, 2015. [9] 钱伯章.一步将CO2和甲烷转化为更高价值的燃料和化学品的等离子体新方法[J].天然气化工(C1化学与化工), 2017, 42(5):125. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201705027.htmQIAN Bo-zhang. A new plasma method for converting CO2 and methane into higher value fuels and chemicals in one step[J]. Nat Gas Chem Ind, 2017, 42(5):125. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201705027.htm [10] KHADIR N, KHODJA K, BELASRI A. Methane conversion using a dielectric barrier discharge reactor at atmospheric pressure for hydrogen[J].Plasma Sci Technol, 2017, 19(9):81-90. http://d.old.wanfangdata.com.cn/Periodical/dlztkxyjs-e201709009 [11] HU S H, WANG B W, LV Y J, YAN W J.Conversion of methane to C2 hydrocarbons and hydrogen using a gliding arc reactor[J]. Plasma Sci Technol, 2013, 15(6):555-561. doi: 10.1088/1009-0630/15/6/13 [12] SHIGERU K, KOHEI U, YASUSHI S, KAORU F, TOMOHIRO N, KEN O.Reaction mechanism of methane activation using non-equilibriumpulseddischarge at room temperature[J]. Fuel, 2003, 82(18):2291-2297. doi: 10.1016/S0016-2361(03)00163-7 [13] 王皓, 宋凌珺, 李兴虎, 岳丽蒙.介质阻挡放电等离子体甲烷部分氧化重整制氢[J].物理化学学报, 2015, 31(7):1406-1412. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201507023WANG Hao, SONG Ling-jun, LI Xing-hu, YUE Li-meng.Hydrogen production from partial oxidation of methane by dielectric barrier discharge plasma reforming[J]. Acta Phys-Chim Sin, 2015, 31(7):1406-1412. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201507023 [14] 张浩, 朱凤森, 李晓东, 吴昂键, 薄拯, 岑可法.旋转滑动弧氩等离子体裂解甲烷制氢[J].燃料化学学报, 2016, 44(2):192-200. doi: 10.3969/j.issn.0253-2409.2016.02.009ZHANG Hao, ZHU Feng-sen, LI Xiao-dong, WU Ang-jian, BO Zheng, CEN Ke-fa. Rotating gliding arc plasma assisted hydrogen production from methane decomposition in argonr[J]. J Fuel Chem Technol, 2016, 44(2):192-200. doi: 10.3969/j.issn.0253-2409.2016.02.009 [15] WANG Y F, TSAI C H, CHANG W Y, KUO Y M. Methane steam reforming for producing hydrogen in an atmospheric-pressure microwave plasma reactor[J]. Intl J Hydrogen Energy, 2010, 35(1):135-140. doi: 10.1016/j.ijhydene.2009.10.088 [16] 徐锋, 朱丽华, 李创.低温等离子体活化转化煤层甲烷机理的光谱诊断[J].发光学报, 2017, 38(3):372-379. http://d.old.wanfangdata.com.cn/Periodical/fgxb201703016XU Feng, ZHU Li-hua, LI Chuang. Mechanism of activation and conversion of coalbed methane under cold plasma by optical emission spectroscopy[J]. Chin J Lumin, 2017, 38(3):372-379. http://d.old.wanfangdata.com.cn/Periodical/fgxb201703016 [17] 魏波, 骆仲泱, 徐飞, 赵磊, 高翔, 岑可法.脉冲电晕放电中OH自由基的发射光谱研究[J].光谱学与光谱分析, 2010, 30(2):293-296. doi: 10.3964/j.issn.1000-0593(2010)02-0293-04WEI Bo, LUO Zhong-yang, XU Fei, ZHAO Lei, GAO Xiang, CEN Ke-fa. Study of emission spectroscopy of OH radicals in pulsed corona discharge[J]. Spectrosc Spect Anal, 2010, 30(2):293-296. doi: 10.3964/j.issn.1000-0593(2010)02-0293-04 [18] 罗丽霞, 吴卫东, 朱永红, 唐永建. CH4/H2和CH4/He体系等离子体发射光谱分析[J].强激光与粒子束, 2008, 20(6):899-902. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qjgylzs200806005LUO Li-xia, WU Wei-dong, ZHU Yong-hong, TANG Yong-jian. Spectrum analysis of plasma in CH4/H2 and CH4/He systems[J]. High Power Laser Part Beams, 2008, 20(6):899-902. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qjgylzs200806005 [19] 刘永卫.甲烷和水蒸汽介质阻挡放电转化研究[D].天津: 天津大学, 2008. http://cdmd.cnki.com.cn/Article/CDMD-10056-2009073124.htmLIU Yong-wei. Study on methane-steam conversion with dielectric-barrier discharge[D]. Tianjin: Tianjin University, 2008. http://cdmd.cnki.com.cn/Article/CDMD-10056-2009073124.htm [20] ZHAO G B, JOHN S, ZHANG J J, WANG L N. Methane conversion inpulsed corona discharge reactors[J]. Chem Eng J, 2006, 125(2):67-79. https://www.sciencedirect.com/science/article/pii/S138589470600324X [21] 闫文娟.介质阻挡微等离子体纯CH4转化制低碳烃[D].天津: 天津大学, 2008. http://cdmd.cnki.com.cn/Article/CDMD-10056-1014036622.htmYAN Wen-juan. Dielectric barrier pure plasma pure CH4 conversion to low carbon hydrocarbons[D]. Tianjin: Tianjin University, 2008. http://cdmd.cnki.com.cn/Article/CDMD-10056-1014036622.htm -





 下载:
下载: