High active Pd/MgO catalyst for CO oxidative coupling to dimethyl oxalate
-
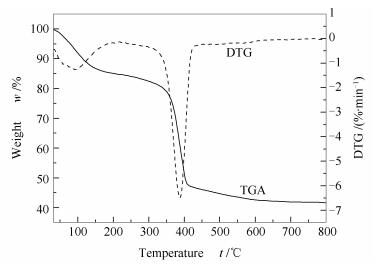
摘要: 利用溶液法结合高温煅烧处理合成MgO载体,通过浸渍法制备Pd/MgO催化剂并对其进行CO氧化偶联制草酸二甲酯催化性能研究。通过X射线粉末衍射、CO2程序升温脱附、比表面仪、热重分析、扫描电镜、透射电镜和微型催化评价装置对合成的样品进行结构和性能表征。结果表明,合成的MgO载体是一种Lewis碱性很强的纳米片结构,Pd纳米颗粒高度分散在MgO载体上,粒径小且分布均一。此MgO纳米片作为载体制备的Pd/MgO催化剂在较低的Pd负载量(0.5%)下表现出优异的CO氧化偶联催化性能,在反应温度130 ℃时CO单程转化率高达65%,草酸二甲酯选择性96%,稳定性超过100 h,明显越于工业催化剂(Pd/α-Al2O3),具有潜在的工业应用前景。Abstract: The MgO support was first synthesized by the solution method, with which the Pd/MgO nanocatalyst was then prepared by the impregnation method. The structure and performance of prepared catalyst were characterized by XRD, CO2-TPD, BET, TG, SEM, TEM and catalytic evaluation device. The research result shows that the synthesized MgO support consists of uniform nanosheets and strong Lewis basic sites. The active Pd nanoparticles of Pd/MgO catalyst are highly dispersed on the surface of MgO support with small and homogeneous size. Moreover, the Pd/MgO nanocatalyst with a low Pd loading (0.5%) exhibits an excellent catalytic performance with 65% CO conversion, 96% selectivity to DMO and more than 100 h on stream at 130 ℃. for CO oxidative coupling to dimethyl oxalate, much higher than industrial catalyst (Pd/α-Al2O3), which has a good prospect of industrial application.
-
Key words:
- Pd/MgO /
- coal to ethylene glycol /
- CO oxidative coupling /
- dimethyl oxalate /
- catalytic performance
-
图 2 MHC前驱体的XRD谱图和底部垂直线条表示碱式碳酸镁(MgCO3)4·Mg(OH)2·5H2O标准衍射峰(JCPDS标准卡片:23-1218);MgO和Pd/MgO催化剂的XRD谱图
Figure 2 XRD patterns of MHC precursors (a), in which vertical bars at the bottom denote the standard data for zinc hydroxide carbonate (MgCO3)4·Mg(OH)2·5H2O (JCPDS, No.23-1218); XRD patterns of MgO and Pd/MgO catalyst (b)
图 6 在0.5% Pd负载量时,反应温度对Pd/MgO催化剂性能的影响(a);在反应温度130 ℃时,Pd负载量对Pd/MgO催化剂性能的影响(b)
Figure 6 Catalytic performances of Pd/MgO with 0.5% Pd loading at different reaction temperatures (a); catalytic performances of Pd/MgO catalysts with different Pd loadings at 130 ℃ (b)
—●—: CO conversion; —■—: DMO selectivity
表 1 Pd/MgO催化剂与工业催化剂的CO氧化偶联催化性能对比a
Table 1 CO oxidative coupling to dimethyl oxalate (DMO) on different catalystsa

-
[1] ZHAO T J, CHEN D, DAI Y C, YUAN W K, HOLMEN A. Synthesis of dimethyl oxalate from CO and CH3ONO on carbon nanofiber supported palladium catalysts[J]. Ind Eng Chem Res, 43(16): 4595-4601. doi: 10.1021/ie030728z [2] YIN A Y, GUO X Y, DAI W L, FAN K N. High activity and selectivity of Ag/SiO2 catalyst for hydrogenation of dimethyl oxalate[J]. Chem Commun, 2010, 46(24): 4348-4350. doi: 10.1039/c0cc00581a [3] CHEN L F, GUO P J, QIAO M H, YAN S R, LI H X, SHEN W, XU H L, FAN K N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal, 2008, 257(1): 172-180. doi: 10.1016/j.jcat.2008.04.021 [4] HE Z, LIN H Q, HE P, YUAN Y Z. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal, 2011, 277(1): 54-63. doi: 10.1016/j.jcat.2010.10.010 [5] ZHU Y Y, WANG S R, ZHU L J, GE X L, LI X B, LUO Z Y. The Influence of copper particle dispersion in Cu/SiO2 catalysts on the hydrogenation synthesis of ethylene glycol[J]. Catal Lett, 2010, 135(3/4): 275-281. doi: 10.1007/s10562-010-0298-z [6] HU Q, FAN G L, YANG L, LI F.Aluminum-doped zirconia-supported copper nanocatalysts: Surface synergistic catalytic effects in the gas-phase hydrogenation of esters[J]. Chem Cat Chem, 2014, 6(12): 3501-3510. https://www.deepdyve.com/lp/wiley/aluminum-doped-zirconia-supported-copper-nanocatalysts-surface-n1tQknGIwB [7] YUE H R, ZHAO Y J, MA X B, GONG J L.Ethylene glycol: properties, synthesis, and applications[J]. Chem Soc Rev, 2012, 41(11): 4218-4244. doi: 10.1039/c2cs15359a [8] 周张锋, 李兆基, 潘鹏斌, 林凌, 覃业燕, 姚元根.煤制乙二醇技术进展[J].化工进展, 2010, 29(11): 2003-2009. http://www.cnki.com.cn/Article/CJFDTOTAL-SDHW201104011.htmZHOU Zhang-feng, LI Zhao-ji, PAN Peng-bin, LIN Ling, QIN Ye-yan, YAO Yuan-gen. Progress in technologies of coal-based ethylene glycol synthesis[J]. Chem Ind Eng Prog, 2010, 29(11): 2003-2009. http://www.cnki.com.cn/Article/CJFDTOTAL-SDHW201104011.htm [9] YAMAMOTO Y. Vapor phase carbonylation reactions using methyl nitrite over Pd catalysts[J]. Catal Surv Asia, 2010, 14(3/4): 103-110. https://www.researchgate.net/publication/226587798_Vapor_Phase_Carbonylation_Reactions_Using_Methyl_Nitrite_Over_Pd_Catalysts [10] XU Z N, SUN J, LIN C S, JIANG X M, CHEN Q S, PENG S Y, WANG M S, GUO G C. High-performance and long-lived Pd nanocatalyst directed by shape effect for CO oxidative coupling to dimethyl oxalate[J]. ACS Catal, 2013, 3(2): 118-122. doi: 10.1021/cs300759h [11] PENG S Y, XU Z N, CHEN Q S, CHEN Y M, SUN J, WANG Z Q, WANG M S, GUO G C. An ultra-low Pd loading nanocatalyst with high activity and stability for CO oxidative coupling to dimethyl oxalate[J]. Chem Commun, 2013, 49(51): 5718-5720. doi: 10.1039/c3cc00219e [12] ZHAO X G, LIN Q, XIAO W D. Characterization of Pd-CeO2/alpha-alumina catalyst for synthesis of dimethyl oxalate[J]. Appl Catal A: Gen, 2005, 284(1/2): 253-257. http://www.sciencedirect.com/science/article/pii/S0926860X05001006 [13] LIN Q, JI Y, JIANG Z D, XIAO W D. Effects of precursors on preparation of Pd/alpha-alumina catalyst for synthesis of dimethyl oxalate[J]. Ind Eng Chem Res, 2007, 46(24): 7950-7954. doi: 10.1021/ie070640b [14] JIANG X Z, SU Y H, LEE B J, CHIEN S H. A study on the synthesis of diethyl oxalate over Pd/alpha-Al2O3 catalysts[J]. Appl Catal A: Gen, 2001, 211(1): 47-51. doi: 10.1016/S0926-860X(00)00837-1 [15] JIN E L, HE L L, ZHANG Y L, RICHARD A R, FAN M H. A nanostructured CeO2 promoted Pd/alpha-alumina diethyl oxalate catalyst with high activity and stability[J]. RSC Adv, 2014, 4(90): 48901-48904. doi: 10.1039/C4RA08170F [16] 高正虹, 胡超权, 李振花, 何琲, 许根慧.一氧化碳偶联制草酸二乙酯用Pd-Fe/α-Al2O3催化剂的表面结构[J].催化学报, 2004, 25(3): 205-209. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200403009&dbname=CJFD&dbcode=CJFQGAO Zheng-hong, HU Chao-quan, LI Zhen-hua, HE Fei, XU Gen-hui. Surface structure of Pd-Fe/α-Al2O3 catalyst for CO coupling to diethyl oxalate[J]. Chin J Catal, 2004, 25(3): 205-209. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200403009&dbname=CJFD&dbcode=CJFQ [17] 张炳楷, 黄存平, 郭廷煌, 王金木.一氧化碳催化偶联合成草酸二甲酯[J].化学研究与应用, 1990, 2(3): 21-25. http://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ199003002.htmZHANG Bing-kai, HUANG Cun-ping, GUO Ting-huang, WANG Jin-mu.Catalytic synthesis of oxalate diester by carbon monoxide coupling[J]. Chem Res Appl, 1990, 2(3): 21-25. http://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ199003002.htm [18] 林茜, 计扬, 谭俊青, 肖文德. Pd/α-Al2O3催化CO偶联制草酸二甲酯的反应机理[J].催化学报, 2008, 29(4): 325-329. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200804003&dbname=CJFD&dbcode=CJFQLIN Qian, JI Yang, TAN Jun-qing, XIAO Wen-de. Mechanism of CO coupling to dimethyl oxalate over Pd/α-Al2O3[J]. Chin J Catal, 2008, 29(4): 325-329. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200804003&dbname=CJFD&dbcode=CJFQ [19] UCHIUMI S, ATAKA K, MATSUZAKI T. Oxidative reactions by a palladium-alkyl nitrite system[J]. J Organomet Chem, 1999, 576(1/2): 279-289. https://www.researchgate.net/publication/239138669_Oxidative_Reactions_by_a_Palladium-Alkyl_Nitrite_System [20] 王春迎, 杜志明, 丛晓民, 田秀丽.碱式碳酸镁的热分解研究[J].应用化工, 2008, 37(6):657-660. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200806021.htmWANG Chun-ying, DU Zhi-ming, CONG Xiao-min, TIAN Xiu-li. Investigation in thermal decomposition of basic magnesium carbonate[J]. Appl Chem Ind, 2008, 37(6):657-660. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200806021.htm [21] 李琳, 张露明, 张煜华, 李金林.镍负载量对Ni/MgO(111) 催化甲烷二氧化碳重整反应性能影响[J].燃料化学学报, 2015, 43(3): 315-322. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18590.shtmlLI Lin, ZHANG Lu-ming, ZHANG Yu-hua, LI Jin-lin. Effect of Ni loadings on the catalytic properties of Ni/MgO(111) catalyst for the reforming of methane with carbon dioxide[J]. J Fuel Chem Technol, 2015, 43(3): 315-322. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18590.shtml [22] LIU Z, CORTES-CONCEPCION J A, MUSTIAN M, AMIRIDIS M D. Effect of basic properties of MgO on the heterogeneous synthesis of flavanone[J]. Appl Catal A: Gen, 2006, 302(2): 232-236. doi: 10.1016/j.apcata.2006.01.007 [23] LI S, CHEN C H, ZHAN E S, LIU S B, SHEN W J. Chirality inversion in enantioselective hydrogenation of isophorone over Pd/MgO catalysts in the presence of (S)-proline: Effect of Pd particle size[J]. J Mol Catal A: Chem, 2009, 304(1/2): 88-94. http://www.sciencedirect.com/science/article/pii/S1381116909000417 [24] NIU Z Q, ZHEN Y R, GONG M, PENG Q, NORDLANDER P, LI Y D. Pd nanocrystals with single-, double-, and triple-cavities: Facile synthesis and tunable plasmonic properties[J]. Chem Sci, 2011, 2(12): 2392-2395. doi: 10.1039/c1sc00449b -





 下载:
下载: