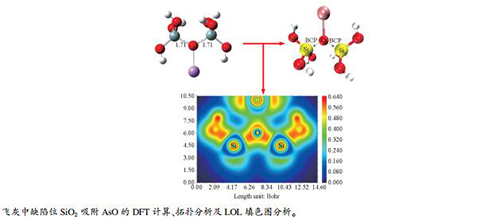
Adsorption mechanism of trace As on the defect sites of SiO2 in fly ash
-
摘要: 为了认识痕量元素As在飞灰中的富集特性,利用密度泛函理论研究了砷的典型氧化物AsO在飞灰中的主要成分SiO2模型上的吸附机理,对优化后的吸附构型进行能量计算、AIM理论、Mulliken电荷分析以及定域化轨道指示函数(LOL)填色图分析,剖析了AsO与SiO2表面的相互作用。结果表明,AsO在无定型SiO2表面的缺陷位的吸附能均大于50 kJ/mol,吸附构型均为典型的化学吸附。在无定型SiO2缺陷活性位点形成的As-Si键、Si-O键和As-O键强度较大,均属于共价键;SiO2与AsO之间为共价相互作用。Abstract: To understand the enrichment characteristics of trace As in fly ash, the adsorption mechanism of AsO, a typical arsenic oxide, in SiO2, the main component of fly ash, was investigated by using density functional theory; energy calculation, AIM theory, Mulliken charge analysis and Localized Orbital Locator(LOL) color map were performed on the optimized adsorption configuration, in order to analyze the interaction between AsO and SiO2. The results show that the adsorption energy of AsO on the defect sites of amorphous SiO2 is higher than 50 kJ/mol, typical for the configuration of chemical adsorption. The bonds of As-Si, Si-O and As-O formed at the active defect sites of the amorphous SiO2 have high strength, belonging to the covalent bond; that is, the interaction between SiO2 and AsO is covalent.
-
Key words:
- density functional theory /
- adsorption /
- AsO /
- SiO2 /
- AIM theory /
- LOL
-
表 1 SiO2单体吸附AsO构型的相互作用能
Table 1 Interaction energies between AsO and SiO2 of various adsorption configurations
Configuration Eads /(kJ·mol-1) Configuration Eads /(kJ·mol-1) SiO2-AsO A1 -214.14 SiO2-AsO C -386.76 A2 -248.49 D -408.62 B1 -83.04 E -427.50 B2 -210.77 表 2 SiO2单体吸附AsO构型的吸附热
Table 2 Adsorption heat of AsO on SiO2 with different configurations
Configuration Hads /(kJ·mol-1) Configuration Hads /(kJ·mol-1) SiO2-AsO A1 225.08 SiO2-AsO C 376.52 A2 250.65 D 404.94 B1 84.00 E 432.04 B2 217.07 表 3 吸附构型中Si-As键临界点(BCP)的拓扑分析性质
Table 3 Topological parameters of the BCP of Si-As bonds in various adsorption configurations
Configuration Bond ρ ▽2ρ G V H |V|/G Mulliken As/O △ SiO2-AsO A1 Si1-As8 0.242 -0.013 0.085 -0.154 -0.204 2.12 0.150 -0.322 A2 Si1-O9 0.249 0.342 0.089 -0.183 -0.344 2.15 -0.831 -0.359 B1 Si8-As15 0.210 0.145 0.029 -0.060 -0.048 2.07 0.329 -0.143 Si1-As15 0.211 0.134 0.029 -0.061 -0.047 2.10 0.329 -0.143 B2 Si1-O16 0.242 0.385 0.074 -0.153 -0.203 2.08 -0.793 -0.321 Si8-O16 0.243 0.387 0.072 -0.151 -0.201 2.09 -0.794 -0.322 C O2-As9 0.290 0.359 0.104 -0.229 -0.565 2.20 0.947 0.475 D O11-As15 0.321 0.406 0.135 -0.348 -0.733 2.58 0.954 0.482 E O5-As27 0.323 0.343 0.152 -0.419 -0.665 2.76 1.043 0.571 -
[1] 赵永椿, 马斯鸣, 杨建平.燃煤电厂污染物超净排放的发展及现状[J].煤炭学报, 2015, 40(11):2629-2640. http://d.old.wanfangdata.com.cn/Periodical/dtsj201620015ZHAO Yong-chun, MA Si-ming, YANG Jian-ping. Development and status quo of ultra-clean pollutant emissions from coal-fired power plants[J]. J China Coal Soc, 2015, 40(11):2629-2640. http://d.old.wanfangdata.com.cn/Periodical/dtsj201620015 [2] 郭欣, 郑楚光, 陈丹. 300MW煤粉锅炉砷排放特征的实验研究[J].环境科学学报, 2006, 27(4):631-634. http://d.old.wanfangdata.com.cn/Periodical/hjkx200604005GUO Xin, ZHENG Chu-guang, CHEN Dan. Experimental Study on arsenic emission characteristics of 300MW pulverized coal-fired boiler[J]. J Environ Sci-China, 2006, 27(4):631-634. http://d.old.wanfangdata.com.cn/Periodical/hjkx200604005 [3] 王泉海, 邱建荣, 温存.氧燃烧方式下痕量元素形态转化的试验和模拟研究[J].工程热物理学报, 2006, 27(s2):199-202. http://d.old.wanfangdata.com.cn/Periodical/gcrwlxb2006z2052WANG Quan-hai, QIU Jian-rong, WUN Cun. Experimental and simulation study on morphological transformation of trace elements under oxygen combustion[J]. J Eng Thermophys, 2006, 27(s2):199-202. http://d.old.wanfangdata.com.cn/Periodical/gcrwlxb2006z2052 [4] DING Z H, ZHENG B S, ZHUANG M. Mode of occurrence of arsenic in high-As coals from endemic arsenicosis areas in southwestern Guizhou Province, China[J]. J Coal Sci Eng, 2007, 13(2):194-198. http://www.cqvip.com/Main/Detail.aspx?id=24615571 [5] 董静兰, 马凯.富氧气氛下煤与生物质掺烧时砷的释放研究[J].动力工程学报, 2017, 37(3):237-241. http://d.old.wanfangdata.com.cn/Periodical/dlgc201703011DONG Jing-lan, MA Kai. Study on arsenic release from coal-biomass combustion under oxygen-enriched atmosphere[J]. Chin J Power Eng, 2017, 37(3):237-241. http://d.old.wanfangdata.com.cn/Periodical/dlgc201703011 [6] 高正阳, 吕少昆, 吉硕.碳基表面吸附铅的机理[J].环境工程学报, 2016, 10(7):3848-3852. http://d.old.wanfangdata.com.cn/Periodical/hjwrzljsysb201607076GAO Zheng-yang, LÜ Shao-kun, JI Shuo. Mechanism of lead adsorption on carbon-based surfaces[J]. J Chin Environ Eng, 2016, 10(7):3848-3852. http://d.old.wanfangdata.com.cn/Periodical/hjwrzljsysb201607076 [7] 高正阳, 吕少昆.二氧化锰改性活性炭吸附汞的机理研究[J].动力工程学报, 2015, 35(11):923-928. doi: 10.3969/j.issn.1674-7607.2015.11.010GAO Zheng-yang, LÜ Shao-kun. Mechanism of adsorption of mercury by manganese dioxide modified activated carbon[J]. Chin J Power Eng, 2015, 35(11):923-928. doi: 10.3969/j.issn.1674-7607.2015.11.010 [8] 郭欣, 郑楚光, 吕乃霞.簇模型CaO(001)面上吸附汞与氯化汞的密度泛函理论研究[J].中国电机工程学报, 2005, 25(13):101-104. doi: 10.3321/j.issn:0258-8013.2005.13.019GUO Xin, ZHENG Chu-guang, LÜ Nai-xia. Density functional theory study of adsorption of mercury and mercury over the CaO(001) Surface of cluster model[J]. Proc CSEE, 2005, 25(13):101-104. doi: 10.3321/j.issn:0258-8013.2005.13.019 [9] 王鹏, 吴江, 任建兴.飞灰未燃尽碳对吸附烟气汞影响的试验研究[J].动力工程学报, 2012, 32(4):332-337. doi: 10.3969/j.issn.1674-7607.2012.04.012WANG Peng, WU Jiang, REN Jian-xing. Experimental study on the effect of unburned carbon from fly ash on mercury adsorption in flue gas[J]. Chin J Power Eng, 2012, 32(4):332-337. doi: 10.3969/j.issn.1674-7607.2012.04.012 [10] 王军, 蒋建国, 隋继超.垃圾焚烧飞灰基本性质的研究[J].环境科学学报, 2006, 27(11):141-145. http://d.old.wanfangdata.com.cn/Periodical/hjkx200611026WANG Jun, JIANG Jian-guo, SUI Ji-chao. Study on the basic properties of waste incineration fly ash[J]. J Environ Sci-China, 2006, 27(11):141-145. http://d.old.wanfangdata.com.cn/Periodical/hjkx200611026 [11] 孙俊民, 韩德馨, 姚强.燃煤飞灰的显微颗粒类型与显微结构特征[J].电子显微学报, 2001, 20(2):140-147. doi: 10.3969/j.issn.1000-6281.2001.02.012SUN Jun-min, HAN De-xin, YAO Qiang. Microscopic particle types and microstructure characteristics of coal-fired fly ash[J]. J Electron Micro, 2001, 20(2):140-147. doi: 10.3969/j.issn.1000-6281.2001.02.012 [12] WILLIAMS R P, RIESSEN A V. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD[J]. Fuel, 2010, 89(12):3683-3692. doi: 10.1016/j.fuel.2010.07.031 [13] NURIA L, FRANCESC I, GIANFRANCO P. Adsorption of Cu, Pd, and Cs Atoms on Regular and Defect Sites of the SiO2 Surface[J]. J Am Chem Soc, 2017, 121(4):813-821. doi: 10.1021/ja981753c [14] FERULLO R M, GARDA G R, BELELLI P G. Deposition of small Cu, Ag and Au particles on reduced SiO2[J]. J Mol Struct (Theochem), 2006, (769):217-223. http://www.sciencedirect.com/science/article/pii/S0166128006002478 [15] 吕鑫.簇-表面类比: 金属氧化物簇模型探讨[D].厦门: 厦门大学, 2004.LÜ Xin. Cluster-Surface Analogy: Discussion on Metal Oxide Cluster Model[D]. Xiamen: Xiamen University, 2004. [16] MUKHOPADHYAY S, SUSHKO P V. Correlation between the atomic structure, formation energies, and optical absorption of neutral oxygen vacancies in amorphous silica[J]. Phys Rev B, 2005, 71(23):5204. http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=PRBMDO000071000023235204000001&idtype=cvips&gifs=Yes [17] KRISHNAN R, BINKLEY J S, SEEGER R. Self-consistent molecular-orbital methods-A basis set for correlated wave functions[J]. Chem Phys, 1980, 72(1):650-654. http://www.tandfonline.com/servlet/linkout?suffix=CIT0112&dbid=16&doi=10.1080%2F00268970701757875&key=10.1063%2F1.438955 [18] WEIGEND F, AHLRICHS R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn:Design and assessment of accuracy[J], Phys Chem Chem Phys, 2005, (7):3297-3305. http://www.tandfonline.com/servlet/linkout?suffix=CIT0030&dbid=16&doi=10.1080%2F01932691.2017.1298040&key=10.1039%2Fb508541a [19] GAUSSIAN 09, REVISIOND.01, FRISCH M J, TRUCKS G W, SCHLEGEL H B. Gaussian, Inc., Wallingford CT, 2016. [20] LU T. Multiwfn:A multifunctional wavefunction analyzer[J]. J Comput Chem, 2012, 33(5):580-592. doi: 10.1002/jcc.v33.5 [21] TANG T H, CUI Y P. A theoretical study of some X-H-π hydrogen-bonded complexes using the theory of atoms in molecules[J]. Can J Chem, 2011, 74(6):1162-1170. doi: 10.1139/v96-130#.W-OmfPkfR8Q [22] BADER R F. A quantum theory of molecular structure and its applications[J]. Chem Rev, 1991, 91(5):893-928. doi: 10.1021/cr00005a013 [23] CREMER D, KRAKA E. Chemical bonds without bonding electron density-Does the difference electron density analysis suffice for a description of the chemical bond[J]. Angew Chem, 1984, 23(23):627-628. doi: 10.1002/anie.198406271/pdf [24] ESPINOSA E, ALKORTA I, ELGUERO J. From weak to strong interactions:A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X-H F-Y systems[J]. J Chem Phys, 2002, 117(12):5529-5542. doi: 10.1063/1.1501133 [25] 董兰, 蒋树斌, 郑申声. Ben(n=1~6)团簇吸附H2的密度泛函研究[J].原子能科学技术, 2010, 44(12):1414-1419. http://d.old.wanfangdata.com.cn/Periodical/yznkxjs201012002DONG Lan, JIANG Shu-bin, ZHENG Shen-sheng. Density functional theory for hydrogen molecule adsorption of Ben(n=1~6) clusters[J]. Atomic Energy Sci Technol, 2010, 44(12):1414-1419. http://d.old.wanfangdata.com.cn/Periodical/yznkxjs201012002 [26] 叶天旭, 徐永强, 张予辉. Co/MoS2催化剂加氢脱硫活性的量子化学研究[J].中国石油大学学报(自然科学版), 2005, 29(4):119-121. doi: 10.3321/j.issn:1000-5870.2005.04.028YE Tian-xu, XU Yong-qiang, ZHANG Yu-hui. Quantum chemistry study on hydrogenation desulfurization activity of Co/MoS2 catalyst[J]. J Chin Univ Pet(Nat Sci Ed), 2005, 29(4):119-121. doi: 10.3321/j.issn:1000-5870.2005.04.028 [27] JACOBSEN H J. Localized-orbital locator (LOL) profiles of transition-metal hydride a[J]. Can J Chem, 2009, 87(7):965-973. doi: 10.1139/V09-060 -





 下载:
下载: