Effect of potassium carbonate on coal ash sintering and mineral transformation in H2O-H2-CO-CO2 atmosphere
-
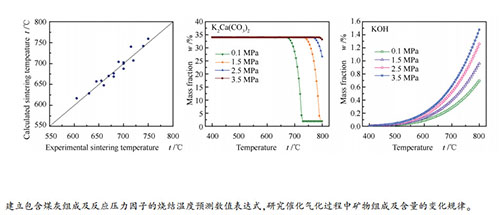
摘要: 在水蒸气气化气氛(水蒸气-氢气-一氧化碳-二氧化碳混合气氛)下考察了反应压力对负载碳酸钾煤灰烧结温度的影响,建立了包含煤的灰分、煤灰化学组成、催化剂负载量及反应压力因素的煤灰烧结温度预测关系式,预测结果与实测烧结温度的误差在±15℃(2%)范围内。利用X射线衍射仪和FactSage热力学计算软件对不同气氛和压力下煤灰中的矿物组成及含量的变化规律进行了分析。结果表明,碳酸钾与煤灰中的硬石膏、方解石反应生成硫酸钾和碳酸钾钙;水蒸气气化气氛下硫酸钾和赤铁矿被还原,碳酸钾钙的分解温度随反应压力的增大而升高;负载催化剂煤灰中氢氧化钾的量随温度和压力的提高而增加,不同压力下煤灰的最低烧结温度与氢氧化钾的含量有关,当氢氧化钾的含量达到一定值时,不同压力下对应的温度与实验测得的煤灰烧结温度接近。Abstract: The influence of reaction pressure on the sintering temperature of potassium carbonate loaded coal ash was investigated in H2O-H2-CO-CO2 atmosphere. An empirical equation for sintering temperature calculation was derived based on the content of ash, the ash composition, the catalyst loading and the reaction pressure. The deviation between the calculated value and the experimental value was within ±15 ℃ (2%). The transformation of two coal ashes was investigated by X-ray diffractometer and thermodynamic calculation. It was found that potassium carbonate could react with anhydrite and calcite in coal to produce potassium sulfate and butschliite. The potassium sulfate and hematite could be reduced in the H2O-H2-CO-CO2 atmosphere, and the decomposition temperature of butschliite increased with the increase of pressure. Moreover, the content of potassium hydroxide in the potassium loaded coal ash increased with the increase of temperature and pressure. The minimum sintering temperatures of coal ash under different pressures were related to the content of potassium hydroxide; when the contents of potassium hydroxide in ashes reached to a certain value, the calculated minimum sintering temperature was very close to the experimental one under different pressures.
-
图 1 加压压差法煤灰烧结温度测定装置示意图
Figure 1 Schematic diagram of pressurized sintering temperature determination apparatus
1-constant pressure valve; 2-mass flowmeter; 3-high-pressure water pump; 4-preheater-heater; 5-cylinder ash column; 6-gas distributor; 7-electric furnace; 8-differential manometer; 9-thermocouple; 10-gas liquid separator; 11-back pressure regulator; 12-volumetric flow meter
图 5 鄂尔多斯原煤灰及负载碳酸钾煤灰不同条件下反应后的XRD谱图
Figure 5 XRD patterns of EEDS raw coal ash and potassium carbonate loaded coal ash under different conditions
(a): EEDS raw coal ash; (b): EEDS-K coal ash a: EEDS raw coal ash; b: EEDS-N2-3.5 MPa; c: EEDS-mixture-3.5 MPa; d: EEDS-K ash; e: EEDS-K-N2-3.5 MPa; f: EEDS-K-mixture-0.1 MPa; g: EEDS-K-mixture-3.5 MPa; Q: quartz-SiO2; A: anhydrite-CaSO4; C: calcite-CaCO3; H: hematite-Fe2O3; Mu: muscovite-K0.77Al1.93(Al0.5Si3.5)O10(OH)2; An: anathite-CaAl2SiO8; Ma:magnetite-Fe3O4; Ar: arcanite-K2SO4; CaO: calcium oxide-CaO; B: butschliite-K2Ca(CO3)2; PC: potassium carbonate-K2CO3; PH: potassium carbonate hydrate-K2CO3 ·1.5H2O; PA: potassium aluminate silicate-K0.85Al0.85Si0.15O2; PS: potassium silicate-K2Si4O9; KA: kaliophilite-KAlSiO4
图 6 文山原煤灰及负载碳酸钾煤灰不同条件下反应后的XRD谱图
Figure 6 XRD patterns of WS raw coal ash and potassium carbonate loaded coal ash under different conditions
(a): WS raw coal ash; (b): WS-K coal ash a: WS raw coal ash; b: WS-N2-3.5 MPa; c: WS-mixture-3.5 MPa; d: WS-K ash; e: WS-K-N2-3.5 MPa; f: WS-K-mixture-0.1 MPa; g: WS-K-mixture-3.5 MPa; Q: quartz-SiO2; A: anhydrite-CaSO4; C: calcite-CaCO3; H: hematite-Fe2O3; I: illite-K(Al4Si2O9(OH)3); An: anathite-CaAl2SiO8; Mu: muscovite-K0.77Al1.93(Al0.5Si3.5)O10(OH)2; Ma: magnetite-Fe3O4; Ar: arcanite-K2SO4; CaO: calcium oxide-CaO; B: butschliite-K2Ca(CO3)2; PH: potassium carbonate hydrate-K2CO3 ·1.5H2O; PC: potassium carbonate-K2CO3; PA: potassium aluminate silicate-K0.85Al0.85Si0.15O2; PS: potassium silicate-K2Si4O9; KA: kaliophilite-KAlSiO4
表 1 煤样的工业分析和元素分析
Table 1 Proximate and ultimate analyses of coal samples
Sample Proximate analysis wd/% Ultimate analysis wd/% A V FC C H N St O* WS 18.29 39.73 41.98 53.78 3.91 1.61 1.24 21.17 EEDS 4.79 35.43 59.78 73.83 4.37 0.66 0.90 15.45 A: ash; V: volatile matter; FC: fixed carbon; d: dry base; St: total sulfur; *: by difference 表 2 煤灰的化学组成
Table 2 Chemical composition of coal ashes
Sample Content w/% SiO2 Al2O3 TiO2 CaO Fe2O3 MgO K2O Na2O SO3 WS 33.52 15.58 0.57 20.96 10.75 1.34 1.73 0.21 13.62 EEDS 29.09 11.34 0.72 21.28 17.48 0.76 0.48 0.70 15.24 表 3 模拟计算负载碳酸钾煤灰的化学组成
Table 3 Calculated composition of potassium carbonate loaded coal ashes
Sample Content w/% K2Ca(CO3)2 K2CO3 KAlSiO4 K2SO4 K2Si2O5 Fe2O3 Mg2SiO4 Na2Ca2Si3O9 Ca3Fe2Si3O12 CaOMgOSiO2 WS K-ash 34.09 18.73 18.54 13.93 9.64 4.12 0.90 0.05 - - EEDS K-ash 9.87 30.44 11.27 32.81 - 2.05 - 1.30 11.31 0.95 -
[1] HIRSCH R L, GALLAGHER J E Jr, LESSARD R R, WESSELHOFT R D. Catalytic coal gasification:An emerging technology[J]. Science, 1982, 215(4529):121-127. doi: 10.1126/science.215.4529.121 [2] VERAA M J, BELL A T. Effect of alkali metal catalysts on gasification of coal char[J]. Fuel, 1978, 57(4):194-200. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=pb/wz2UjXR1yvtiMN5cRB2833OngiCvi/EuU/jYJJs0= [3] YEBOAH Y D, XU Y, SHETH A, GODAVARTY A, AGRAWAL P K. Catalytic gasification of coal using eutectic salts:identification of eutectics[J]. Carbon, 2003, 41(2):203-214. [4] 樊利霞, 李克忠, 张荣, 毕继诚.负载碳酸钾煤焦上CO甲烷化反应的研究[J].燃料化学学报, 2014, 42(9):1047-1052. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201409004FAN Li-xia, LI Ke-zhong, ZHANG Rong, BI Ji-cheng. Methanation of CO over coal char loaded with K2CO3[J]. J Fuel Chem Technol, 2014, 42(9):1047-1052. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201409004 [5] 李伟伟, 李克忠, 康守国, 郑岩, 张荣, 毕继诚.煤催化气化中非均相反应动力学的研究[J].燃料化学学报, 2014, 42(3):290-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201403006LI Wei-wei, LI Ke-zhong, KANG Shou-guo, ZHENG Yan, ZHANG Rong, BI Ji-cheng. Heterogeneous reaction kinetics of catalytic coal gasification[J]. J Fuel Chem Technol, 2014, 42(3):290-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201403006 [6] 毛燕东, 金亚丹, 李克忠, 毕继诚, 李金来, 辛峰.煤催化气化工艺中内蒙王家塔烟煤灰烧结温度的影响因素分析[J].化工学报, 2015, 66(3):1080-1087. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb2015030031MAO Yan-dong, JIN Ya-dan, LI Ke-zhong, BI Ji-cheng, LI Jin-lai. XIN Feng. Analysis of influencing factors on sintering temperature of Inner Mongolia Wangjiata bituminous coal ash during catalytic coal gasification[J]. CIESC J, 2015, 66(3):1080-1087. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb2015030031 [7] 毛燕东, 金亚丹, 李克忠, 毕继诚, 李金来, 辛峰.煤催化气化条件下不同煤种煤灰烧结行为研究[J].燃料化学学报, 2015, 43(4):402-409. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201504004MAO Yan-dong, JIN Ya-dan, LI Ke-zhong, BI Ji-cheng, LI Jin-lai, XIN Feng. Sintering behavior of different coal ashes in catalytic coal gasification process[J]. J Fuel Chem Technol, 2015, 43(4):402-409. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201504004 [8] 沙兴中, 杨南星.煤的气化与应用[M].上海:华东理工大学出版社, 1995.SHA Xing-zhong, YANG Nan-xing. Coal Gasification and Its Application[M]. Shanghai:East China University of Science and Technology Press, 1995. [9] 芦涛.催化气化结渣及钾迁移特性的基础研究[D].太原: 中国科学院山西煤炭化学研究所, 2015. http://d.wanfangdata.com.cn/thesis/Y2956501LU Tao. Fundamental research on agglomeration and potassium migration characteristics during catalytic coal gasification[D]. Taiyuan: Institute of Coal Chemistry, Chinese Academy of Sciences, 2015. http://d.wanfangdata.com.cn/thesis/Y2956501 [10] 王勤辉, 揭涛, 李小敏, 骆仲泱, 景妮洁, 岑可法.反应气氛对不同煤灰烧结温度影响的研究[J].燃料化学学报, 2010, 38(1):17-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201001004WANG Qin-hui, JIE Tao, LI Xiao-min, LUO Zhong-yang, JING Ni-jie, CEN Ke-fa. Experiments of the effects of reaction atmosphere on the coal ash sintering temperature[J]. J Fuel Chem Technol, 2010, 38(1):17-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201001004 [11] 李风海, 黄戒介, 房倚天, 王洋.小龙潭褐煤灰烧结温度影响因素的研究[J].洁净煤技术, 2011, 17(3):57-60. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jjmjs201103018LI Feng-hai, HUANG Jie-jie, FANG Yi-tian, WANG Yang. Research on the influencing factors of sintering temperature of Xiaolongtan lignite ashes[J]. Clean Coal Technol, 2011, 17(3):57-60. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jjmjs201103018 [12] 王勤辉, 景妮洁, 骆仲泱, 李小敏, 揭涛.灰成分影响煤灰烧结温度的实验研究[J].煤炭学报, 2010, 35(6):1015-1020. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtxb201006028WANG Qin-hui, JING Ni-jie, LUO Zhong-yang, LI Xiao-min, JIE Tao. Experiments on the effect of chemical components of coal ash on the sintering temperature[J]. J China Coal Soc, 2010, 35(6):1015-1020. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtxb201006028 [13] HU H W, ZHOU K, MENG K S, SONG L B, LIN Q Z. Effects of SiO2/Al2O3 ratios on sintering characteristics of synthetic coal ash[J]. Energies, 2017, 10(2):242. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=MDPI000000198789 [14] CHENG X L, WANG Y G, LIN X C, BI J C, ZHANG R, BAI L. Effects of SiO2-Al2O3-CaO/FeO low-temperature eutectics on slagging characteristics of coal ash[J]. Energy Fuels, 2017, 31(7):6748-6757. doi: 10.1021/acs.energyfuels.7b00535 [15] LU T, LI K Z, ZHANG R, BI J C. Addition of ash to prevent agglomeration during catalytic coal gasification in a pressurized fluidize bed[J]. Fuel Process Technol, 2015, 134:414-423. doi: 10.1016/j.fuproc.2015.02.024 [16] WANG L, HUSTAD J E, GRONLI M. Sintering characteristics and mineral transformation behaviors of corn cob ashes[J]. Energy Fuels, 2012, 26(9):5905-5916. doi: 10.1021/ef300215x [17] JING N J, WANG Q H, CHENG L M, LUO Z Y, CEN K F. The sintering behavior of coal ash under pressurized conditions[J]. Fuel, 2013, 103:87-93. doi: 10.1016/j.fuel.2011.09.025 [18] JING N J, WANG Q H, LUO Z Y, CEN K F. Effect of chemical composition on sintering behavior of Jincheng coal ash under gasification atmosphere[J]. Chem Eng Commun, 2012, 199(2):189-202. doi: 10.1080/00986445.2011.582531 [19] 郑绍聪, 宁平, 马丽萍, 杜亚雷, 张伟, 牛学奎.不同气氛下磷石膏热分解的反应特性[J].武汉理工大学学报(交通科学与工程版), 2010, 34(3):580-583. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=whjtkjdxxb201003038ZHENG Shao-cong, NING Ping, MA Li-ping, DU Ya-lei, ZHANG Wei, NIU Xue-kui. Reaction properties of thermal decomposition of phosphogypsum in different atmospheres[J]. J Wuhan Univ Technol (Transp Sci Eng), 2010, 34(3):580-583. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=whjtkjdxxb201003038 [20] 陈升, 刘少文.氢气还原分解硫酸钙的热力学研究[J].化学工业与工程技术, 2012, 33(5):7-11. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxgyygcjs201205002CHEN Sheng, LIU Shao-wen. Thermodynamic study on reductive decomposition of calcium sulfate with hydrogen[J]. J Chem Ind Eng, 2012, 33(5):7-11. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxgyygcjs201205002 [21] ENNACIRI Y, EL ALAOUI-BELGHITI H, BETTACH M. Comparative study of K2SO4 production by wet conversion from phosphogypsum and synthetic gypsum[J]. J Mater Res Technol, 2019, 8(3):2586-2596. http://www.sciencedirect.com/science/article/pii/S2238785418306446 [22] AREFIEV A V, PODBORODNIKOV I V, SHATSKIY A F, LITASOV K D. Synthesis and Raman spectra of K-Ca double carbonates:K2Ca(CO3)2 butschliite, fairchildite, and K2Ca2(CO3)3 at 1 atm[J]. Geochem Int, 2019, 57(9):981-987. doi: 10.1134/S0016702919090039 [23] ARCEO H B, GLASSER F P. Fluxing reactions of sulfates and carbonates in cement clinkering II. The system CaCO3-K2CO3[J]. Cement Concrete Res, 1995, 25(2):339-344. http://www.sciencedirect.com/science/article/pii/0008884695000194 -





 下载:
下载: