Synthesis of dispersed molybdenum disulfide nano-catalysts and their performance in the hydrogenation of simulated oil slurry
-
摘要: 以二烷基二硫代氨基甲酸钼(Mo-DTC)和六羰基钼(Mo(CO)6)为前驱体、水热法合成了分散型纳米MoS2,采用X-ray射线衍射(XRD)、透射电子显微镜(TEM)、X射线光电子能谱分析(XPS)和程序升温脱附法(NH3-TPD)等方法对其进行了表征。利用三种烯烃(辛烯、苯乙烯、反式二苯乙烯)、苯并噻吩和蒽等构建模拟油浆体系,结合气相色谱-质谱(GC-MS)分析,对分散型纳米MoS2的模拟油浆加氢处理催化性能进行了研究。结果表明,不同预处理条件下制备出的催化活性样品均为2H-MoS2,但各样品的结晶度、颗粒尺寸、硫化程度及其酸性质等均有所不同,其中,总酸量差别较小;以Mo-DTC和Mo(CO)6为前驱体的优选硫化条件分别为380℃/30 min和370℃/30 min,所得到的催化剂对烯烃和噻吩的加氢活性较高。其中,Mo-DTC基纳米MoS2催化剂的烯烃加氢饱和转化率高达98.10%,加氢脱硫率为94.51%,而蒽的部分加氢饱和转化率则较低,为29.47%,且无八氢蒽(8HN)或全氢蒽的生成。Mo(CO)6基纳米MoS2催化剂的加氢效果则略差,烯烃加氢饱和转化率为94.01%,加氢脱硫率为89.01%,对蒽的加氢饱和转化率为24.20%,无8HN或全氢蒽的生成。总体而言,由Mo-DTC所制备的MoS2催化剂具有烯烃高效饱和、含硫化合物高效脱硫、芳烃浅度加氢饱和的效果,且油浆加氢处理反应的选择性及催化稳定性均更高。Abstract: A series of dispersed nano molybdenum disulfide (MoS2) catalysts were prepared with molybdenum dialkyl dithiocarbamate (Mo-DTC) and molybdenum hexacarbonyl (Mo(CO)6) as the precursors by hydrothermal methods and characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and temperature programmed desorption (NH3-TPD). By using a simulated oil slurry containing three kinds of olefins (octane, styrene and trans-dibenzylethene), benzothiophene and anthracene, the catalytic performance of nano MoS2 in the hydrogenation was investigated, with the help of gas chromatography-mass spectrometry (GC-MS). The results indicate that all the prepared catalysts are in the form of 2H-MoS2; however, their crystallinity, particle size, vulcanization degree, and acid property are influenced by the pretreatment conditions; the preferred vulcanization conditions for the Mo-DTC-and Mo(CO)6-based MoS2 catalysts are 380 ℃/30 min and 370 ℃/30 min, respectively, to achieve a relatively high activity in the hydrogenation of olefins and benzothiophene. Over the Mo-DTC-based nano-MoS2 catalyst, the saturation conversion of olefins hydrogenation is 98.10% and the hydrodesulfurization rate is 94.51%, whereas the saturation conversion of anthracene hydrogenation is 29.47%, without forming octahydroanthracene (8HN) or perhydroanthracene. In contrast, the activity of Mo(CO)6-based nano-MoS2 catalyst is slightly lower, with the saturation conversion of olefins hydrogenation being 94.01% and the hydrodesulfurization rate being 89.01%; similarly, the saturation conversion for anthracene hydrogenation is 24.20%, without 8HN or perhydroanthracene in the product. As a whole, in comparison with the Mo(CO)6-based MoS2 catalyst, the nano MoS2 catalyst derived from Mo-DTC displays higher efficiency in both olefins saturation and sulfur-containing compounds desulfurization, and low degree hydrogenation of aromatic hydrocarbons; moreover, it also exhibits higher hydro-treating selectivity for the catalytic cracking slurry and higher stability during hydrogenation.
-
Key words:
- nano-MoS2 /
- hydrodesulfurization /
- olefin saturation /
- anthracene /
- benzothiophene
-
图 5 不同预处理条件下合成MoS2的XPS谱图
(a)/(b): Mo-DTC-based MoS2 synthesized at 380 ℃ for 30 min; (c)/(d): Mo-DTC-based MoS2 synthesized at 370 ℃ for 90 min; (e)/(f): Mo(CO)6-based MoS2 synthesized at 370 ℃ for 30 min; (g)/(h): Mo(CO)6-based MoS2 synthesized at 380 ℃ for 90 min
Figure 5 XPS spectra of MoS2 catalysts prepared under different pretreatment conditions
图 7 模拟油浆加氢饱和产物的GC-MS谱图
1: n-octane; 2: ethylbenzene; 3: cumene; 4: n-propylbenzene; 5: 1, 2, 3, 4-tetrahydro-1-methyl-naphthalene; 6:1-methylnaphthalene; 7: 1, 2-diphenylethane; 8: 9, 10-dihydroanthracene; 9: 1, 2, 3, 4-tetrahydroanthracene; 10: anthracene
Figure 7 GC-MS spectra of the hydrogenation saturation products of simulated slurry over the Mo-DTC (380 ℃/3 h)
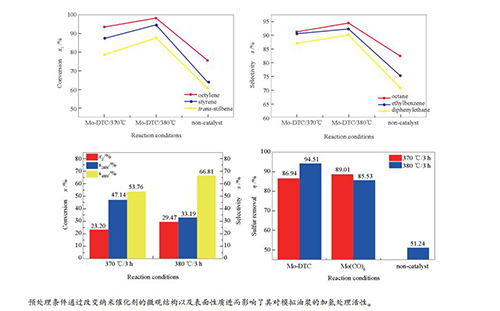
图 8 不同反应条件下Mo-DTC基MoS2对模拟油浆中的烯烃加氢饱和效果
(a): hydrogenation saturation conversion rate of various olefins; (b): selectivity of hydrogenated saturated product alkanes a: pretreatment with Mo-DTC-based MoS2 at 370 ℃ for 90 min; b: pretreatment with Mo-DTC-based MoS2 at 380 ℃ for 30 min; c: pretreatment without catalyst
Figure 8 Hydrogenation saturation of olefins in simulated slurry oil over the Mo-DTC-based MoS2 catalyst under different reaction conditions
图 9 不同反应条件下Mo(CO)6基MoS2对模拟油浆的烯烃加氢饱和效果
(a): hydrogenation saturation conversion rate of various olefins; (b): selectivity of hydrogenated saturated product alkanes a: pretreatment with Mo(CO)6-based MoS2 at 370 ℃ for 30 min; b: pretreatment with Mo(CO)6-based MoS2 at 380 ℃ for 30 min; c: pretreatment without catalyst
Figure 9 Hydrogenation saturation of olefins in simulated slurry oil over the Mo(CO)6-based MoS2 catalyst under different reaction conditions
图 13 加氢处理反应后MoS2的XRD谱图(a)和加氢处理反应前后结晶系数α变化(b)
a: Mo-DTC based MoS2 pretreatment under 380 ℃ for 30 min; b: Mo(CO)6 based MoS2 pretreatment under 370 ℃ for 30 min A: Mo(CO)6-based MoS2; B: Mo-DTC-based MoS2
Figure 13 XRD patterns of the MoS2 catalysts after hydro-treating reaction (a) and change in crystallinity factor (α) before and after hydro-treating reaction (b)
-
[1] 王红军, 马锋, 童晓光, 刘祚冬, 张新顺, 吴珍珍, 李登华, 王勃, 谢寅符, 杨柳燕.全球非常规油气资源评价[J].石油勘探与开发, 2016, 43(6):850-862. http://d.old.wanfangdata.com.cn/Periodical/syktykf201606002WANG Hong-jun, MA Feng, TONG Xiao-guang, LIU Zuo-dong, ZHANG Xin-shun, WU Zhen-zhen, LI Deng-hua, WANG Bo, XIE Yin-fu, YANG Liu-yan. Global unconventional oil and gas resources evaluation[J]. Adv Pet Explor Dev, 2016, 43(6):850-862. http://d.old.wanfangdata.com.cn/Periodical/syktykf201606002 [2] 郭爱军, 郑文林, 焦守辉, 陈坤, 刘贺, 王宗贤, 王子豪.催化裂化油浆中烯烃分布研究[J].燃料化学学报, 2018, 46(4):413-418. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19195.shtmlGUO Ai-jun, ZHENG Wen-lin, JIAO Shou-hui, CHEN Kun, LIU He, WANG Zong-xian, WANG Zi-hao. Study on olefin distribution of catalytic cracking slurry[J]. J Fuel Chem Technol, 2018, 46(4):413-418. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19195.shtml [3] 尚猛, 李传, 邓文安, 田广华, 阙国和.渣油悬浮床加氢裂化油溶性催化剂的性能[J].石油学报(石油加工), 2011, 27(3):361-366. doi: 10.3969/j.issn.1001-8719.2011.03.006SHANG Meng, LI Chuan, DENG Wen-an, TIAN Guang-hua, QUE Guo-he. Performance of residual oil hydrocracking oil-soluble catalyst[J]. Acta Pet Sin (Pet Process Sect), 2011, 27(3):361-366. doi: 10.3969/j.issn.1001-8719.2011.03.006 [4] 倪术荣, 徐伟池, 姜维, 张全国, 郭金涛.非负载型加氢脱硫催化剂研究进展[C]//全国工业催化技术及应用年会. 2016.NI Shu-rong, XU Wei-chi, JIANG Wei, ZHANG Quan-guo, GUO Jin-tao. Research progress on unsupported hydrodesulfurization catalysts[C]//National Industrial Catalysis Technology and Application Annual Meeting. 2016. [5] PANARITI N, BIANCO A D, PIERO G D, MARCHIONNA M, CARNITI P. Petroleum residue upgrading with dispersed catalysts:Part 2. Effect of operating conditions[J]. Appl Catal A:Gen, 2000, 204(2):215-222. doi: 10.1016/S0926-860X(00)00532-9 [6] 刘晨光, 阙国和.孤岛渣油在分散型催化剂存在下加氢裂化反应的研究[J].石油炼制与化工, 1993, 24(3):57-62. http://d.old.wanfangdata.com.cn/Thesis/Y152868LIU Chen-guang, QUE Guo-he. Study on hydrocracking reaction of Gudao residue in the presence of dispersed catalyst[J]. Pet Process Petrochem, 1993, 24(3):57-62. http://d.old.wanfangdata.com.cn/Thesis/Y152868 [7] CLOSE M R, PETERSEN J L, KUGLER E L. Synthesis and characterization of nanoscale molybdenum sulfide catalysts by controlled gas phase decomposition of Mo(CO)6and H2S[J]. Inorg Chem, 1999, 38(7):1535-1542. doi: 10.1021/ic980700t [8] 王亚男, 李广学, 刘银.二硫化钼催化剂的制备与应用[J].广东化工, 2009, 36(3):37-41. http://d.old.wanfangdata.com.cn/Periodical/gdhg200903011WANG Ya-nan, LI Guang-xue, LIU Yin. Preparation and application of molybdenum disulfide catalyst[J]. Guangdong Chem Ind, 2009, 36(3):37-41. http://d.old.wanfangdata.com.cn/Periodical/gdhg200903011 [9] ROMERO-RIVERA R, VALLE M D, ALONSO G, FLORES E, CASTILLON F, FUENTES S, CRUZ-REYES J. Cyclohexene hydrogenation with molybdenum disulfide catalysts prepared by ex situ, decomposition of ammonium thiomolybdate-cetyltrimethylammonium thiomolybdate mixtures[J]. Catal Today, 2008, 130(2):354-360. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=acc4c47f9b64870bef9779bb9db3c1c2 [10] BACKES C, BENERN C, CHEN X, LAFARGUE P, LAPIACE P, FREELEY M, DUESBERG G S, COLEMA J N, MCDONALD A R. Functionalization of liquid-exfoliated two-dimensional 2H-MoS2[J]. Angew Chem Int Ed, 2015, 54(9):2638-2642. doi: 10.1002/anie.201409412 [11] TANG G, WANG Y, CHEN W, TANG H, LI C. Hydrothermal synthesis and characterization of novel flowerlike MoS2 hollow microspheres[J]. Mater Lett, 2013, 100:15-18. doi: 10.1016/j.matlet.2013.02.103 [12] XIE J, ZHANG J, LI S, GROTE F, ZHANG X, ZHANG H, WANG R, LEI Y, PAN B, XIE Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution[J]. J Am Chem Soc, 2014, 136(4):17881-17888. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4d93c0efc67a5a254b3c25799588d58d [13] PENG Y, MENG Z, ZHONG C, LU J, YU W, YANG Z, QIAN Y. Hydrothermal synthesis of MoS2 and its pressure-related crystallization[J]. J Solid State Chem, 2001, 159(1):170-173. doi: 10.1006/jssc.2001.9146 [14] WATANABE I, OTAKE M, YOSHIMOTO M, SAKANISHI K, KORAIY, MOCHIDA I. Behaviors of oil-soluble molybdenum complexes to form very fine MoS2 particles in vacuum residue[J]. Fuel, 2002, 81(11):1515-1520. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5d27bc62af572b0c2d5f30447d255753 [15] 张倩倩, 邓文安, 李传, 沐宝泉.不同有机钼催化剂在重油加氢反应中的差异及其原因初探[J].石油炼制与化工, 2015, 46(5):61-65. doi: 10.3969/j.issn.1005-2399.2015.05.013ZHANG Qian-qian, DENG Wen-an, LI Chuan, MU Bao-quan. Preliminary study on the differences and causes of different organic molybdenum catalysts in heavy oil hydrogenation[J]. Pet Process Petrochem, 2015, 46(5):61-65. doi: 10.3969/j.issn.1005-2399.2015.05.013 [16] SAKANISHI K, WATANABE I, MOCHIDA I. Characterization of soluble Mo complex catalyst during hydro-treatment of vacuum residues[J]. ACS Symp, 2005:197-203. https://www.researchgate.net/publication/286426210_Characterization_of_Soluble_Mo_Complex_Catalyst_during_Hydrotreatment_of_Vacuum_Residues [17] LI J H, WAN D E, MA H J. Ionic liquid assisted hydrothermal synthesis of MoS2 double-shell polyhedral cages with enhanced catalytic hydrogenation activities[J]. RSC Adv, 2017, 7(38):23523-23529. doi: 10.1039/C7RA02482G [18] LI M, WANG D E, LI J H. Facile hydrothermal synthesis of MoS2 nano-sheets with controllable structures and enhanced catalytic performance for anthracene hydrogenation[J]. RSC Adv, 2016, 6(75):71534-71542. doi: 10.1039/C6RA16084K [19] 任萍, 兰新哲, 周军, 宋永辉, 张秋利.水热法制备二硫化钼微球花及其结构表征[J].有色金属(冶炼部分), 2011, (10):47-49. doi: 10.3969/j.issn.1007-7545.2011.10.014REN Ping, LAN Xin-zhe, ZHOU Jun, SONG Yong-hui, ZHANG Qiu-li. Hydrothermal synthesis and characterization of MoS2flower-sphere[J]. Nonferrous Met Extr Metall, 2011, (10):47-49. doi: 10.3969/j.issn.1007-7545.2011.10.014 [20] 黄继武.多晶材料X射线衍射-实验原理、方法与应用[M].北京:冶金工业出版社, 2006:119-132.HUANG Ji-wu. X-ray Diffraction of Polycrystalline Materials-Experimental Principle, Method and Application[M]. Beijing:Metallurgical Industry Press, 2006:119-132. [21] YANG Y Q, TYE C T, SMITH K J. Influence of MoS2 catalyst morphology on the hydride-oxygenation of phenols[J]. Catal Commun, 2008, 9(6):1364-1368. doi: 10.1016/j.catcom.2007.11.035 [22] WU Zhuang-zhi. Preparation and properties of nanostructured molybdenum disulfide (tungsten)[M]. Changsha:Central South University, 2012. [23] DREW M G B, MITCHELL P C H, KASZTELAN S. Computer modelling of a molybdenum disulfide catalyst[J]. Polyhedron, 1990, 8(86):697-702. http://cn.bing.com/academic/profile?id=18f27517dc967535a0b637d31d9f2f7f&encoded=0&v=paper_preview&mkt=zh-cn [24] 李敏, 刘忠杉, 王冬娥, 潘振栋, 马怀军, 姜玉霞, 田志坚.纳米二硫化钼的制备及其蒽加氢性能[J].石油学报(石油加工), 2018, 34(2):253-260. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201802006LI Min, LIU Zhong-shan, WANG Dong-e, PAN Zhen-dong, MA Huai-jun, JIANG Yu-xia, TIAN Zhi-jian. Preparation of nanometer molybdenum disulfide and its hydrogenation performance[J]. Acta Pet Sin(Pet Process Sect), 2018, 34(2):253-260. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201802006 [25] HAO L, XIONG G, LIU L P, LONG H Y, JIN F Y, WANG X S. Preparation of highly dispersed desulfurization catalysts and their catalytic performance in hydrodesulfurization of dibenzothiophene[J]. Chin J Catal, 2016, 37(3):412-419. doi: 10.1016/S1872-2067(15)61017-8 [26] LIU K K, ZHANG W, LEE Y H, LIN Y C, CHANG M T, SU C Y, CHANG C S, LI H, SHI Y M. Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates[J]. Nano Lett, 2012, 12(3):1538-1544. doi: 10.1021/nl2043612 [27] WANG H W, SKELDON P, THOMPSON G E. XPS studies of MoS2 formation from ammonium tetrathiomolybdate solutions[J]. Surf Coat Technol, 1997, 91(3):200-207. doi: 10.1016/S0257-8972(96)03186-6 [28] RAO C N R, NAG A. Inorganic analogues of graphene[J]. Eur J Inorg Chem, 2010, (27):4244-4250. http://d.old.wanfangdata.com.cn/Conference/8706614 [29] BAKER M A, GILMORE R, LENARDI C, GISSLER W. XPS investigation of preferential sputtering of S from MoS2 and determination of MoSx stoichiometry from Mo and S peak positions[J]. Appl Surf Sci, 1999, 150(14):255-262. https://www.sciencedirect.com/science/article/pii/S0169433299002536 [30] 徐忠民, 苏运来, 李全芝, 胡家芬. Pd/HM催化剂的制备及其表面酸性质[J].催化学报, 1994, 15(2):152-156. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400002112XU Zhong-min, SU Yun-lai, LI Quan-zhi, HU Jia-fen. Preparation of Pd/HM catalysts and their surface acid properties[J]. J Catal, 1994, 15(2):152-156. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199400002112 [31] BENRABAA R, LOFBERG A, RUBBENS A, BORDES R E, VANNIER R N, BARAMA A. Structure, reactivity and catalytic properties of nanoparticles of nickel ferrite in the dry reforming of methane[J]. Catal Today, 2013, 203(5):188-195. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6b61646d1000118100a69f7baf07a7d1 [32] LI M, WANG D E, LI J H, PAN Z D, MA H J, JIANG Y X, TIAN Z J, LU A H. Surfactant-assisted hydrothermally synthesized MoS2 samples with controllable morphologies and structures for anthracene hydrogenation[J]. Chin J Catal, 2017, 38(3):597-606. doi: 10.1016/S1872-2067(17)62779-7 [33] 万元洋, 刘威, 黄路露, 沈义, 孙昱.多环芳烃加氢催化剂的研究[J].燃料与化工, 2018, 49(1):36-38. http://d.old.wanfangdata.com.cn/Periodical/rlyhg201801015WAN Yuan-yang, LIU Wei, HUANG Lu-lu, SHEN Yi, SUN Yu. Study on polycyclic aromatic hydrogenation catalysts[J]. Fuel Chem Process, 2018, 49(1):36-38. http://d.old.wanfangdata.com.cn/Periodical/rlyhg201801015 [34] NAGAI M, MIYAO T, TUBOI T. Hydrodesulfurization of dibenzothiophene on alumina-supported molybdenum nitride[J]. Catal Lett, 1993, 18(1/2):9-14. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2504735de5d1671356a9ef7c7c00af86 -





 下载:
下载: