Study on adsorption desulfurization performance of porous microsphere nickel zinc composite adsorbent
-
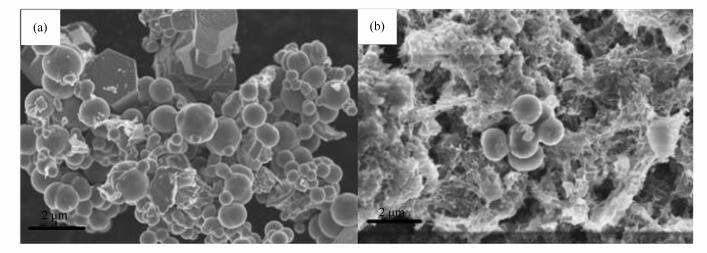
摘要: 考察了葡萄糖量对氧化锌微球形貌的影响,分别采用等体积浸渍法和水热法制备含Ni质量分数为5%的NiO/ZnO微球吸附剂,使用N2吸附-脱附、扫描电子显微镜(SEM)、X射线衍射(XRD)等手段对NiO/ZnO微球吸附剂进行结构和形貌分析,并研究了制备方法对NiO/ZnO微球吸附剂物化性质的影响,再将其经H2还原后制得Ni/ZnO微球吸附剂,在模型汽油中使用噻吩作为含硫化合物,通过固定床反应器进行反应吸附脱硫性能研究。实验表明,与等体积浸渍法制备的负载型NiO/ZnO微球吸附剂相比,水热法制备的复合型NiO-ZnO微球吸附剂的比表面积和孔体积分别高达(40.45 m2/g)和(0.096 cm3/g),还原后所得复合型Ni-ZnO微球吸附剂具有更好的脱硫活性,在吸附温度350℃、压力1.0 MPa、进料液体积空速6 h-1及氢气与模拟油体积比为60的条件下,可以将模拟油中的硫质量含量从1.5×10-4降至10-5以下。并且拥有很好的再生能力,经过多次再生后仍保持很高的脱硫率,具有潜在的工业应用价值。Abstract: The effects of glucose on the morphology of the microspheres of zinc oxide were first studied, and then 5% NiO/ZnO microspheric adsorbents were prepared by impregnation method and hydrothermal synthesis. The morphology and structure of NiO/ZnO microspheres were analyzed by N2 adsorption, scanning electron microscopy (SEM), X-ray diffraction (XRD) methods, and the effects of the preparation method of NiO/ZnO microspheric adsorbents on the physicochemical properties were examined. Furthermore, Ni/ZnO microsphere adsorbent was prepared by reduction with H2, over which the performance of adsorption desulfurization was tested in a fixed bed reactor by using thiophene as the sulfur compounds in model gasoline.The results show that the specific surface area and pore volume of the NiO-ZnO microspheric adsorbents prepared by hydrothermal method are as high as 40.45 m2/g and 0.096 cm3/g, respectively, in contrast to the ones prepared by the impregnation method, and the composite Ni-ZnO microspheric adsorbents exhibit a favorable desulfurization activity. Under the conditions including a temperature of 350℃, a pressure of 1.0 MPa, a volume space velocity of 6 h-1, and a hydrogen to simulated oil volume ratio of 60, the sulphur contents in simulated oil decrease from 1.5×10-4 to less than 10-5. Also, the adsorbents boast a good regeneration capacity and a potential application value in industry.
-
Key words:
- composites /
- microsphere /
- adsorbent /
- adsorption desulfurization /
- zincoxide /
- mesoporous structure
-
表 1 不同样品的BET比表面积和孔体积
Table 1 BET specific surface area and pore volume of different samples
Sample ABET/(m2·g-1) vp/(cm3·g-1) ZnO 37.76 0.083 Supported NiO/ZnO 35.92 0.075 Composite NiO-ZnO 40.45 0.096 -
[1] BABICH I V, MOULIJN J A. Science and technology of novel processes for deep desulfurization of oil refinery streams:a review[J]. Fuel, 2003, 82(6):607-631. doi: 10.1016/S0016-2361(02)00324-1 [2] BEALE A M, GIBSON E K, O'BRIEN M G, JACQUES S D M. Chemical imaging of the sulfur-induced deactivation of Cu/ZnO catalyst bodies[J]. J Catal, 2014, 314(20):94-100. http://eprints.gla.ac.uk/146902/ [3] BABE C, TAYAKOUT-FAYOLLE M, GEANTET C, VRINAT M, BERGERET G, HUARD T, BAZER-BACHI D, Crystallite size effect in the sulfidation of ZnO by H2S:Geometric and kinetic modelling of the transformation[J]. Chem Eng Sci, 2012, 82:73-83. doi: 10.1016/j.ces.2012.07.026 [4] HE W, LI Y, CHEN Z, W YANG, ZHANG R. One-step solution synthesis of monodispersed ZnO nanowhiskers at low-temperature[J]. Mater Lett, 2006, 60(s17-18):2299-2301. [5] FANG Z, TANG K, SHEN G, CHEN D, KONG R. Self-assembled ZnO 3D flowerlike nanostructures[J]. Mater Lett, 2006, 60(20):2530-2533. doi: 10.1016/j.matlet.2006.01.034 [6] YANG Y, YAN H, FU Z, YANG B, ZUO J. Enhanced photoluminescence from three-dimensional ZnO photonic crystals[J]. Sol St Comm, 2006, 139(5):218-221. doi: 10.1016/j.ssc.2006.06.003 [7] YU J, YU X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres[J]. Environ Sci Technol, 2008, 42(13):4902-7. doi: 10.1021/es800036n [8] 郭婷婷, 刘彦平, 叶灵娟, 刘栋.单分散ZnO微球的合成及表征[J].科学技术与工程, 2011(25):6141-6144. doi: 10.3969/j.issn.1671-1815.2011.25.032GUO Ting-ting, LIU Yan-ping, YE Ling-juan, LIU Dong. Synthesis and characterization of monodispersed ZnO microsphere[J]. Sci Technol Eng, 2011(25):6141-6144. doi: 10.3969/j.issn.1671-1815.2011.25.032 [9] KUANG Q, JIANG Z Y, XIE Z X. LIN S C.Tailoring the optical property by a three-dimensional epitaxial heterostructure:a case of ZnO/SnO2[J]. J Am Chem Soc, 2005, 127(33):11777-84. doi: 10.1021/ja052259t [10] ZHAO A, LUO T, CHEN L, LIU Y, LI X G. T G. Synthesis of ordered ZnO nanorods film on zinc-coated Si substrate and their photoluminescence property[J]. Mater Chem Phy, 2006, 99(1):50-53. doi: 10.1016/j.matchemphys.2005.10.013 [11] VAFAEE M, GHAMSARI M S. Preparation and characterization of ZnO nanoparticles by a novel sol-gel route[J]. Mater Lett, 2007, 61(14/15):3265-3268. http://www.oalib.com/references/8825404 [12] SUN X M, LIU J F, LI Y D. Use of carbonaceous polysaccharide microspheres as templates for fabricating metal oxide hollow spheres.[J]. Chem Eur J, 2006, 12:2039-2047. doi: 10.1002/(ISSN)1521-3765 [13] 景茂祥. 氧化铝/镍复合材料制备新工艺及结构和性能研究[D]. 江苏大学, 2007.JING Mao-xiang. Study on New Technology, Structure and Properties of Alumina/Nickel Composites[D]. Jiangsu University, 2007. [14] LI W J, SHI E W, ZHONG W Z, YIN Z W. Growth mechanism and growth habit of oxide crystals[J]. J. Cryst Growth, 1999, 203(1/2):186-196. doi: 10.1007/BF02879530 [15] SONG P, WANG Q, YANG Z. Acetone sensing characteristics of ZnO hollow spheres prepared by one-pot hydrothermal reaction[J]. Mater Lett, 2012, 86(11):168-170. doi: 10.1007/978-3-319-12898-6_1 [16] 徐志兵, 严家平, 吴亮.氧化锌空心球的制备及其光催化性能研究[J].人工晶体学报, 2012, 41(6):1722-1725. http://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201715007.htmXU Zhi-bing, YAN Jia-ping, WU Liang. Preparation and photocatalytic activity of zinc oxide hollow spheres[J].J Synth Cryst, 2012, 41(6):1722-1725. http://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201715007.htm [17] 邓文雅, 赵宗彬, 沈琳, 邱介山.氧化锌空心球的制备及光致发光特征[J].功能材料, 2007, 38(9):1559-1562. http://www.cnki.com.cn/Article/CJFDTOTAL-GNCL200709048.htmDENG Wen-ya, ZHAO Zong-bin, SHEN Lin, QIU Jie-shan. Preparation and photoluminescence characteristics of zinc oxide hollow spheres[J]. Fun Mat, 2007, 38(9):1559-1562. http://www.cnki.com.cn/Article/CJFDTOTAL-GNCL200709048.htm [18] ROUQUEROL F, ROUQUEML J, SING K. Adsorption by Powders and Porous Solides:Principles Methodology And Applications, Academic Press:Harcourt Brace & Company, 1999. [19] Jing Z H, Zhan J H. Fabrication and gas-sensing properties of porous ZnO nanoplates[J]. Ad Mat, 2008, 20:4547-4551. doi: 10.1002/adma.v20:23 [20] Xu L, Huang W Q, Wang L L, Tian Z A, Hu W Y. Insights into enhanced visible-light photocatalytic hydrogen evolution of g-C3N4 and highly reduced graphene oxide composite:The role of oxygen[J]. Chem Mat, 2015, 27(5). http://www.sciencedirect.com/science/article/pii/S0169433217322900 [21] 赖梨芳, 杨骞, 纪云洲, 王利娟, 戎红仁.不规则形ZnO纳米棒的制备及其光催化性能研究[J].化学研究与应用, 2012, 24(6):866-872. http://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ201206006.htmLai Li-fang, Yang Qian, JI Yun-zhaou, Wang Li-juan, Rong Hong-ren. Preparation and photocatalytic performance of irregular ZnO nanorods[J]. Chem Res Application, 2012, 24(6):866-872. http://www.cnki.com.cn/Article/CJFDTOTAL-HXYJ201206006.htm [22] 周广林, 孙文艳, 姜伟丽. Ni/ZnO-SiO2-Al2O3吸附剂上加氢汽油的深度临氢吸附脱硫[J].石油炼制与化工, 2013, 44(7):17-21.ZHOU Guang-lin, SUN Wen-yan, JIANG Wei-li. Deep hydrogen adsorption desulfurization of hydrogenated gasoline on Ni/ZnO-SiO2-Al2O3 adsorbent[J]. Petroleum Process Petrochemicals, 2013, 44(7):17-21. [23] JIRATOVA K, JANACEK L. Effect of peptizing on the physical and chemical properties of extruded alumina[J]. Int Chem Eng, 1983, 23(1):167-174. -





 下载:
下载: